Abstract
Background
Tracheostomy site infection can cause numerous problems. Absorbent foam dressing may be able to prevent proliferation of infectious microorganisms by absorbing the tracheostomy stoma exudate.
This study aimed to compare the efficacy of absorbent foam with gauze dressing for prevention of tracheostomy site infection.
Materials and Methods
In this double-blind randomized clinical trial, 80 patients (18 to 60 years) hospitalized in the intensive care unit (ICU) due to severe head injury were randomly divided into two groups and early tracheostomy was done for them during the first 2 days. In the first group, gauze was used as tracheostomy site dressing, while in the second, absorbent foam, was placed. Tracheostomy site was checked daily for any sign of infection and samples were taken from the stoma for culture in case of presence of any sign of infection.
Results
Of a total of 80, 11 had tracheostomy site infection (13.75%), including 7 (17.5%) in the gauze group and 4 (10%) in the foam group. The difference in this regard between the two groups was not significant (P=0.051). Also, the dominant strains in the culture of gauze group were hospital-acquired Gram-negative bacteria (particularly Acinetobacter), while in the foam group, Gram-positives and more commonly Staphylococcus epidermidis were found.
Conclusion
Absorbent foam dressing is not superior to gauze dressings for prevention of tracheostomy site infection.
Keywords: Foam dressing, Infection, Tracheostomy
INTRODUCTION
Prolonged intubation (more than 1-5 weeks) can result in many complications (1) directly related to long intubation (2). Thus, 10% of ICU patients will eventually require tracheostomy (3). The first tracheostomy was performed in a patient with airway obstruction due to diphtheria in the 20th century (4). Today, several indications are considered for tracheostomy (5). Some studies have reported shorter hospital stay, decreased mechanical ventilation period, lower incidence of nosocomial pneumonia (6–8), less work of breathing and lower peak inspiratory pressures, auto-PEEP and airway resistance due to early tracheostomy (9–11).
Recent studies found no significant difference in prevalence of pneumonia and outcome among early and late tracheotomy patients (≤ 10 versus > 10 days) (12–16). The rate of mortality, length of hospital stay and duration of mechanical ventilation did not differ either (17, 18). Stoma infection is one complication of tracheostomy that may occur due to the activity of several bacteria (19). To prevent this, the site of stoma should be constantly monitored in terms of secretion, odor, inflammation and redness of the skin around the stoma (20). Irrigation, use of appropriate dressing and topical application of antibacterial agents are commonly recommended to prevent infection at the tracheostomy site.
Wound dressings absorb secretions and prevent moisture at the wound site (21).
To absorb secretions of the tracheostomy site, simple gauze, foam, or hydro fiber dressings may be used (22).
Absorbent dressing foams may be maintained at the tracheostomy site for 5 to 7 days, unless they are soaked with blood or secretions. The inner surface of foam, which is in contact with the wound, is soft, hydrophilic and absorbent, while the outer layer is impermeable (23).
The main hypothesis of this study was that absorbent foam dressings would significantly decrease the tracheostomy site infection by absorbing tracheostomy site exudates.
The aim of this study was to compare the efficacy of foam dressing with gauze dressing for prevention of tracheostomy stoma infection during a 7-day period following tracheostomy. Also, this study sought to assess the relationship between the type of bacterial strain causing the infection at the tracheostomy site and dressing type.
MATERIALS AND METHODS
Study design
This double-blind randomized controlled clinical trial evaluated and compared the efficacy of foam and gauze dressings for prevention of tracheostomy stoma infection.
Setting
This study was performed at a trauma centre in Kerman Shahid Bahonar Hospital.
Inclusion criteria
We enrolled 80 severe head trauma patients admitted to the ICU of Shahid Bahonar Hospital in Kerman.
We included intubated patients due to severe head trauma (Glasgow coma score <8) who were 18 to 60 years old.
