Abstract
CD34 is a transmembrane phosphoglycoprotein, first identified on hematopoietic stem and progenitor cells. Clinically, it is associated with the selection and enrichment of hematopoietic stem cells for bone marrow transplants. Due to these historical and clinical associations, CD34 expression is almost ubiquitously related to hematopoietic cells, and it is a common misconception that CD34-positive (CD34+) cells in nonhematopoietic samples represent hematopoietic contamination. The prevailing school of thought states that multipotent mesenchymal stromal cells (MSC) do not express CD34. However, strong evidence demonstrates CD34 is expressed not only by MSC but by a multitude of other nonhematopoietic cell types including muscle satellite cells, corneal keratocytes, interstitial cells, epithelial progenitors, and vascular endothelial progenitors. In many cases, the CD34+ cells represent a small proportion of the total cell population and also indicate a distinct subset of cells with enhanced progenitor activity. Herein, we explore common traits between cells that express CD34, including associated markers, morphology and differentiation potential. We endeavor to highlight key similarities between CD34+ cells, with a focus on progenitor activity. A common function of CD34 has yet to be elucidated, but by analyzing and understanding links between CD34+ cells, we hope to be able to offer an insight into the overlapping properties of cells that express CD34. Stem Cells 2014;32:1380–1389
Keywords: CD34, Stem cell, Progenitor, Mesenchymal, Stromal, Epithelial, Endothelial
Introduction
CD34 is predominantly regarded as a marker of hematopoietic stem cells (HSC) and hematopoietic progenitor cells. However, CD34 is now also established as a marker of several other nonhematopoietic cell types, including vascular endothelial progenitors 1 and embryonic fibroblasts 2. Accumulating evidence demonstrates CD34 expression on several other cell types, including multipotent mesenchymal stromal cells (MSC), interstitial dendritic cells, and epithelial progenitors 3–6, but there remains limited recognition of the role of CD34-positive (CD34+) cells outside of each individual specialty. Despite consistent evidence of expression by many cell types, there is still a misconception that CD34 represents a cell of hematopoietic origin, and experimentally, CD34+ cells are often regarded as hematopoietic contamination and subsequently disregarded.
This review presents evidence establishing CD34 as a general marker of progenitor cells. We explore common traits, such as marker expression, morphology and differentiation potential, and endeavor to draw focus toward the many, disparate cell types that express CD34, and in the process highlight key similarities. CD34 expression across different cell types and the associated implications has not previously been presented, although selected literature has reviewed expression within individual cell groups. Although a common function of CD34 has yet to be elucidated, analyzing and understanding the links between cells offers an insight into the role of CD34 in identifying progenitor cells from many tissue types. A summary of the properties of all the CD34+ cell types discussed in this review can be found in Table1.
Table 1.
Summary of different CD34+ cell types
| CD34+ cell type | Associated markers | Differentiation potential | Properties | Reference |
|---|---|---|---|---|
| HSC and progenitors | HLA-DR, CD38, CD117 (c-kit), CD45, CD133 | Hematopoietic cells, cardiomyocytes, hepatocytes | Large nucleus, little cytoplasm, high proliferative capacity | 7, 8 |
| MSC | Stro-1, CD73, CD90, CD105, CD146, CD29, CD44, CD271 | Adipogenic, osteogenic, chondrogenic, myogenic, angiogenic | CD34+ MSC form a higher proportion of CFU-f colonies than CD34−. CD34+ MSC exhibit a high proliferative capacity. Fibroblastic cells | 9–13 |
| Muscle satellite cells | CD56, Myf5, Desmin, M-cadherin, CD90, CD106, Flk-1, VEGFR, MyoD, CD146 | Myogenic, adipogenic, osteogenic, chondrogenic | The CD56+CD34+ population may represent a more primitive or pluripotent stem cell. In vivo, CD34+ cells are located near the basal lamina. Small and round | 14–17 |
| Keratocytes | CD34, CD133, l-selectin, keratocan, ALDH | Fibroblastic, myofibroblastic, adipogenic, osteogenic, chondrogenic, corneal epithelial, corneal endothelial | Dendritic morphology. In vitro population acquires an MSC phenotype | 18–21 |
| Interstitial cells | CD117, vimentin, Desmin, Connexin-43, PDGFRβ | Not yet fully elucidated | Triangular or spindle-shaped with large nucleus and long cytoplasmic processes. CD34+ population may have a stem cell/progenitor role in the bladder, intestine, and reproductive organs | 22–24 |
| Fibrocyte | CD45, CD80, CD86, MHC class I and II | Fibroblastic, myofibroblastic, adipogenic, osteogenic, chondrogenic | Small spindle shape. CD34 is lost in culture and upon maturation | 25–27 |
| Epithelial progenitors | CD49f, CD10, CD146, CD71, S100a4, Dkk3, CD133, CD117, ALDH, CD90 | Dermal epithelial cells, neural mesenchymal | Predominantly described in HF niche in skin | 28–33 |
| Endothelial cells | CD146, VE-cadherin, CD133, CD117, CD14, CD31 | Angiogenesis | Elongated with filopodia. Lack tight junctions. CD34 is present on luminal membrane processes and is expressed on filopodia during in vivo angiogenesis. Quiescent in vivo/low proliferation activity | 1, 34, 35 |
Abbreviations: ALDH, aldehyde dehydrogenase; CD, cluster of differentiation; CFU-F, colony forming units fibroblast; Flk-1, fetal liver kinase-1; HF, hair follicle; HLA-DR, human leukocyte antigen-DR; HSC, hematopoietic stem cells; MSC, multipotent mesenchymal stromal cells; Myf5, myogenic factor 5; MyoD, myogenic differentiation 1; MHC, major histocompatibility complex; PDGFRβ, platelet derived growth factor receptor β; VEGFR, vascular endothelial growth factor receptor.
