Abstract
Background
Stroke patients should be cared for in accordance with evidence-based guidelines. The extent of implementation of guidelines for the acute care of stroke patients in Germany has been unclear to date.
Methods
The regional quality assurance projects that cooperate in the framework of the German Stroke Registers Study Group (Arbeitsgemeinschaft Deutscher Schlaganfall-Register, ADSR) collected data on the care of stroke patients in 627 hospitals in 2012. The quality of the acute hospital care of patients with stroke or transient ischemic attack (TIA) was assessed on the basis of 15 standardized, evidence-based quality indicators and compared across the nine participating regional quality assurance projects.
Results
Data were obtained on more than 260 000 patients nationwide. Intravenous thrombolysis was performed in 59.7% of eligible ischemic stroke patients patients (range among participating projects, 49.7–63.6%). Dysphagia screening was documented in 86.2% (range, 74.8–93.1%). For the following indicators, the defined targets were not reached for all of Germany: anti-aggregation within 48 hours, 93.4% (range, 86.6–96.4%); anticoagulation for atrial fibrillation, 77.6% (range, 72.4–80.1%); standardized dysphagia screening, 86.2% (range, 74.8–93.1%); oral and written information of the patients or their relatives, 86.1% (range, 75.4–91.5%). The rate of patients examined or treated by a speech therapist was in the target range.
Conclusion
The defined targets were reached for most of the quality indicators. Some indicators, however, varied widely across regional quality assurance projects. This implies that the standardization of care for stroke patients in Germany has not yet been fully achieved.
Stroke is one of the most common disorders in Germany, with an estimated 200 000 first events and 66 000 recurrent events in 2008 (1). Stroke is also one of the most common causes of morbidity and mortality in adults. More than 40% of patients have a poor outcome after three months after their first stroke—defined as “dead” or “dependent” (2). Evidence-based approaches to the early treatment, secondary prevention, and rehabilitation of stroke patients exist, such as:
Thrombolysis for patients after cerebral infarction,
Antiplatelet therapy in patients with cerebral infarction or transient ischemic attack (TIA),
Anticoagulation for patients with atrial fibrillation (3).
Since 1994 a number of regional stroke registers have been established for the purpose of external quality assurance in acute inpatient stroke treatment.
Since 1999 these regional registers have been collaborating within the framework of the Arbeitsgemeinschaft Deutscher Schlaganfall-Register (ADSR, German Stroke Registers Study Group). The ADSR was established in order to standardize the collection of data in the area of acute hospital stroke care and in order to develop consistent, standardized quality indicators (5). Additionally, data from the participating registers are pooled regularly and jointly scientifically evaluated. The first data pooling exercise was undertaken for the years 2000 to 2005 and supported by the German stroke competence network (5).
This article aims to present the quality indicators for acute hospital care of stroke patients in Germany, on the basis of the pooled results from the ADSR from 2012.
Methods
The German Stroke Registers Study Group (ADSR)
The ADSR is a voluntary association of regional quality assurance projects regarding the disease entity that is stroke. Currently, nine regional quality assurance projects are collaborating (the stroke registers for Bavaria, Baden-Württemberg, Berlin, Hamburg, Hesse, North Rhine, Northwest Germany, Rhineland-Palatinate, Schleswig-Holstein), as well as the population based stroke register Erlangen (www.adsr.uni-wuerzburg.de). The registers participating in the ADSR compare the regional and supraregional stroke care of the participating hospitals under scientific, quality relevant, and epidemiological aspects, while anonymity is guaranteed. Furthermore, regular joint data poolings are undertaken under the auspices of the designated ADRS methodology center (Institut für Klinische Epidemiologie und Biometrie [IKE-B], Universität Würzburg—the Institute of Clinical Epidemiology and Biometry [ICE-B], University of Würzburg). The data are collected mainly electronically, with defined completeness and plausibility checks, by the respective quality assurance projects. Only data sets that have been checked for plausibility are submitted to the data pooling center.
Development of the quality indicators
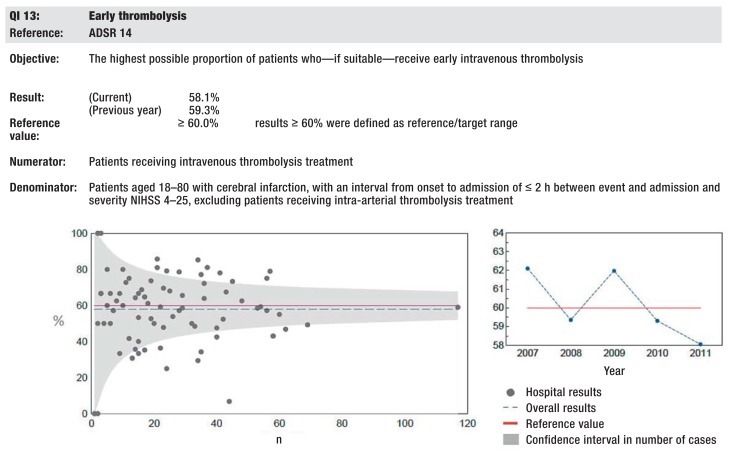
In 2003 a multidisciplinary quality indicator board of the ADSR was founded. In 2006 this board for the first time published standardized, evidence-based indicators that had been developed using a standardized process for the purpose of measuring quality indicators of acute stroke care in Germany (5). Among others, this process entailed a systematic literature review, independent external validation, as well as a prospective pilot study (5). The data set specification and the accompanying data collection manual were finalized on the basis of the pilot study‘s results. Since 2007 this manual has been consistently used by the regional registers (5). The developed quality indicators are regularly updated by the quality indicator board. In 2010, five additional patient-related quality indicators were introduced. Furthermore, target ranges for defined quality indicators were developed, using the results of the regional quality assurance projects and a standardized consensus process (1). The data specification, instructions for completion, and calculation rules for the quality indicators are regularly updated by the ADSR‘s technical committee. Currently a set of 19 evidence-based indicators is being used to monitor the quality of stroke care in the participating hospitals. The definitions of the quality indicators are listed in the eBox. The next update of the quality indicators is planned for 2014. The eFigure shows exemplarily how the results are reflected back to the participating hospitals.