Exclusion criteria
Patients who had diabetes, pneumonia, liver or renal failure or chronic cardio-pulmonary diseases, those taking antibiotics before undergoing tracheostomy, subjects with obvious signs of infection, burns in the neck area, being allergic to foam, infection in other body parts requiring antibiotic therapy and those with a previous history of tracheostomy were excluded.
Randomization
The patients were randomly divided into two groups using online random allocation software (www.allocationsoftware.com).
Sample size
The sample size (n=80) was calculated based on similar previous studies and consultation with a statistician.
Blinding
The patients and the attending intensivists responsible for data collection were blinded to the group allocation of patients.
Interventions
In the first group, immediately after tracheostomy, regular gauze was used for stoma dressing, while absorbent foam was placed for the second group. All patients were tracheostomized by one surgeon with the same technique and we used the same type of tracheostomy tube with the same material from the same manufacturer for all patients. Also, all patients were treated with the same antibiotic regimen (as prophylaxis for neurosurgery). Surgical stoma was washed with saline solution every 8 hours. The gauze on the tracheostomy site was exchanged daily. However, the foam dressing remained until the end of the study period (7 days), according to the manufacturer's recommendations unless it was soaked with blood or secretions. The tracheostomy site was checked for inflammation, swelling, heat, redness and purulent discharge at the time of dressing change by someone unaware of the type of dressing used. In case of presence of any sign of infection, a sample was taken from the stoma site and sent to the laboratory for microbial culture (the lab technician was also blinded to the type of dressing). The culture results were recorded. Patients who had clinical signs and symptoms of infection confirmed by a positive microbial culture were considered as having tracheostomy site infection and appropriate antibiotic regimen based on the type of bacteria was initiated for them.
Statistical analysis
Data were analyzed using SPSS 17. Chi-Square test was applied for comparing the two groups. Level of significance was set at P< 0.05.
Ethics
The study was approved by the Ethics Committee of Kerman University of Medical Sciences (ethical code: KA/91/63) and registered in the clinical trials research center of Iran (code number: N5201205215426). Written informed consent was obtained from the legal guardians of patients.
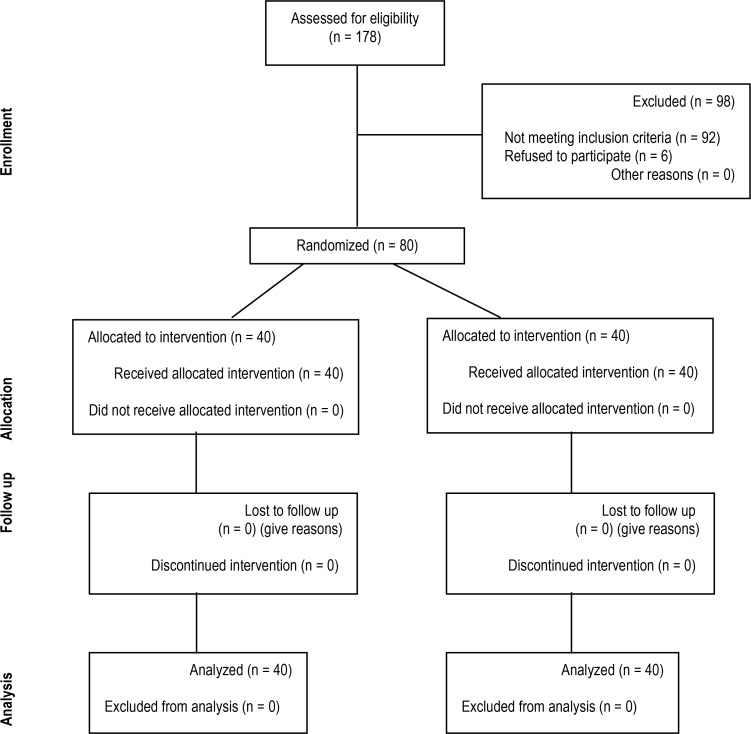
Figure 1 shows the algorithm of participation in trial of foam dressing for prevention of tracheostomy stoma infection.