Structure and Function of CD34
CD34 is a transmembrane phosphoglycoprotein, first identified in 1984 on hematopoietic stem and progenitor cells 36. It has a molecular weight of approximately 115 kDa and possesses an extracellular domain that is heavily sialylated, O-linked glycosylated, and contains some N-linked glycosylation sites. There is a single transmembrane helix and a cytoplasmic tail that contains PDZ (PSD-95-Dlg-ZO-1)-domain binding motifs 3,37. The most commonly described ligand for CD34 is l-Selectin (CD62L), however, the adapter protein CrkL, known for adhesion regulation, also binds CD34 38,39.
Although the structure of CD34 is well-investigated, there is still relatively little known about its function. Studies in hematopoietic cells suggest roles in cytoadhesion and regulation of cell differentiation and proliferation 40,41. Lymphocytes exhibit l-selectin-mediated adhesion to CD34 surface proteins in the vascular endothelium 38,42 and in addition, it has been hypothesized that CD34 plays a role in trafficking of HSC to niches within the bone marrow (BM) 41. However in contrast, CD34 has also been associated with blocking of adhesion, particularly involving mast cells 43.
CD34 and Hematopoietic Cells
The expression of CD34 on hematopoietic progenitors and the properties of these cells have been discussed in depth previously 7,44,45 and are not covered in detail in this review. In clinical practice, CD34 expression is evaluated to ensure rapid engraftment in BM transplants and can also be used as a selective marker in cell sorting to enrich a population of immature hematopoietic cells 46,47. Although sometimes assumed to be solely a stem cell marker, the detection of CD34 in BM or blood samples represents a hematopoietic stem/progenitor mix, of which the majority of cells are progenitor 44. Human HSC are further separated from CD34+ progenitor cells by low expression of CD90 and a lack of expression of CD38, human leukocyte antigen-DR, and a panel of mature hematopoietic lineage markers (lin−) 7. CD34+ HSC are able to differentiate into all cells of the hematopoietic lineage and have a high proliferative capacity 7,8. Evidence suggests that CD34+ HSC and progenitors have the ability to differentiate in vivo into other lineages, including respiratory epithelial cells 48, hepatocytes 49, and cardiomyocytes 50. Thus far, the properties of CD34+ HSC have not been directly linked to the properties of CD34+ non-HSC.
CD34 and Stromal Cells
Multipotent MSC
MSC are found in most adult tissues and are a prevalent and versatile cell type, studied extensively for regenerative medicine applications 51. Although their in vitro mesenchymal differentiation potential is well-reported, MSC are also frequently associated with other properties including paracrine wound healing, niche forming abilities, immune privilege, and immunomodulation 52,53. Nomenclature of MSC is a controversial topic, with differing opinions on whether cells should be identified as mesenchymal stem cells or multipotent mesenchymal stromal cells, among many other names 54. In this review, we use the term multipotent mesenchymal stromal cells but include a broad range of references that refer generically to mesenchymal stem and progenitor cells. This section includes research on MSC from different tissue sources and discusses both freshly isolated and culture expanded MSC.
Two recent reviews have discussed the expression of CD34 on MSC, both with an emphasis on adipose-derived MSC 4,55. While Scherberich et al. 55 concentrated on the structure and function of CD34 in relation to MSC, Lin et al. 4 challenged the opinion that CD34 is a negative marker of MSC. The latter review discussed evidence demonstrating that CD34 was an important marker in early MSC research, highlighting research by Simmons and Torok-Storb showing that freshly isolated, CD34+ BM MSC form greater proportions of fibroblastic colonies (colony forming units fibroblast) than their CD34− counterparts 9. The review also made the observation that CD34+ MSC were originally used in screening for additional MSC immunogens, leading to the identification of Stro-1 56. They concluded that CD34 should be considered a positive marker of MSC, with a particular association to vasculature and suggested that these cells be known as vascular stem cells 4. While Lin et al. highlights several key issues in the definition of an MSC, the review only briefly touches upon a connection between CD34 and progenitor cells.
This same review was also critical of the position paper, published in 2006, by the International Society for Cellular Therapy (ISCT) 57, which outlined a minimal criteria for cells to be considered MSC. These criteria, based predominantly on MSC extracted from BM, describe a plastic-adherent, culture expanded cell population and have subsequently been widely adopted by the research community. One ISCT criterion states that to be considered MSC, a cell population must be largely negative for CD34 (≤2% of the population). This is due to the consideration that CD34 is a marker of hematopoietic cells, alongside a number of previous studies that show an absence of CD34 in cultured MSC 58–60. In support of the ISCT, establishing well-defined criteria has created a clear benchmark for the depiction of MSC populations, allowing more accurate and reproducible comparisons between research groups. MSC populations demonstrate high levels of heterogeneity and this diversity exists not only within different tissue sources but also between MSC from a single source and clonal MSC 60,61. Hence, prior to the establishment of these criteria, many disparate cell types were described as MSC, leading to uncertainty over MSC properties and characteristics. However, in unifying and defining characterization, it is almost certain that stromal progenitors expressing markers that are considered negative, or not included in the ISCT criteria, are being overlooked by researchers, and more importantly, actively removed when following these guidelines.
Although prevailing opinion states that MSC are CD34−, in vitro characterization predominantly takes place following culture, usually after several passages. Consequently, this is not representative of the in vivo or initially extracted cell phenotype. Freshly extracted stromal cells, from various tissues, have been shown to contain CD34+ cells 9–11,63. The expression of CD34 has been demonstrated more extensively on MSC isolated from sources other than BM, such as adipose tissue, and therefore is more widely accepted by researchers in these areas 10,61,64. CD34+ MSC are present immediately following extraction but rapidly diminish in numbers after a short time in culture 12,13,65. For example, when analyzing adipose-derived MSC at P0, Mitchell et al. described an average of 59.2% of adherent cells expressing CD34, which decreased to 5% at passage 2 and subsequently disappeared 64. This change in CD34 expression upon culture is also mirrored in changes of expression of other surface antigens. Freshly extracted adipose MSC also show low expression of CD73, CD90, and CD105 that increase upon culture; this is of interest due to the ISCT classification of these as definitive MSC markers 10,64. There are also decreases in expression of other markers associated with MSC, such as CD106, CD146, and CD271 12,13,64,66. This change in MSC phenotype upon culture is perhaps indicative of a differentiation process or response to environmental changes.