eBox. Definition of quality indicators.
1. Antithrombotic therapy—antiaggregation ≤ 48 h after stroke
Numerator: Number of patients receiving platelet inhibitors within the first 48 hours after the stroke
Denominator: All patients with TIA or cerebral infarction. Excluded are: patients receiving anticoagulation, patients <18 years, and patients with interval onset to admission >48 h.
Reference/target range: ≥95%
2. Antithrombotic therapy—antiaggregation as secondary prophylaxis
Numerator: Number of patients receiving platelet inhibitors at discharge
Denominator: All patients with TIA or cerebral infarction. Patients receiving anticoagulation, patients <18 years, and patients with discharge status “deceased” are excluded.
Reference/target range: ≥95%
3. Antithrombotic therapy—anticoagulation in atrial fibrillation
Numerator: Number of patients receiving therapeutic anticoagulation at discharge or with a recommendation for anticoagulation in their discharge notes
Denominator: All patients with TIA or cerebral infarction and atrial fibrillation, who are discharged home or to a rehabilitation hospital and are mobile (operationalized by using categories 10–15 in item “change of position from bed to chair” and categories 10–15 in item “locomotion” in the Barthel index at discharge) and minimally impaired (operationalized by using Rankin scale 0–3 at discharge); patients <18 are excluded.
Reference/target range: ≥80%
4. Brain imaging in patients with suspected stroke
Numerator: Number of patients receiving brain imaging (CCT and/or NMR)
Denominator: All documented patients
Reference/target range: ≥95%
5. Vascular imaging in cerebral infarction and TIA
Numerator: All patients with extracranial vascular imaging (Doppler ultrasound and/or duplex sonography and/or digital subtraction angiography and/or magnetic resonance/computed tomography angiography)
Denominator: All patients with cerebral infarction or TIA
Reference/target range: ≥90%
6. Screening for dysphagia
Numerator: All patients with swallowing test according to protocol with minimal requirements (for example, “includes stepwise water swallowing test of at least 50/mL”)
Denominator: All patients with stroke and a minimum stay ≥ 1 day; patients with TIA and patients with impaired consciousness are excluded
Reference/target range: ≥90%
7. Early rehabilitation—physiotherapy/occupational therapy
Numerator: Number of patients examined by and/or receiving treatment from a physiotherapist and/or occupational therapist ≤ day 2 after admission
Denominator: All patients with documented paresis and notable functional impairment (Rankin scale ≥ 3 and/or sum Barthel index ≤ 70 within the first 24 hours after admission) with a minimum stay of ≥ 1 day; patients who are comatose at admission and patients with a diagnosis of TIA are excluded
Reference/target range: ≥90%
8. Early rehabilitation—speech therapy
Numerator: Number of patients who were examined by and/or received treatment from a speech therapist ≤ day 2 after admission
Denominator: All patients with documented aphasia and/or dysarthria and/or dysphagia at admission and a minimum stay of ≥ 1 day. Patients who are comatose at admission and patients with TIA are excluded
Reference/target range: ≥80%
9. Early mobilization
Numerator: Number of patients who were mobilized within 2 days after admission
Denominator: All patients who changed position from bed to chair “with support” or found it “impossible” (operationalized by categories 0–10 in item “change of position from bed to chair” in the Barthel index within the first 24 hours after admission) with a minimum stay ≥ 1 day; patients with TIA and/or intracranial pressure and/or ventilation and/or coma at admission are excluded
Reference/target range: ≥90%
10. Information for patients and their relatives
Numerator: Number of patients (or their relatives) who received verbal and written information before discharge
– On the disease course/prevention by the doctor
– On support offers by social/care services
Denominator: Patients with a minimum length of stay ≥ 1 day; patients with a diagnosis of TIA and patients with a discharge status “deceased” were excluded
Reference/target range: ≥90%
11. Patients with brain imaging within 1 h of admission in patients admitted within 2 h after stroke onset
Numerator: All patients with interval admission to first imaging procedure (CCT and/or MRI) ≤ 1 h
Denominator: All patients with interval between onset and admission ≤ 2 h and sufficient stroke severity to receive intravenous thrombolysis (NIHSS 4–25) and age between 18 and 80 years
Reference/target range: ≥90%
12. Early systemic thrombolytic therapy in eligible patients
Numerator: All patients receiving intravenous thrombolysis
Denominator: All patients with cerebral infarction and a time interval between onset and admission of ≥ 2 hours, as well as sufficient stroke severity to conduct intravenous thrombolysis (NIHSS 4–25), aged between 18 and 80 years. Patients with intra-arterial thrombolysis are excluded.