Figure 1.
Participation in Trial of foam dressing for prevention of tracheostomy stoma infection.
RESULTS
Baseline patient characteristics
A total of 80 patients were entered in the study (n=40 in each group). The mean age of patients was 36.71 ± 8.04 years. This value was 35.33 ± 2.06 years in the gauze and 38.10 ± 12.85 years in the foam group (P=0.35).
Of a total of 80 patients, 66 were males (82.5%) and 14 were females (17.5%). There were 31 males and 9 females in the gauze group and 35 males and 5 females in the foam group (Table 1).
Table 1.
Patient demographics
| Items | Gauze | Percent% | Foam | Percent% | P-value |
|---|---|---|---|---|---|
| Male (count) | 31 | 77.5% | 35 | 87.5% | 0.089 |
| Female (count) | 9 | 13.5% | 5 | 12.5% | 0.068 |
| Age (Mean ± SD) | 35.33±2.06 | 38.10±12.85 | 0.35 | ||
No significant difference was seen between the two groups in terms of gender or mean age (P>0.05).
Of a total of 80 patients, 11 had clinical signs of inflammation and infection at the tracheostomy stoma with positive culture (13.75%).
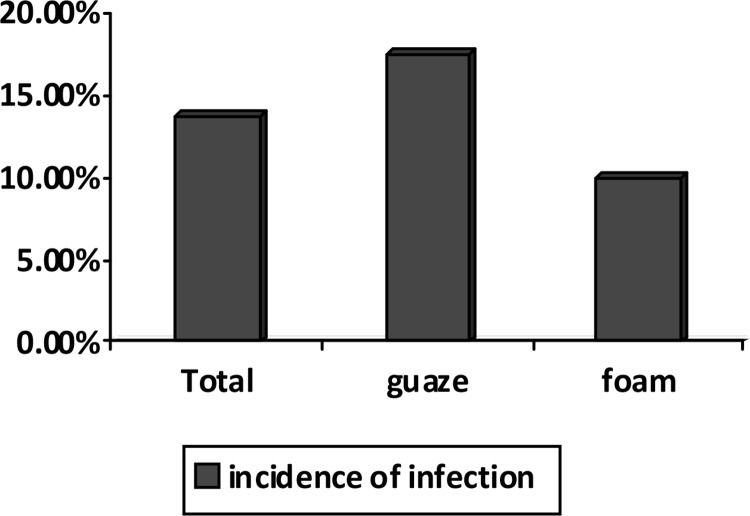
Of 40 patients in the gauze dressing group, 7 (17.5%) had tracheostomy site infection while this rate was 4 (10%) in the foam group (Figure 2).
Figure 2.
Comparison of the incidence of stoma infection between the two groups
Although the rate of stoma infection in the gauze group was higher than in the foam group, this difference was not statistically significant (P=0.051).
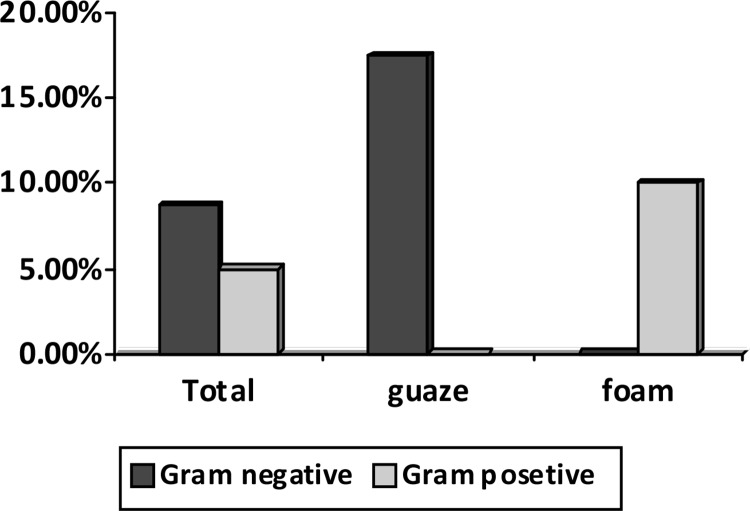
Microbial cultures of patients in the gauze group revealed Gram-negative nosocomial bacterial species: Acinetobacter in 4 (10%), Klebsiella in 2 (5%) and Pseudomonas aeruginosa in one (2.5%), whereas Gram-positive bacteria were dominant in the foam group: 4 (10%) positive cultures among 40 patients, Staphylococcus epidermidis in 3 (7.5%) and Staphylococcus aureus in one (2.5%) (Figure 3).