CD34+ cells represent a proportion of the total MSC population, and this subset of cells possesses distinct characteristics. CD34 expression is associated with high colony forming efficiency and long-term proliferative capacity 9,12,63. CD34+ MSC have been associated with typical MSC markers, alongside differentially expressed markers such as CD271 and Stro-1, and markers commonly related to other cell types including CD45 and CD133 10,12,13,41,42,67,68. CD34+ MSC have been shown to possess a greater propensity for endothelial transdifferentiation 14,69. CD34 is also found on embryonic stem cell derived MSC, further suggesting it to be a marker of early human MSC 15.
Muscle Satellite Cells
CD34 is commonly used as a marker of both human and mouse muscle satellite cells, also known as muscle stem cells 16,70. Muscle satellite cells are small, stromal progenitor cells that give rise to mature skeletal muscle cells. In vivo, muscle satellite cells are quiescent, unless activated to provide myonuclei for myofibers, by increased weight-bearing exercise or trauma. Activation of these cells, also considered to be differentiation, corresponds to a complete downregulation of CD34 71. CD34 has been suggested to play a fundamental role in the regulation of muscle progenitor cell differentiation and establishment and maintenance of a satellite cell population 71.
CD34 is not expressed on all muscle satellite cells but is used in identification alongside other markers including CD56 16,17. Developmentally, the earliest known myogenic precursors do not express CD34 and during muscle development CD34 expression is first detected with the appearance of committed muscle satellite cells 72. This, alongside the simultaneous expression of myogenic markers such as Myf5 and M-cadherin, is suggestive of CD34+ satellite cells demonstrating a prior commitment to the myogenic fate 71.
Alternative evidence proposes that CD34+ cells have the potential to be more than myogenic precursors. Distinct MSC-like cells, that demonstrate in vitro mesenchymal differentiation, have been identified in muscle satellite locations by characterizing CD34 expression 73. Similar to MSC, CD34 expression on muscle satellite cells does not persist during culture and disappears as the cells differentiate. It has been suggested that CD34+ cells residing in the interstitial spaces of muscle are related to endothelial cells, due to the expression profile CD56+CD34+CD144+ 18. These myoendothelial cells show an enhanced ability to regenerate muscle, compared to cells that express only myogenic or endothelial markers in an in vivo mouse model. They also differentiate in vitro down the osteogenic and chondrogenic lineages.
The different observed properties of muscle satellite cells may be explained by the presence of distinct subsets of CD34+ cells, with distinct differentiation potentials 17. The markers that are coexpressed alongside CD34 have an impact on differentiation. For example, CD34+ cells that coexpress the endothelial marker CD31 display angiogenic differentiation. However, CD34+CD31− cell populations demonstrate greater potential to differentiate down the adipogenic and myogenic lineage 17.
Corneal Keratocytes
The corneal stroma is populated with quiescent cells known as keratocytes, which exhibit a dendritic morphology with extensive cellular contacts 19. Similar to muscle satellite cells, CD34 is a well-established marker for quiescent keratocytes in vivo (Fig. 1) 74,75. As a result of corneal trauma, keratocytes adjacent to the wound take on a more fibroblastic phenotype and CD34 expression rapidly disappears, as cells become “activated” 20. Upon activation, they shift to a fibroblastic phenotype and are associated with stromal tissue remodeling and scar formation 76. Activation also occurs in vitro, when keratocytes are cultured on tissue culture plastic, particularly in serum-containing medium 65,77. Keratocyte transformation to the fibroblast phenotype was previously thought to be irreversible in vitro, but recent evidence demonstrates that careful recapitulation of the niche-like environment and tailoring of culture conditions has the potential to revert fibroblastic cells back to a native keratocyte phenotype 78–80. However, it has yet to be investigated whether CD34 expression is restored.
Figure 1.
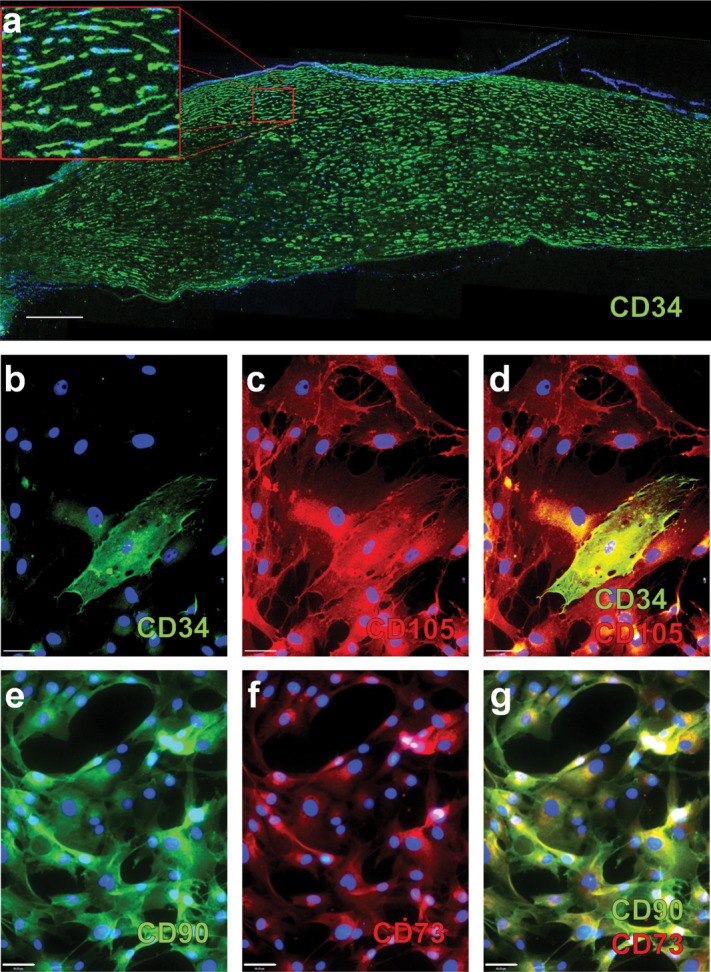
CD34 expression by keratocytes. (A): Immunofluorescence showing CD34 expression by keratocytes in a section of human cornea, counterstained with DAPI. Scale bar = 500 μm. (B–G): Keratocyte phenotype after in vitro culture. Human keratocytes were extracted from the corneal stroma and cultured for 5 days in Medium 199 containing 20% fetal bovine serum. (B–D): Immunocytochemistry identifying individual cells expressing CD34, among cells expressing CD105. (E–G): Keratocytes in culture also express markers associated with MSC, such as CD90 and CD73. All images counterstained with Hoechst 33258. Scale bar = 46 μm.