Reference/target range: ≥60%
13. Pneumonia rate after stroke
Numerator: Number of patients with pneumonia as a complication
Denominator: All patients with cerebral infarction
Reference/target range: —
14. In-hospital mortality after acute stroke
Numerator: Number of patients with discharge status deceased on day 7
Denominator: All patients with cerebral infarction. Patients with length of stay ≤ 7 days and who were discharged into another acute hospital, other department, rehabilitation ward, or care home are excluded
Reference/target range: —
15. Mortality after thrombolysis (since 2010)
Numerator: Patients who died within 7 days after thrombolysis
Denominator: All patients receiving thrombolysis
Reference/target range: —
16. Revascularization of carotid artery stenosis (since 2010)
Numerator: Patients with a recommendation for revascularization in their referral letter, or referral to revascularization, or who have undergone revascularization
Denominator: All patients with cerebral infarction/TIA and symptomatic carotid artery stenosis ≥ 70%
Reference/target range: —
17. Door to needle time ≤ 60 min if time between onset and admission ≤ 2 h (since 2010)
Numerator: Patients with a time window from admission to start of thrombolysis ≤ 60 minutes
Denominator: All patients receiving thrombolysis
Reference/target range: —
18. Treatment in stroke unit (since 2010)
Numerator: Patients admitted to stroke unit
Denominator: All patients with stroke/TIA who were admitted to the hospital
Reference/target range: —
19. Discharge destination rehabilitation of patients with impairments affecting everyday life (since 2010)
Numerator: All patients with the discharge destination of outpatient or inpatient rehabilitation (to start imminently, organized and registered by the hospital, not necessarily direct transfer to rehabilitation)
Denominator: All patient with stroke, mRS 2–5 at discharge, without prior stay in a care home, age <80. Patients transferred to another acute hospital or ward are excluded
Reference/target range: —
CCT, cranial computed tomography
NIHSS, National Institutes of Health Stroke Scale
MRI, magnetic resonance imaging
NMR, nuclear magnetic resonance
mRS, modified Rankin scale
TIA, transient ischemic attack
eFigure.
Exemplary illustration of the quality indicators (QI) in registers‘ reports using the example of QI early thrombolysis in Bavaria in 2011 on the basis of a funnel plot (www.baq-bayern.de). QI, quality indicator; ADSR: German Stroke Registers Study Group; NIHSS, National Institutes of Health Stroke Scale
Statistical analyses
The characteristics of the patients documented in the participating registers were extensively described (Table 1). The quality of acute hospital care was operationalized on the basis of standardized developed quality indicators. The definition of the quality indicators, including inclusion criteria and exclusion criteria and possible risk adjustment, is shown in the eBox. Multiple logistic regression analyses were performed, with the respective quality indicators as dependent variables and the registers, age, sex, and stroke subtype (if applicable) as independent variables, in order to calculate adjusted P values for differences between the participating registers (Table 2). As sensitivity analyses, the quality indicators were additionally calculated for registers that participated compulsorily in 2012 (Baden-Württemberg, Hamburg, Hesse, Rhineland-Palatinate) and compared with the overall average.
Table 1. Patient characteristics.
| Total | Range total | Data from quality assurance projects with mandatory documentation | |||
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| Age. years. mean (SD) | 72.5 (13.3) | 71.6 (13.4) | 73.6 (12.9) | 73.2 (13.3) | |
| Women % | 49.5 | 48.9 | 50.4 | 49.6 | |
| Stroke type % | |||||
| Ischemic stroke | 64.9 | 60 | 70.3 | 61.8 | |
| Intracranial hemorrhage | 6.5 | 3.9 | 8.5 | 7.9 | |
| Subarachnoid hemorrhage | 0.5 | 0.3 | 2 | 0.5 | |
| Transient ischemic attack (TIA) | 26.6 | 24.6 | 33 | 27.2 | |
| Unknown | 1.4 | 0.1 | 4.7 | 2.7 | |
| Comorbidities % | |||||
| Atrial fibrillation | 25.6 | 22.8 | 28.1 | 24.3 | |
| Diabetes | 26 | 21 | 28.4 | 23.8 | |
| Hypertension | 80.5 | 62.7 | 84.6 | 75.2 | |
| Intravenous thrombolysis after cerebral infarction % | 13.6 | 9.5 | 15.1 | 11.8 | |
| Door to needle time in hours %* | |||||
| ≤ 0.5 | 30.7 | 15.1 | 51.1 | 28.4 | |
| >0.5–1 | 49.1 | 32.7 | 56.4 | 50.6 | |
| >1–2 | 14.7 | 10.7 | 24.9 | 13.7 | |
| >2–3 | 3.6 | 2.7 | 6.8 | 4.7 | |
| >3–6 | 1.6 | 0.4 | 2.9 | 1.9 | |
| >6 | 0.4 | 0.1 | 1.6 | 0.8 | |
| Time to admission in hours % | |||||
| ≤2 | 27.4 | 24 | 31.1 | 27.8 | |
| >2–3 | 12.1 | 9.9 | 13.7 | 12 | |
| >3–6 | 20 | 16.9 | 22.3 | 20.3 | |
| >6–24 | 21 | 19.2 | 26.5 | 21.4 | |
| >24–48 | 7.5 | 7.3 | 8.4 | 7.6 | |
| >48 | 11.9 | 9.8 | 14.3 | 10.9 | |
| Length of stay. Days. mean (SD) | 8.8 (7.5) | 8.2 | 10.1 | 8.7 (8.0) | |
*Data not consistently documented in one quality assurance project
Table 2. Target ranges and results of the 15 quality indicators.