Figure 3.
Comparison of stoma culture results between the two groups
DISCUSSION
Tracheostomy tube insertion is associated with increased risk of acquired antibiotic-resistant bacterial infections (24). The primary role of wound dressings is to absorb exudates. Generally, absorbent foam or gauze is used for wound dressing (25).
Many experimental and clinical studies have evaluated the effect of dressings on depth and size of wounds, risk of infection, and amount of exudates (26, 27). The results of the current study showed no significant difference between foam and gauze dressings for prevention of tracheostomy site infection (P=0.051).
Also, in the foam group, Gram-positive bacteria and mainly Staphylococcus epidermidis were the dominant bacterial strains causing infection, whereas in the gauze group, the nosocomial Gram negative bacteria were the dominant strains causing tracheostomy site infection.
Jones and Milton reported that absorbent foam dressings were more effective for tracheostomy stoma healing due to their superior moisture absorption (23).
The incidence of stoma infection after tracheostomy has reported to be 0% to 63% in the literature (28, 29). A study by Brook and colleagues showed that 16% of the tracheostomy stoma infections were caused by aerobic bacteria, 8% by anaerobic bacteria, and 76% by a combination of aerobic and anaerobic bacteria. The most commonly isolated strains were Peptostreptococci, Pseudomonas aeruginosa, Fusobacterium, and Bacteriocides (19). In our study, the stoma infection rate was 13.75%. This rate was 17.5% in the gauze group and 10% in the foam group. Chuang and colleagues carried out a clinical trial to compare gauze and a solid pectin-based skin barrier and evaluate clinical outcomes and cost-effectiveness of care for tracheostomy wounds. They suggested that using a solid skin barrier for tracheostomy care was associated with lower occurrence of impaired skin integrity and higher satisfaction among nurses compared to gauze (30).
In a clinical trial conducted by Ubbink and colleagues, the foam and hydrogel dressings were compared with gauze in 285 patients admitted to the hospital. They concluded that the occlusive and moist-environment dressing principle in the clinical surgical setting did not lead to faster wound healing than gauze dressing (31). Effects of antimicrobial foam dressings have been investigated in several studies, but only a few studies have exclusively compared the prevalence of tracheostomy stoma infection between absorbent foam and gauze dressings, which is commonly used in the tracheostomy site.
Results of the current study indicated that the incidence of tracheostomy stoma infection in the gauze and foam dressing groups was not significantly different (P= 0.051). The inefficacy of foam dressing to reduce the rate of infection at the tracheostomy site compared to more superficial wounds may be attributed to the lack of its direct contact with the surface of stoma wound and the smaller surface of tracheostomy wound compared to its depth. Foam dressing needs to be in contact with the wound surface in order to best exert its absorbent effect and reduce bacterial accumulation and proliferation. Limitations: One of the major limitations of the current study was short-term evaluation of patients. Since many ICU patients need to have tracheostomy tubes for more than 7 days (the period of this study), it is possible that the pathogenic bacterial species or rate of infection change over longer periods due to the impact of dressing.