The function of CD34 expression in keratocytes has not yet been elucidated, although it has been speculated that CD34 plays roles in regulation of differentiation, adhesion, and quiescence 75. Although it has been suggested that CD34+ keratocytes are of hematopoietic origin 81, these cells do not form hematopoietic colonies when cultured in semisolid medium and CD34+ cells from these cultures display a plastic adherent, dendritic morphology 65. Although CD34+ cells disappear during in vitro culture, they can still be seen in early cultures, when cultured in medium 199 (Fig. 1B). These cells also express typical stromal markers such as CD105, CD90, and CD73 (Fig. 1C–1G). In vitro, keratocytes have been shown to display characteristics of MSC 21,82, and after several passages, when CD34 has disappeared, conform to the requisite ISCT criteria 65. Therefore, it has been hypothesized that the keratocyte is an MSC progenitor found in the corneal stroma 65,83. Additionally, recent investigations have revealed that CD34+ keratocytes possess the ability to transdifferentiate into corneal epithelial 77 and endothelial cells 22,74. This has led to the hypothesis that CD34 is a marker for a tissue-specific progenitor that resides in the stroma, which could give rise to all corneal lineages and MSC.
Interstitial Cells and Fibrocytes
CD34+ stromal cells have been described in organs including the thyroid, dermis, tonsils, uterus, and testes 84. CD34+ cells in these cases are termed dendritic interstitial cells or fibrocytes. Although their function remains unclear, these cells display similar properties to CD34+ MSC, keratocytes, and muscle satellite cells, and it has been suggested that these cells function as progenitor cells for specific tissues.
The CD34+ interstitial cells of Cajal (ICC) are mesenchymal in origin and were first identified in the gastrointestinal (GI) tract 85. The prevailing belief is that ICC are responsible for modulating smooth muscle contraction through electrical and chemical signaling as pacemaker cells. However, the discovery of ICC in other tissues, including the pancreas, myocardium, bladder, urethra, and blood vessels, has brought into question the functional role of these cells 86.
Although not all ICC express CD34, immunoreactivity has been shown in a subset of ICC in the GI tract in mice and humans 86. In humans, CD34+ populations have been described in the human detrusor muscle of the bladder, uterus, and fallopian tubes 23,24. Investigations have identified a possible role for CD34+ ICC as uncommitted progenitor cells 25. Moreover, ICC in the bladder of mice showed colocalization of c-kit and CD34 26 and authors have suggested a functional relationship between CD34+ ICC and mast cells. This supports the notion that CD34 expression relates to plasticity of the ICC cell type and a progenitor function.
Fibrocytes are mesenchymal progenitor cells that are often considered distinct from MSC. Despite this, they are quiescent cells that circulate in the bloodstream and upon trauma are recruited to the site of injury, where the cells play roles in inflammation and wound healing 27,87. These cells have been shown to produce myofibroblastic, fibroblastic, adipogenic, chondrogenic, and osteogenic phenotypes in vitro 27,28. Fibrocytes are currently not recognized as MSC due to their cell surface marker profile which includes markers associated with leukocytes, including CD34, CD45, CD80, CD86, and major histocompatibility complex class I and II 27,28. As with the aforementioned stromal cells, CD34 expression disappears during in vitro culture and in vivo upon maturity and differentiation 87. In common with other stromal cells, it has been suggested that both keratocytes and fibrocytes are derived from leukocytes due to a CD34+CD45+ cell surface marker profile 81.
CD34 and Epithelial Cells
The expression of CD34 by epithelial progenitors in the skin is widely accepted 29,88. In vivo, each unit of epithelium contains a hair follicle (HF) and within the HF there is a stem cell niche that maintains the epithelial layers of the skin. Trauma to the epidermis results in migration of stem cells from this niche to the injury site. Within the niche there are populations of multipotent epithelial stem cells and a subpopulation of these cells is CD34+ 29,89. The location of the CD34+ cells in the HF is one of contention, depending on species. The majority of studies have been conducted on mice and in these cases the CD34+ population resides in the outer root sheath (ORS) bulge, alongside other recognized stem cells 6,29. Human HFs are considerably more difficult to study, due to small size and low cell numbers. However, indications show that the CD34+ cells are not located in the ORS bulge, but are below the bulge zone, in the suprabulbar region 89–91. In humans, CD34+ cells have also been identified in the skin between the HF, in the basal interfollicular epidermis, but these cells are less well-investigated 92. It has been suggested that because the CD34+ cells are not present in the adult human ORS bulge, with the majority of other stem cells, they are the progeny of bulge stem cells 92.
HFs are constantly cycling through stages of active hair growth (anagen), destruction (catagen), and rest (telogen). During these stages, cell surface marker expression and phenotype within the HF is altered 6. CD34 expression is seen in human HFs during anagen but not during catagen and telogen 89. The authors suggest that this indicates that CD34 function relates only to epithelial cells that are proliferating and also relates to adhesion of the root sheath cells to the surrounding stroma. It is worth noting that although human CD34+ cells are not located in the bulge with other stem cells, they are located in the area of the HF that demonstrates the most clonogenic activity 30. In human HFs, cells that express CD34 do not express cytokeratin 15 (CK15), another commonly used marker of epithelial progenitor cells 31, suggesting either more than one population of progenitor cells or a hierarchy of cells at different stages of differentiation 89. CD34+ cells of the HF are multipotent and able to generate a fully stratified epidermis 6,32. There is some evidence to show that HF stem cells, including CD34+ cells, may be pluripotent, demonstrating transdifferentiation into neural and mesenchymal lineages 33,93.