| Quality indicator | Target range% | Total.% | Quality assurance projects with mandatory participation. % | Total range | p value*1 | |
|---|---|---|---|---|---|---|
| Minimum | Maximum | |||||
| Antiaggregation ≤ 48h after stroke | ≥ 95 | 93.4 | 91.7 | 86.6 | 96.4 | <0.0001 |
| Antiaggregation as secondary prevention | ≥ 95 | 95.2 | 93.7 | 87.4 | 98 | <0.0001 |
| Anticoagulation in atrial fibrillation | ≥80 | 77.6 | 75.2 | 72.4 | 80.1 | <0.0001 |
| Brain imaging in patients with suspected stroke*2 | ≥95 | 99.4 | 99 | 98.6 | 99.9 | <0.0001 |
| Vascular imaging in cerebral infarction and transient ischemic attack (TIA) | ≥90 | 93.8 | 89.8 | 84.8 | 96.7 | <0.0001 |
| Dysphagia screening | ≥90 | 86.2 | 88.4 | 74.8 | 93.1 | <0.0001 |
| Early rehabilitation—physiotherapy/occupational therapy | ≥90 | 94.4 | 93.3 | 90.4 | 97.8 | <0.0001 |
| Early rehabilitation—speech therapy | ≥80 | 88.5 | 85.9 | 81.7 | 91.3 | <0.0001 |
| Early mobilization*3 | ≥90 | 91.4 | 91.3 | 86.8 | 92.7 | <0.0001 |
| Information for patients and their relatives *3 | ≥90 | 86.1 | 87.6 | 75.4 | 91.5 | <0.0001 |
| Patients with brain imaging within 1 h of admission in patients admitted within 2 h after stroke onset *3 | ≥90 | 95.5 | 95.1 | 93.5 | 96 | <0.01 |
| Early systemic thrombolysis in eligible patients*3 | ≥60 | 59.7 | 56.9 | 49.7 | 63.6 | <0.0001 |
| Door to needle time ≤ 60min. when intervall onset/admission ≤ 2h | – | 98.7 | 98.3 | 95.6 | 99.2 | <0.001 |
| Treatment in a stroke unit*4 | – | 77.2 | 68 | 65.9 | 84.1 | <0.0001 |
| Discharge destination rehabilitation of patients with disabilities impairing everyday activities*4 | – | 75.1 | 64.9 | 54.7 | 80.1 | – |
In bold print: quality indicators for which target ranges were not achieved in the nationwide mean 2012
*1p values each relate to one test for significant differences between registers with regard to likelihood of success of achieving one quality indicator
*2Computed tomography or magnetic resonance imaging
*3Data not consistently documented in one quality assurance project
*4Data not included in the following registers: Hamburg. Schleswig–Holstein. Rhineland–Palatinate
Results
Characteristics of participating registers
Nationwide in 2012, the registers collected the data of more than 260 800 stroke patients from 627 hospitals (eTable). Participation in the documentation in 2012 was extensively mandatory in Baden-Württemberg, Hamburg, Hesse, and Rhineland-Palatinate for hospitals treating stroke patients. Since 2006, participation in an ADSR quality assurance project has also been mandatory for hospitals that are certified regional or supraregional stroke units according to criteria of the German Stroke Society and the German Stroke Foundation (6). Since January 2012, a documentation rate in one of the quality assurance projects participating in the ADSR of >90% of all patients with acute stroke who were treated in the department has been a controlled criterion in the context of such stroke unit certification (see current certification criteria of the German Stroke Society, www.dsg-info.de [in German]).
eTable. Characteristics of participating quality assurance projects (2012).