In addition, in most cases particularly in the gauze group, the culture results were reported in poly-microbial form and only the dominant strain was compared between the two groups. More comprehensive studies are required to clarify this issue. Suggestions: Although the results of the current study demonstrated no significant difference in the rate of tracheostomy site infection between the foam and gauze dressings, a similar project is recommended to be done in a larger scale.
REFERENCES
- 1.Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J (Clin Res Ed) 1984;288(6422):965–8. doi: 10.1136/bmj.288.6422.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touat L, Fournier C, Ramon P, Salleron J, Durocher A, Nseir S. Intubation-related tracheal ischemic lesions: incidence, risk factors, and outcome. Intensive Care Med. 2013;39(4):575–82. doi: 10.1007/s00134-012-2750-6. [DOI] [PubMed] [Google Scholar]
- 3.Fischler L, Erhart S, Kleger GR, Frutiger A. Prevalence of tracheostomy in ICU patients. A nation-wide survey in Switzerland. Intensive Care Med. 2000;26(10):1428–33. doi: 10.1007/s001340000634. [DOI] [PubMed] [Google Scholar]
- 4.Borman J, Davidson JT. A history of tracheostomy: si spiritum ducit vivit (Cicero) Br J Anaesth. 1963;35:388–90. doi: 10.1093/bja/35.6.388. [DOI] [PubMed] [Google Scholar]
- 5.Alali AS, Scales DC, Fowler RA, Mainprize TG, Ray JG, Kiss A, et al. Tracheostomy timing in traumatic brain injury: a propensity-matched cohort study. J Trauma Acute Care Surg. 2014;76(1):70–6. doi: 10.1097/TA.0b013e3182a8fd6a. discussion 76-8. [DOI] [PubMed] [Google Scholar]
- 6.Bösel J. Tracheostomy in stroke patients. Curr Treat Options Neurol. 2014;16(1):274. doi: 10.1007/s11940-013-0274-1. [DOI] [PubMed] [Google Scholar]
- 7.Choi HJ, Paeng SH, Kim ST, Lee KS, Kim MS, Jung YT. The Effectiveness of Early Tracheostomy (within at least 10 Days) in Cervical Spinal Cord Injury Patients. J Korean Neurosurg Soc. 2013;54(3):220–4. doi: 10.3340/jkns.2013.54.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis K, Jr, Campbell RS, Johannigman JA, Valente JF, Branson RD. Changes in respiratory mechanics after tracheostomy. Arch Surg. 1999;134(1):59–62. doi: 10.1001/archsurg.134.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Moscovici da Cruz V, Demarzo SE, Sobrinho JB, Amato MB, Kowalski LP, Deheinzelin D. Effects of tracheotomy on respiratory mechanics in spontaneously breathing patients. Eur Respir J. 2002;20(1):112–7. doi: 10.1183/09031936.02.01342001. [DOI] [PubMed] [Google Scholar]
- 10.Lin MC, Huang CC, Yang CT, Tsai YH, Tsao TC. Pulmonary mechanics in patients with prolonged mechanical ventilation requiring tracheostomy. Anaesth Intensive Care. 1999;27(6):581–5. doi: 10.1177/0310057X9902700604. [DOI] [PubMed] [Google Scholar]
- 11.Blot F, Similowski T, Trouillet JL, Chardon P, Korach JM, Costa MA, et al. Early tracheotomy versus prolonged endotracheal intubation in unselected severely ill ICU patients. Intensive Care Med. 2008;34(10):1779–87. doi: 10.1007/s00134-008-1195-4. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120(2):555–61. doi: 10.1378/chest.120.2.555. [DOI] [PubMed] [Google Scholar]
- 13.Nseir S, Di Pompeo C, Jozefowicz E, Cavestri B, Brisson H, Nyunga M, et al. Relationship between tracheotomy and ventilator-associated pneumonia: a case control study. Eur Respir J. 2007;30(2):314–20. doi: 10.1183/09031936.06.00024906. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Li Y, Ariani F, Chen X, Lin J. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One. 2014;9(3):e92981. doi: 10.1371/journal.pone.0092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(7502):1243. doi: 10.1136/bmj.38467.485671.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32(8):1689–94. doi: 10.1097/01.ccm.0000134835.05161.b6. [DOI] [PubMed] [Google Scholar]