The majority of research into CD34 expression in epithelial cells has been performed on skin tissue. However, there is a population of CD34+ stem cells residing in the epithelial ducts of the salivary gland 94,95. These cells retain their proliferative ability when cultured in 3D structures known as salispheres and demonstrate differentiation into cells and structures reminiscent of the salivary gland. There are also some links between CD34+ stromal cells and epithelial cells. CD34+ keratocytes have been shown to express the epithelial cell marker CK3 96, and CD34+ cells from human fetal liver express biliary epithelial markers CK7, CK8, and CK18 97.
CD34 and Endothelial Cells
CD34 is widely regarded as a marker of vascular endothelial progenitor cells 1,98. These BM-derived cells are found circulating in peripheral blood 99 and their usefulness in proangiogenic therapies has been extensively researched 98,99. The properties of CD34+ endothelial cells are often linked with hematopoietic cells, as both cell types can be isolated from peripheral blood using CD34 as an antigen. CD34+ cells isolated from peripheral blood have been studied for use in neovascularization therapies 100 and furthermore have shown ability to differentiate into cardiomyocytes 50 and osteoblasts 34.
A review published several years ago by Matsumoto et al. 35 discussed a possible overlap between endothelial progenitor cells and osteoblasts. There is a circulating CD34+ population of endothelial/skeletal progenitor cells, thought to originate in BM, capable of differentiating into both osteoblasts and endothelial cells. Investigations are ongoing using the circulating CD34+ cells to treat cases of nonunion fractures, which often suffer from delayed healing times due to inadequate blood supply around the injury site.
There is a subset of noncirculating adult endothelial cells that are also CD34+, most notably located within smaller blood vessels, while most endothelial cells in larger veins and arteries are CD34− 1. In contrast to the typical cobblestone morphology of endothelial cells, CD34+ cells are more elongated and lack tight junctions 101. CD34 expression is predominantly found on the luminal membrane of cellular processes but may also be seen on the abluminal membrane of cells found at the tips of vascular sprouts 1,102. In addition, CD34+ endothelial cells are quiescent and are thought to be involved in migration and adhesion 1.
Human umbilical vein endothelial cells (HUVEC) are CD34+ in vivo, however, when cultured in vitro, expression is lost after several passages and only a small population of CD34+ cells are retained 1. CD34+ HUVEC have distinct morphological characteristics, including numerous filopodia 101. Angiogenic stimuli provokes migration of these cells, and it has been proposed that this CD34+ subpopulation is homologous to sprouting tip cells, a specialized type of endothelial cell present at the leading edge during in vivo angiogenesis 101. CD34 is strongly expressed on the filopodia of these tip cells at sites of active angiogenesis and evidence yet again emphasizes the important functional role for CD34 in progenitor cell activity.
CD34 Antibody Selection
Careful selection of the antibody used for CD34 sorting and identification is of utmost importance during investigations. Some CD34 monoclonal antibodies (mAbs) select for epitopes that are sensitive to cleavage with neuraminidase (sialidase) and glycoprotease 103. Therefore, they are dependent on sialic acid residues remaining on the antigen. For this reason, an epitope classification for CD34 mAbs was devised, as seen in Table2. There are over 30 different mAbs for CD34 targeting different epitopes, and antibody clone used by studies discussed in this review can be seen in Table2. The most commonly used clones are MY10, a class Ib mAb, that is partially dependent on the presence of sialic acid residues and QBEnd10, a class II mAB, that has been shown to successfully detect both sialylated and desialylated CD34 103. The QBEnd10 clone is the antibody of choice for immunohistochemical protocols as it is resistant to denaturation and as the epitope is at the N-terminus of the CD34 molecule it is ideally suited to selection protocols such as fluorescence-activated cell sorting and magnetic-activated cell sorting. Class III epitopes, such as 8G12 retain high specificity for all glycoforms of the CD34 antigen and so are suitable for many protocols including flow cytometry and immunofluorescence 103. Polyclonal antibodies should be avoided for CD34 sorting and immunoblotting due to the possibilities of nonspecific labeling and high variation between antibody batches.
Table 2.
CD34 antibody selection: epitope specificity and clones
| Epitope class | Clone | Cell type | Assays performed | Reference |
|---|---|---|---|---|
| Ia. Sensitive to neuraminidase and glycoprotease | 12.8 | Hematopoietic cells, MSC, endothelial progenitors | Immunoblots, immunostaining, FACS | 19, 47 |
| BI.3C5 | MSC, keratocytes, endothelial progenitors | Immunoblots, immunostaining, FACS | 1, 9, 75 | |
| Ib. Partially sensitive to neuraminidase and glycoprotease | ICH3 | MSC, keratocytes, endothelial progenitors | Immunoblots, immunostaining, FACS | 1, 9, 75, 102 |
| MY10 | Hematopoietic cells, MSC, interstitial cells, endothelial progenitors | Immunoblots, immunostaining, FACS, Western blots | 1, 5, 9, 36, 39, 56, 84 | |
| II. Resistant to neuraminidase and sensitive to glycoprotease | QBEnd10 | MSC, muscle satellite cells, keratocytes, interstitial cells, HF epithelial progenitors, endothelial progenitors. | Immunoblots, immunostaining, flow cytometry | 1, 16, 23, 25, 65, 77, 86, 89, 101, 102 |
| III. Resistant to neuraminidase and glycoprotease | 563 | HF epithelial progenitors | Immunostaining | 90 |
| 581 | Muscle satellite cells, keratocytes | Immunostaining, flow cytometry | 16, 75 | |
| 8G12 | Muscle satellite cells, haematopoietic cells, MSC | Immunostaining, flow cytometry, FACS | 8, 12, 40, 44, 62, 64, 73 | |
| TUK3 | Endothelial progenitors | Immunoblots | 1 | |
| 115.2 | Endothelial progenitors | Immunoblots | 1 | |
| Monoclonal (clone not stated) | MSC | Immunostaining, flow cytometry | 10, 58, 59, 63, 69 | |
| Polyclonal | HF epithelial | Flow cytometry | 91 | |
| Antibody type not stated | MSC, muscle satellite cells, keratocytes, HF epithelial progenitors, salivary epithelial progenitors, endothelial progenitors | Immunostaining, flow cytometry, FACS | 13–15, 18, 21, 30, 34, 60, 67, 68, 70, 74, 82, 83, 92, 94 | |
Abbreviations: FACS, fluorescence-activated cell sorting; HF, hair follicle; MSC, multipotent mesenchymal stromal cells.