| Quality assurance project | Region | Mandatory participation | Number of participating institutions | Number of patients | Responsible body | Contact |
|---|---|---|---|---|---|---|
| Baden–Württemberg | Entire federal state | Yes | 149 | 39756 | Office for Quality Assurance in Hospitals (GeQiK) Stuttgart at Baden-Württembergische Hospital Federation | Dr. Ingo Bruder |
| Bavaria | Entire federal state | No | 82 | 41034 | Bavarian Permanent Working Party for Quality Assurance (BAQ). Bavarian Hospital Federation | Dr. Melanie Eßer |
| Berlin | Entire federal state | No | 17 | 11011 | Berlin Stroke Register. Berlin Medical Association | Dr. Barbara Hoffmann |
| Hamburg | Entire federal state | Yes | 16 | 8488 | Association for Quality Assurance Hamburg (EQS) | Ralf Hohnhold |
| Hesse | Entire federal state | Yes | 86 | 23916 | Institute of Quality Assurance Hesse (GQH); Hesse Hospital Federation | Dr. Björn Misselwitz |
| North Rhine | Parts of North Rhine–Westphalia | No | 35 | 21528 | The Quality Assurance in the Care of Stroke Patients Project. North Rhine Medical Association | Dr. Alfred Janssen |
| Northwest Germany | Parts of North Rhine–Westphalia. Lower Saxony. Mecklenburg– Western Pomerania. Saxony. Thuringia. Saxony–Anhalt. Saarland. Brandenburg | No | 155 | 89438 | The Quality Assurance in Stroke Care for Northwest Germany Project. Institute of Epidemiology and Social Medicine. University of Münster | Prof. Klaus Berger |
| Rhineland–Palatinate | Entire federal state | Yes | 74 | 19114 | Institute of Quality Assurance Rhineland–Palatinate / SQMed | Dr. Christoph Burmeister |
| Schleswig-Holstein | Entire federal state | No | 13 | 6563 | Quality Association for Acute Stroke Treatment Schleswig–Holstein (QugSS). University Hospital Schleswig–Holstein. Institute of Social Medicine and Epidemiology | Dr. Christine Matthis |
| ADSR | All quality assurance projects | – | 627 | 260848 |
ADSR. German Stroke Registers Study Group
Patient characteristics
The mean patient age was 73 years, 50% were women (Table 1). The distribution of stroke subtypes, comorbidities, length of stay, admission times, and the proportion of patients receiving thrombolysis are shown in Table 1. Wide ranges in some of the individual parameters were observed between registers, especially in the proportions of different stroke subtypes and the admission and “door to needle” times.
Quality indicators
The ADSR‘s quality indicator board defined in 2010 target ranges for most of the indicators. These target ranges, as well as overall means and the lowest and highest values achieved among participating registers, are shown in Table 2. For seven quality indicators the target ranges were achieved in the overall average. For four quality indicators the means of all registers achieved the target ranges (brain imaging, early physiotherapy, early speech therapy, brain imaging within 1 h of admission in patients admitted within 2 h after stroke onset). All quality indicators varied significantly in terms of the extent to which they were met across the different registers. Considerable differences between individual quality assurance projects were seen especially in:
The proportion of eligible patients who received thrombolysis (target: ≥ 60%, total: 59.7%; range 49.7–63.6%),
The proportion of patients who were screened for dysphagia (target: ≥ 90%; total: 86.2%; range 74.8–93.1%),
The administration of indicated anticoagulants in patients with atrial fibrillation (target: ≥ 80%; total: 77.6%; range: 72.4–80.1%).
Not achieved in all of Germany were in particular the defined target ranges in:
The administration of anticoagulants in atrial fibrillation (target: ≥ 80%; total: 77.6%; range 72.4–80.1%),
Standardized screening for dysphagia (target: ≥ 90%; total: 86.2%; range 74.8–93.1%),
Verbal and written information for the patients or their relatives (target: ≥ 90%; total: 86.1%; range: 75.4–91.5%).
With regard to the treatment of dysphagia, the rate of patients examined or treated by a speech therapist was in the target range (target: ≥ 80%; total: 88.5%; range: 81.7–91.3%). No big differences were found between quality assurance projects with mandatory documentation compared with the overall average.
Discussion
The present study describes the quality of acute stroke care in 627 hospitals in Germany in 2012 on the basis of standardized quality indicators that are documented in the framework of regional quality assurance projects. The target ranges defined for the quality indicators are often already achieved at register level. By contrast, however, large differences exist between individual registers. The indicators in which the defined target ranges are currently not yet achieved nationwide include in particular: administration of anticoagulants in atrial fibrillation, standardized screening for dysphagia, and verbal and written information provided to the patients and their relatives. For all quality indicators, significant differences exist in terms of the extent to which they were met across the participating registers.
International comparison
Comparable data for 2012 from other European countries are currently lacking. A comparison with data from the Minnesota Stroke Registry Program from the US, in which 44 hospitals documented the quality of care in 2013, shows comparable results for antithrombotic therapy at discharge (Minnesota: 98.2%, ADSR: 95.2%) as well as for antithrombotic therapy during the first 48 hours (Minnesota: 97.6%; ADSR: 93.4%). The proportion of patients with atrial fibrillation and anticoagulation is notably higher in Minnesota (Minnesota: 94.6%, ADSR: 77.6%), whereas the proportion of patients receiving standardized screening for dysphagia is higher in Germany (Minnesota: 70.8%, ADSR: 86.2%) (7).
Data from earlier years with a standardized extensive quality assurance program—such as Britain (excluding Scotland) or Catalonia/Spain—indicate that in Germany, comparable or higher proportions of patients had received appropriate secondary prevention or were screened for dysphagia. In Britain in 2010, 84% of patients were screened for the presence of dysphagia, and in Catalonia/Spain in 2007, 45.8% of patients were screened (ADSR: 86.2%). 77% of patients with ischemic stroke received antithrombotic therapy in Catalonia/Spain during the first 48 hours; in Britain, that proportion was 93% (ADSR: 93.4%) (8, 9). In the area of secondary prevention at discharge from acute hospital care, the ADSR results are also comparable with earlier rates from Britain and Catalonia/Spain or substantially higher—for example, with regard to antiaggregation at discharge from acute hospital care (Catalonia/Spain: 96.6%; ADSR: 95.2%) or administration of anticoagulants in atrial fibrillation (Catalonia/Spain: 66.2%; ADSR: 95.2%) (8, 9).