- 17.Gomes Silva BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2012;3:CD007271. doi: 10.1002/14651858.CD007271.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Young D, Harrison DA, Cuthbertson BH, Rowan K, TracMan Collaborators Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–9. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 19.Brook I. Microbiological studies of tracheostomy site wounds. Eur J Respir Dis. 1987;71(5):380–3. [PubMed] [Google Scholar]
- 20.Morris LL, McIntosh E, Whitmer A. The importance of tracheostomy progression in the intensive care unit. Crit Care Nurse. 2014;34(1):40–8. doi: 10.4037/ccn2014722. quiz 50. [DOI] [PubMed] [Google Scholar]
- 21.Jeffcoate WJ, Price P, Harding KG. International Working Group on Wound Healing and Treatments for People with Diabetic Foot Ulcers Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev. 2004;(20 Suppl 1):S78–89. doi: 10.1002/dmrr.476. [DOI] [PubMed] [Google Scholar]
- 22.Thomas L. Wachtel. Pearls for Practice: A Novel Tracheostomy Dressing: Extension of a Hydroconductive Wound Dressing. 2013 Feb;59(2):17–18. Available from: http://www.o-wm.com/article/pearls-practice-novel-tracheostomy-dressing-extension-hydroconductive-wound-dressing. [Google Scholar]
- 23.Jones V, Milton T. When and how to use foam dressings. Nurs Times. 2000;96(36 Suppl):2–3. [PubMed] [Google Scholar]
- 24.Tomasz A. Multiple-antibiotic-resistant pathogenic bacteria. A report on the Rockefeller University Workshop. N Engl J Med. 1994;330(17):1247–51. doi: 10.1056/NEJM199404283301725. [DOI] [PubMed] [Google Scholar]
- 25.Birke-Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, et al. Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)--steps towards an international consensus. J Plast Reconstr Aesthet Surg. 2011;64(Suppl):S1–16. doi: 10.1016/j.bjps.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Malmsjö M, Lindstedt S, Ingemansson R. Influence on pressure transduction when using different drainage techniques and wound fillers (foam and gauze) for negative pressure wound therapy. Int Wound J. 2010;7(5):406–12. doi: 10.1111/j.1742-481X.2010.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraccalvieri M, Zingarelli E, Ruka E, Antoniotti U, Coda R, Sarno A, et al. Negative pressure wound therapy using gauze and foam: histological, immunohistochemical and ultrasonography morphological analysis of the granulation tissue and scar tissue. Preliminary report of a clinical study. Int Wound J. 2011;8(4):355–64. doi: 10.1111/j.1742-481X.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glossop AJ, Meekings TC, Hutchinson SP, Webber SJ. Complications following tracheostomy insertion in critically ill patients – experience from a large teaching hospital. JICS. 2011;12(4):301–6. [Google Scholar]
- 29.Engels PT, Bagshaw SM, Meier M, Brindley PG. Tracheostomy: from insertion to decannulation. Can J Surg. 2009;52(5):427–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Chuang WL, Huang WP, Chen MH, Liu IP, Yu WL, Chin CC. Gauze versus solid skin barrier for tracheostomy care: a crossover randomized clinical trial. J Wound Ostomy Continence Nurs. 2013;40(6):573–9. doi: 10.1097/01.WON.0000436431.01159.9f. [DOI] [PubMed] [Google Scholar]
- 31.Ubbink DT, Vermeulen H, Goossens A, Kelner RB, Schreuder SM, Lubbers MJ. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143(10):950–5. 1. doi: 10.1001/archsurg.143.10.950. [DOI] [PubMed] [Google Scholar]