Almost all methods of cell surface marker analysis involve the binding of antibodies to an antigen. Antigen–antibody interactions are noncovalent and can be reversible; therefore, it is important to validate an experiment carefully. Key proteins of interest can be interrogated using multiple methods, such as using an alternative antibody, technique or analysis of gene transcription. To characterize a cell population it is essential to develop a comprehensive characterization process comprising of a profile of markers, both surface and intracellular, functional assays and, as in the case of stem cells, interrogation of their differentiation potentials.
Conclusion
CD34 is a cell surface marker that is expressed by a broad range of cells including hematopoietic, stromal, epithelial, and endothelial cells. Although the function of CD34 as a surface antigen is still unknown, it has been linked to inhibition or facilitation of adhesion, cell proliferation, and regulation of differentiation 40,41,55,103. This review focused on the use of CD34 to identify cells from diverse tissues that have comparable properties (Table3). The discernible link between all CD34+ cell types is progenitor and stem cell activity, and in many cases, the CD34+ population of cells shows a more potent or pronounced differentiation capacity. Research alludes to a correlation between cell plasticity and CD34 expression, with loss of CD34, alongside other cell surface antigens, suggesting lineage commitment from the more positive progenitor cell. Many of these cells also demonstrate a quiescent state in vivo, until activated to differentiate. Although all the cell types discussed throughout this review express CD34, they do not all display identical properties. Many coexpress tissue-specific markers alongside CD34, suggesting that the presence of CD34 may indicate a specific progenitor for that tissue. Evidence for this is certainly apparent in muscle satellite cells, keratocytes, and epithelial progenitors. However, this does not necessarily limit the differentiation capacity of the cells in vitro, with many of the CD34+ cells linked by transdifferentiation ability.
Table 3.
Comparable properties of CD34+ cells
| Comparable properties of CD34+ cells | Described in: |
|---|---|
| Potential stem/progenitor cell population | HSC, MSC, muscle satellite cells, keratocytes, interstitial cells, fibrocytes, HF epithelial stem cells, vascular endothelial progenitors |
| Quiescent in vivo | Muscle satellite cells, keratocytes, interstitial cells, fibrocytes |
| Greater CFU-F capacity and high proliferative capacity in vitro | HSC, MSC, HF epithelial stem cells |
| Loss of CD34 expression upon “activation” or differentiation either in vivo or upon in vitro culture | MSC, muscle satellite cells, keratocytes, fibrocytes, HF epithelial stem cells |
| Coexpression with tissue specific markers | Muscle satellite cells, keratocytes, interstitial cells, HF epithelial stem cells, salivary gland stem cells, vascular endothelial progenitors |
| Transdifferentiation potential | HSC, MSC, muscle satellite cells, keratocytes, HF epithelial stem cells, vascular endothelial progenitors |
Abbreviations: CD, cluster of differentiation; CFU-F, colony forming units fibroblast; HF, hair follicle; HSC, hematopoietic stem cells; MSC, multipotent mesenchymal stromal cells.
Although CD34 may be one marker that is useful in identifying progenitor populations, a single marker alone is not appropriate for the characterization of a cell type. The expression of CD34 by all cell types discussed throughout the review may not be exclusive. Due to a lack of cohesive research, we cannot yet identify another marker that appears on all cells, but markers such as CD90, CD117 (c-kit), CD146, and CD133 have been indicated on more than one cell type. To fully characterize a population of stem cells it is most likely that a specific marker profile would be required, alongside clonal assays, differentiation assays and functional profiling.
The culture and propagation of adherent CD34+ cells in vitro is challenging. This is conceivably due to the association of CD34 with quiescence and adhesion, and the loss of CD34 expression upon differentiation. Culture on tissue culture plastic and in serum-containing medium creates an environment dissimilar to the in vivo environment, forcing the cells to proliferate and differentiate, subsequently losing CD34. If CD34+ cells are to be investigated in vitro, more thought, specialized culture conditions and optimization is required to recapitulate an environment more similar to the in vivo niche.
By compiling this review, we would like to propose that CD34 is considered as a surface antigen suitable for the selection of subpopulations of progenitor cells from larger cell populations, including mesenchymal cells, and is not associated only with hematopoietic and endothelial cells. Recognizing CD34 as a progenitor marker will allow further research into this distinct subset of cells, which potentially possess a pronounced differentiation capacity. If culture and propagation techniques for these cells can be optimized, CD34+ cells from many tissue types may represent a source of progenitor cells that can be exploited clinically in regenerative medicine strategies.
Acknowledgments
This work was supported by grants from The Royal College of Surgeons Edinburgh and Fight for Sight. S.E.D. is supported by funding from the EPSRC Doctoral Training Centre in Regenerative Medicine. The authors would like to thank Mr Owen McIntosh for discussions and assistance with proof reading.