Regarding recanalization therapy after ischemic stroke, the Swedish register RIKS-Stroke reports rates of 13.3%/10.4% for all men/women younger than 80 with ischemic stroke who were treated in Swedish acute hospitals and received recanalization treatment (thrombolysis/thrombectomy) in 2012 (10). In ADSR in 2012, an average of 13.6% of all ischemic strokes were treated with systemic thrombolysis, with the rates between the quality assurance projects ranging from 9.5% to 15.1%.
The usefulness of routine data for quality assurance purposes
The use of so-called routine data (“diagnosis-related groups” [DRG], billing data) to describe quality of care is increasingly the subject of discussion (11). Using routine data for quality assurance has the advantage that these data are easily available and complete. However, in spite of these advantages, in the area of complex disorders—for example, stroke—there are arguments in favor of the additional effort of the independent collection of data that are not included in routine documentation (especially in the acute treatment of stroke) (12, 13). These include the collection of stroke severity data for the purposes of adjusting for case mix, and the standardized documentation of complications or of multidisciplinary measures, such as dysphagia screening. Furthermore, in a prospective data collection of stroke-specific information, certain imprecisions will not occur, such as can happen when using routine data. These include the incorrect coding of diagnoses in the hospital‘s billing system (14), the frequently non-standardized documentation of comorbidities or risk factors, as well as lacking data on defined, stroke-related neurological deficits that necessitate certain diagnostic/therapeutic approaches (for example, early mobilization in impaired mobility) (14). Because of the comprehensive data collection and the large number of documented patients, the ADSR‘s data pool is one of the largest national quality assurance projects in the area of acute stroke care (15).
To improve the relevance of the ADSR data further, a number of improvement measures are required. It is estimated that in Germany, about 70% of first or recurrent strokes are documented in the ADSR‘s collaborating quality assurance projects (11). The completeness of the documentation in the participating hospitals can be determined by using a so-called quality assurance filter, which also enables a comparison between billed and transmitted data sets. This is already being successfully used in a number of registers, such as Hesse (16), Hamburg, Rhineland–Palatinate, and Baden–Württemberg. Similarly, in some registers, data validation procedures were implemented—for example, checking the reliability of the data on a random basis by on-site monitoring. The standardized implementation of quality assurance filters in the remaining registers should be a further objective for the future development of standardized data documentation.
In future, further phases of stroke care should be added to the data collection and comparative evaluation in acute care after stroke, in order to enable cross-sectoral quality assurance. Recently, 18 quality indicators for the area of rehabilitation of stroke patients were proposed in the framework of a model project by the Berlin Stroke Alliance‘s working group “core data set” (17). Since January 2012, these indicators for the specific description of stroke treatment during the rehabilitation phase have been documented in 10 institutions participating in the Berlin Stroke Alliance (see Dohle et al, Evidenzbasierte Qualitätsindikatoren in der neurologischen Rehabilitation – Erfahrungen aus der Berliner Schlaganfallallianz. 86. Kongress der Deutschen Gesellschaft für Neurologie 2013). Comparable projects have also successfully been established in other regions. Data on inpatient rehabilitation after stroke have been collected and evaluated for quality assurance purposes in Hesse since 1998 and in Hamburg since 2012 (18) (see Seidel G et al, Qualitätsmanagement in der neurologischen Frührehabilitation für Schlaganfallpatienten. Neurol Rehabil 2013: 347). Furthermore, only few data are available to date on the care of stroke patients and outcomes after discharge from acute hospital care and inpatient rehabilitation. Some regional registers have performed follow-ups after three months or six months (19– 22). Further follow-up projects on outcomes and quality of care are in the planning stages, based on jointly developed standards (1). Especially as far as the long-term sequelae of stroke are concerned, hardly any data exist in Germany, and the same is true for further outpatient treatment. The Erlangen Stroke Register is currently the ADSR‘s only quality assurance project that collects data on long-term outcomes (23). In order to answer further research questions—such as the identification of predictors for the long-term outcome after stroke—with regard to morbidity and mortality, additional follow-up projects will need to be set up. To this end, relevant data on health and mortality after discharge from the acute hospital will have to be collected in a standardized way using a uniform methodology.
Limitations of the study
The data used in the study were documented primarily for the purpose of quality assurance. They were checked for plausibility before they were sent to the data pooling center, but possible coding errors at hospital level cannot be ruled out since no consistent monitoring of the data was being undertaken. Not all registers implemented a quality assurance filter in 2012 in order to ensure completeness of the documentation. In four registers, documentation is mandatory, whereas this is otherwise required only for the stroke units certified according to the requirements of the German Stroke Society/German Stroke Foundation. In registers without mandatory participation, “hospitals with regional or supraregional stroke unit” are probably over-represented because of the above-mentioned certification requirement. For this reason, registers with mandatory documentation might reflect the German care situation more precisely (at least in the less densely populated states). In spite of all this, no major differences were observed with regard to patient characteristics or the results between registers with mandatory participation and all registers.
Conclusion
Acute hospital care for stroke patients in Germany is excellent, according to data from the ADSR for 2012. The defined target ranges are already being achieved for many of the standardized quality indicators. The wide ranges with significant differences in the degree to which individual quality indicators have been met between individual quality assurance projects show, however, that stroke care in Germany has not been fully standardized yet. No major differences exist for the quality of care between quality assurance projects with mandatory documentation compared with the overall average. The data from the ADSR‘s regional quality assurance projects are a unique data source for finding answers to further scientific questions regarding the improvement of care for stroke patients in Germany—for example, regarding the identification of institutional factors for the quality of care or the investigation of time trends in stroke care.
Key Messages.
Data on acute inpatient care given to more than 260 000 stroke patients were collected in 2012 by 627 hospitals in the participating registers of the German Stroke Registers Study Group (ADSR)
Nationwide, the target ranges were achieved for 7 of 15 quality indicators; for 4 quality indicators the mean values of all registers reached the target ranges.
For all quality indicators, significant differences were observed across the different registers with regard to the degree to which the individual indicators were met.
Considerable differences between the quality assurance projects were observed for intravenous thrombolysis, screening for dysphagia, and anticoagulation in atrial fibrillation.
The defined target ranges were not reached on a nationwide basis for antiplatelet therapy within 48 h, administration of anticoagulants in atrial fibrillation, screening for dysphagia, and information for patients and their relatives. With regard to treatment for dysphagia, the rate of patients examined or treated by a speech therapist was within the target range.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
The authors thank all participating hospitals that participate in the individual registers in the ADSR.
The following stroke registers participate in the German Stroke Registers Study Group (ADSR):
Bavaria stroke register: Prof. Dr. med. P. Hermanek, Dr. med. M. Eßer;
Baden–Württemberg stroke register: Dr. med. I. Bruder;
Berlin Stroke Register: Dr. med. B. Hoffmann, Berlin Medical Association, Priv.-Doz. Dr. med. H.C. Koennecke, Vivantes Klinikum am Friedrichshain;
Stroke Register Hamburg: R. Hohnhold, EQS-Hamburg Landesgeschäftsstelle Qualitätssicherung [regional office for quality assurance] (EQS);
Stroke Register Hesse: Dr. med. B. Misselwitz, A. Reihs;
Stroke Register North Rhine: Dr. med. A. Janssen;
Stroke Register of Northwest Germany: M. Kalic;
Stroke Register Rhineland–Palatinate: Dr. med. C. Burmeister, Dr. med. S. Dienlin;
Stroke Register Schleswig–Holstein: Prof. Dr. med. H. Raspe;
Erlangen Stroke Register: Prof. Dr. med. P. Kolominsky-Rabas MBA
Technical committee of the ADSR: Dr. med. I. Bruder, Dr. med. S. Dienlin, Dr. med. M. Eßer, Prof. Dr. med. P. Heuschmann, Dr. med. B. Misselwitz, A. Reihs
Funding:
Parts of the data pooling exercise were financially supported within the framework of the FP7 program by the European Union European Implementation Score Collaboration [EIS]; No. 223153).
The Erlangen Stroke Register receives funding from the Federal Ministry of Health in the context of the Federal Health Reporting (GBE) initiative (ref IIA5–2013–25413KEU305).
Footnotes
Conflict of interest statement
Prof. Busse has received travel expenses from Boehringer-Ingelheim.
Prof. Seidel has received financial funding for scientific events from Bayer Health Care, Biotronic, Boehringer-Ingelheim, Biogen Idec, Bracco, Bristol-Myers Squibb, Grifols, Pfizer, Philips, Genzyme, Merck Serono, Noras, Novartis, Takeda, Teva, UCB, HOCOMA, Braun, Shire, Talecris, Sanofi Aventis, MSD, Meda, Desitin, Krauth+Timmermann. He is the director of a stroke unit that contributes data to the project and has received author fees for the book “Stroke XXS pocket—Behandlungsmanual zur Schlaganfallbehandlung. He received study support (third party funding) from Asklepios Hospitals, Hamburg.
The other authors declare that no conflict of interest exists.