Author Contributions
L.E.S. and M.J.B.: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; S.E.D.: data analysis and interpretation and manuscript writing; H.S.D. and A.H.: financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 2.Brown J, Greaves MF, Molgaard HV. The gene encoding the stem cell antigen, CD34, is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts. Int Immunol. 1991;3:175–184. doi: 10.1093/intimm/3.2.175. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 4.Lin CS, Ning H, Lin G, et al. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14:1159–1163. doi: 10.3109/14653249.2012.729817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama H, Enzan H, Miyazaki E, et al. Differential expression of CD34 in normal colorectal tissue, peritumoral inflammatory tissue, and tumour stroma. J Clin Pathol. 2000;53:626–629. doi: 10.1136/jcp.53.8.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanpain C, Lowry WE, Geoghegan A, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Huss R. Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells. 2000;18:1–9. doi: 10.1634/stemcells.18-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Servida F, Soligo D, Caneva L, et al. Functional and morphological characterization of immunomagnetically selected CD34+ hematopoietic progenitor cells. Stem Cells. 1996;14:430–438. doi: 10.1002/stem.140430. [DOI] [PubMed] [Google Scholar]
- 9.Simmons PJ, Torok-Storb B. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991;78:2848–2853. [PubMed] [Google Scholar]
- 10.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 11.Ferraro GA, De Francesco F, Nicoletti G, et al. Human adipose CD34(+) CD90(+) stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J Cell Biochem. 2013;114:1039–1049. doi: 10.1002/jcb.24443. [DOI] [PubMed] [Google Scholar]
- 12.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 13.Kuci S, Kuci Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Francesco F, Tirino V, Desiderio V, et al. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One. 2009;4:e6537. doi: 10.1371/journal.pone.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopher RA, Penchev VR, Islam MS, et al. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. 2010;47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinanan AC, Hunt NP, Lewis MP. Human adult craniofacial muscle-derived cells: Neural-cell adhesion-molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnol Appl Biochem. 2004;40:25–34. doi: 10.1042/BA20030185. [DOI] [PubMed] [Google Scholar]
- 17.Dupas T, Rouaud T, Rouger K, et al. Fetal muscle contains different CD34+ cell subsets that distinctly differentiate into adipogenic, angiogenic and myogenic lineages. Stem Cell Res. 2011;7:230–243. doi: 10.1016/j.scr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Zheng B, Cao B, Crisan M, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 19.Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993;106(Pt 2):685–691. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- 20.West-Mays JA, Dwivedi DJ. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38:1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polisetty N, Fatima A, Madhira SL, et al. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 22.Hatou S, Yoshida S, Higa K, et al. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem Cells Dev. 2013;22:828–839. doi: 10.1089/scd.2012.0286. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen H, Hansen A, Smedts F, et al. CD34-positive interstitial cells of the human detrusor. APMIS. 2007;115:1260–1266. doi: 10.1111/j.1600-0643.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 24.Popescu LM, Ciontea SM, Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann N Y Acad Sci. 2007;1101:139–165. doi: 10.1196/annals.1389.022. [DOI] [PubMed] [Google Scholar]
- 25.Popescu LM, Ciontea SM, Cretoiu D, et al. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005;9:479–523. doi: 10.1111/j.1582-4934.2005.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W, Zeidel ML, Hill WG. Cellular expression profile for interstitial cells of cajal in bladder - a cell often misidentified as myocyte or myofibroblast. PLoS One. 2012;7:e48897. doi: 10.1371/journal.pone.0048897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost. 2009;101:613–618. [PMC free article] [PubMed] [Google Scholar]
- 28.Choi YH, Burdick MD, Strieter RM. Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. Int J Biochem Cell Biol. 2010;42:662–671. doi: 10.1016/j.biocel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 31.Waseem A, Dogan B, Tidman N, et al. Keratin 15 expression in stratified epithelia: Downregulation in activated keratinocytes. J Invest Dermatol. 1999;112:362–369. doi: 10.1046/j.1523-1747.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez-Rivera A, Pavon-Rodriguez A, Jimenez-Acosta F, et al. Functional characterization of highly adherent CD34+ keratinocytes isolated from human skin. Exp Dermatol. 2010;19:685–688. doi: 10.1111/j.1600-0625.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 33.Sieber-Blum M, Grim M, Hu YF, et al. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258–269. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 34.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: Proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto T, Kuroda R, Mifune Y, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43:434–439. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Civin CI, Strauss LC, Brovall C, et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 37.Krause DS, Ito T, Fackler MJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994;84:691–701. [PubMed] [Google Scholar]
- 38.Baumheter S, Singer MS, Henzel W, et al. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 39.Felschow DM, McVeigh ML, Hoehn GT, et al. The adapter protein CrkL associates with CD34. Blood. 2001;97:3768–3775. doi: 10.1182/blood.v97.12.3768. [DOI] [PubMed] [Google Scholar]
- 40.Healy L, May G, Gale K, et al. The stem cell antigen CD34 functions as a regulator of hemopoietic cell adhesion. Proc Natl Acad Sci USA. 1995;92:12240–12244. doi: 10.1073/pnas.92.26.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen JS, McNagny KM. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcirculation. 2009;16:487–496. doi: 10.1080/10739680902941737. [DOI] [PubMed] [Google Scholar]
- 42.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 43.Drew E, Merzaban JS, Seo W, et al. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity. 2005;22:43–57. doi: 10.1016/j.immuni.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivanovic Z. Hematopoietic stem cells in research and clinical applications: The "CD34 issue". World J Stem Cells. 2010;2:18–23. doi: 10.4252/wjsc.v2.i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berardi AC, Wang A, Levine JD, et al. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 47.Berenson RJ, Bensinger WI, Hill RS, et al. Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood. 1991;77:1717–1722. [PubMed] [Google Scholar]