References
- 1.Heuschmann PU, Busse O, Wagner M, et al. Frequency and care of stroke in Germany. Akt Neurologie. 2010;37:333–330. [Google Scholar]
- 2.Heuschmann PU, Wiedmann S, Wellwood I, et al. Three-month stroke outcome: The European Registers of Stroke (EROS) Investigators. Neurology. 2010;76:159–165. doi: 10.1212/WNL.0b013e318206ca1e. [DOI] [PubMed] [Google Scholar]
- 3.Deutsche Gesellschaft für Neurologie. AWMF-Leitlinie 030 - 046, Akuttherapie des ischämischen Schlaganfalls. www.awmf.org/uploads/tx_szleitlinien/030-046l_S1_Akuttherapie_des_ischmischen_Schlaganfalls_2012_1.pdf. (last accessed on 1. August 2014)
- 4.Heuschmann PU, Kolominsky Rabas PL, Kugler C, et al. Qualitätssicherung in der Schlaganfall-Behandlung: das Basismodul der Arbeitsgemeinschaft Deutscher Schlaganfall-Register (ADSR) Gesundheitswesen. 2000;62:547–552. doi: 10.1055/s-2000-13039. [DOI] [PubMed] [Google Scholar]
- 5.Heuschmann PU, Biegler MK, Busse O, et al. Development and implementation of evidence-based indicators for measuring quality of acute stroke care: the Quality Indicator Board of the German Stroke Registers Study Group (ADSR) Stroke. 2006;37:2573–2578. doi: 10.1161/01.STR.0000241086.92084.c0. [DOI] [PubMed] [Google Scholar]
- 6.Nabavi DG, Ringelstein EB, Faiss J, et al. Regional and national stroke units in Germany: amended certification criteria. Nervenarzt. 2012;83:1039–1052. doi: 10.1007/s00115-012-3594-6. [DOI] [PubMed] [Google Scholar]
- 7.Minnesota Stroke Registry. Minnesota Stroke Registry Program Report 2008-2012. Minnesota Department of Health 2014; www.mnstrokeregistry.org/documents/MSRPProgramReport2008-2012.pdf. (last accessed on 1. 8. 2014)
- 8.Royal Collage of Physicians. Prepared on behalf of the Intercollegiate Stroke Working Party: Report for England, Wales and Northern Ireland—National Sentinel Stroke Audit Phase II (clinical audit) 2010. www.rcplondon.ac.uk/sites/default/files/national-sentinel-stroke-audit-2010-public-report-and-appendices_0.pdf. (last accessed on 1. August 2014)
- 9.Abilleira S, Ribera A, Sánchez E, Tresserras R, Gallofré M. The second stroke audit of catalonia shows improvements in many, but not all quality indicators. Int J Stroke. 2012;7:19–24. doi: 10.1111/j.1747-4949.2011.00638.x. [DOI] [PubMed] [Google Scholar]
- 10.Summary of the RIKS Stroke Annual Report 2012. (03.06.2014) www.riks-stroke.org/content/english/pdf/Riks-Stroke%20annual%20report%202012.pdf. (last accessed on 1. August 2014)
- 11.Nimptsch U, Mansky T. Trends in acute inpatient stroke care in Germany-an observational study using administrative hospital data from 2005-2010. Dtsch Arztebl Int. 2012;109:885–892. doi: 10.3238/arztebl.2012.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaebel W, Kowitz S, Fritze J, Zielasek J. Use of health care services by people with mental illness-secondary data from three statutory health insurers and the German statutory pension insurance scheme. Dtsch Arztebl Int. 2013;110:799–808. doi: 10.3238/arztebl.2013.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberlein-Gonska M, Petzold T, Helaß G, Albrecht DM, Schmitt J. The incidence and determinants of decubitus ulcers in hospital care—an analysis of routine quality management data at a university hospital. Dtsch Arztebl Int. 2013;110:550–556. doi: 10.3238/arztebl.2013.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco S, Pistoia F, Carolei A. Stroke tracked by administrative coding data: Is it Fair? Stroke. 2013;44:1766–1768. doi: 10.1161/STROKEAHA.113.001742. [DOI] [PubMed] [Google Scholar]
- 15.Wiedmann S, Norrving B, Nowe T, et al. Variations in quality indicators of acute stroke care in 6 European countries: the European Implementation Score (EIS) Collaboration. Stroke. 2012;43:458–463. doi: 10.1161/STROKEAHA.111.628396. [DOI] [PubMed] [Google Scholar]
- 16.Stolz E, Hamann GF, Kaps M, Misselwitz B. Regional differences in acute stroke admission and thrombolysis rates in the German federal state of Hesse. Dtsch Arztebl Int. 2011;108:607–611. doi: 10.3238/arztebl.2011.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grube MM, Dohle C, Djouchadaret D, et al. Evidence-based quality indicators for stroke rehabilitation. Stroke. 2012;43:142–146. doi: 10.1161/STROKEAHA.111.627679. [DOI] [PubMed] [Google Scholar]
- 18.Arbeitsgruppe Schlaganfall Hessen, Geschäftsstelle Qualitätssicherung Hessen. Stationäre Rehabilitationsbehandlung nach Schlaganfall. Ergebnisse der Hessischen Schlaganfall-Datenbank. Akt Neurol. 2001;28:413–420. [Google Scholar]
- 19.Grube MM, Koennecke HC, Walter G, et al. Association between socioeconomic status and functional impairment 3 months after ischemic stroke: the Berlin Stroke Register. Stroke. 2012;43:3325–3330. doi: 10.1161/STROKEAHA.112.669580. [DOI] [PubMed] [Google Scholar]
- 20.Schneider K, Heise M, Heuschmann P, Berger K. [Situation of life and care in patients with a stroke] Nervenheilkunde. 2009;28:114–118. [Google Scholar]
- 21.Walter A, Seidel G, Thie A, Raspe H. Semi-intensive stroke unit versus conventional care in acute ischemic stroke or TIA-a prospective study in Germany. J Neurol Sci. 2009;287:131–137. doi: 10.1016/j.jns.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Ferbert A, Suenkeler IH von Reutern GM. 6-Monats-Follow-up nach Schlaganfall. Ergebnisse der hessischen Schlaganfalldatenbank. Akt Neurol. 2005;32 [Google Scholar]
- 23.Kolominsky-Rabas PL, Heuschmann PU, Marschall D, et al. Lifetime cost of ischemic stroke in Germany: results and national projections from a population-based stroke registry: the Erlangen Stroke Project. Stroke. 2006;37:1179–1183. doi: 10.1161/01.STR.0000217450.21310.90. [DOI] [PubMed] [Google Scholar]