- 48.Mao Q, Chu S, Ghanta S, et al. Ex vivo expanded human cord blood-derived hematopoietic progenitor cells induce lung growth and alveolarization in injured newborn lungs. Respir Res. 2013;14:37. doi: 10.1186/1465-9921-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang YY, Collector MI, Baylin SB, et al. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Wang D, Estrov Z, et al. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 51.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 52.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 53.Chamberlain G, Fox J, Ashton B, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 54.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 55.Scherberich A, Di Maggio ND, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J Stem Cells. 2013;5:1–8. doi: 10.4252/wjsc.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 57.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 58.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 59.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Phinney DG, Sensebe L. Mesenchymal stromal cells: Misconceptions and evolving concepts. Cytotherapy. 2013;15:140–145. doi: 10.1016/j.jcyt.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 63.Waller EK, Olweus J, Lund-Johansen F, et al. The "common stem cell" hypothesis reevaluated: Human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995;85:2422–2435. [PubMed] [Google Scholar]
- 64.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 65.Branch MJ, Hashmani K, Dhillon P, et al. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53:5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 66.Halfon S, Abramov N, Grinblat B, et al. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 67.Ferraro GA, De Francesco F, Nicoletti G, et al. Human adipose CD34+ CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J Cell Biochem. 2013;114:1039–1049. doi: 10.1002/jcb.24443. [DOI] [PubMed] [Google Scholar]
- 68.Kaiser S, Hackanson B, Follo M, et al. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. 2007;9:439–450. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 69.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 70.Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cossu G, Molinaro M, Pacifici M. Differential response of satellite cells and embryonic myoblasts to a tumor promoter. Dev Biol. 1983;98:520–524. doi: 10.1016/0012-1606(83)90382-2. [DOI] [PubMed] [Google Scholar]
- 73.Lecourt S, Marolleau JP, Fromigue O, et al. Characterization of distinct mesenchymal-like cell populations from human skeletal muscle in situ and in vitro. Exp Cell Res. 2010;316:2513–2526. doi: 10.1016/j.yexcr.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 74.Perrella G, Brusini P, Spelat R, et al. Expression of haematopoietic stem cell markers, CD133 and CD34 on human corneal keratocytes. Br J Ophthalmol. 2007;91:94–99. doi: 10.1136/bjo.2006.097352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joseph A, Hossain P, Jham S, et al. Expression of CD34 and l-selectin on human corneal keratocytes. Invest Ophthalmol Vis Sci. 2003;44:4689–4692. doi: 10.1167/iovs.02-0999. [DOI] [PubMed] [Google Scholar]
- 76.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashmani K, Branch MJ, Sidney LE, et al. Characterisation of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther. 2013;4:75. doi: 10.1186/scrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu J, Du Y, Mann MM, et al. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng Part A. 2013;19:2063–2075. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson SL, Yang Y, El Haj AJ. Corneal stromal cell plasticity: In vitro regulation of cell phenotype through cell-cell interactions in a three-dimensional model. Tissue Eng Part A. 2014;20:225–238. doi: 10.1089/ten.tea.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du Y, Sundarraj N, Funderburgh ML, et al. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–515. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 82.Choong PF, Mok PL, Cheong SK, et al. Mesenchymal stromal cell-like characteristics of corneal keratocytes. Cytotherapy. 2007;9:252–258. doi: 10.1080/14653240701218508. [DOI] [PubMed] [Google Scholar]
- 83.Li GG, Zhu YT, Xie HT, et al. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuroda N, Nakayama H, Miyazaki E, et al. Distribution and role of CD34-positive stromal cells and myofibroblasts in human normal testicular stroma. Histol Histopathol. 2004;19:743–751. doi: 10.14670/HH-19.743. [DOI] [PubMed] [Google Scholar]
- 85.Junquera C, Martinez-Ciriano C, Castiella T, et al. Immunohistochemical and ultrastructural characteristics of interstitial cells of Cajal in the rabbit duodenum. Presence of a single cilium. J Cell Mol Med. 2007;11:776–787. doi: 10.1111/j.1582-4934.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanderwinden JM, Rumessen JJ, De Laet MH, et al. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000;302:145–153. doi: 10.1007/s004410000264. [DOI] [PubMed] [Google Scholar]
- 87.Bucala R. Circulating fibrocytes: Cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39. doi: 10.1016/j.jacr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 88.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poblet E, Jimenez F, Godinez JM, et al. The immunohistochemical expression of CD34 in human hair follicles: A comparative study with the bulge marker CK15. Clin Exp Dermatol. 2006;31:807–812. doi: 10.1111/j.1365-2230.2006.02255.x. [DOI] [PubMed] [Google Scholar]
- 90.Ohyama M, Terunuma A, Tock CL, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh JH, Mohebi P, Farkas DL, et al. Towards expansion of human hair follicle stem cells in vitro. Cell Prolif. 2011;44:244–253. doi: 10.1111/j.1365-2184.2011.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang S, Zhao L, Purandare B, et al. Differential expression of stem cell markers in human follicular bulge and interfollicular epidermal compartments. Histochem Cell Biol. 2010;133:455–465. doi: 10.1007/s00418-010-0684-z. [DOI] [PubMed] [Google Scholar]
- 93.Amoh Y, Li L, Katsuoka K, et al. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banh A, Xiao N, Cao H, et al. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res. 2011;17:7265–7272. doi: 10.1158/1078-0432.CCR-11-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pringle S, Van Os R, Coppes RP. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2012;31:613–619. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- 96.Perrella G, Scott CA, Spelat R, et al. Cultured hyman keratocytes from the limbus and cornea both express epithelial cytokeratin 3: Possible mesenchymal-epithelial transition. Int J Ophthalmic Pathol. 2012;1:1–7. [Google Scholar]
- 97.Lemmer ER, Shepard EG, Blakolmer K, et al. Isolation from human fetal liver of cells co-expressing CD34 haematopoietic stem cell and CAM 5.2 pancytokeratin markers. J Hepatol. 1998;29:450–454. doi: 10.1016/s0168-8278(98)80064-0. [DOI] [PubMed] [Google Scholar]
- 98.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58:148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Brenes RA, Bear M, Jadlowiec C, et al. Cell-based interventions for therapeutic angiogenesis: Review of potential cell sources. Vascular. 2012;20:360–368. doi: 10.1258/vasc.2011.201205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mackie AR, Losordo DW. CD34-positive stem cells: In the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- 101.Siemerink MJ, Klaassen I, Vogels IM, et al. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 2012;15:151–163. doi: 10.1007/s10456-011-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schlingemann RO, Rietveld FJ, de Waal RM, et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990;62:690–696. [PubMed] [Google Scholar]
- 103.Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: An update. J Biol Regul Homeost Agents. 2001;15:1–13. [PubMed] [Google Scholar]


