Abstract
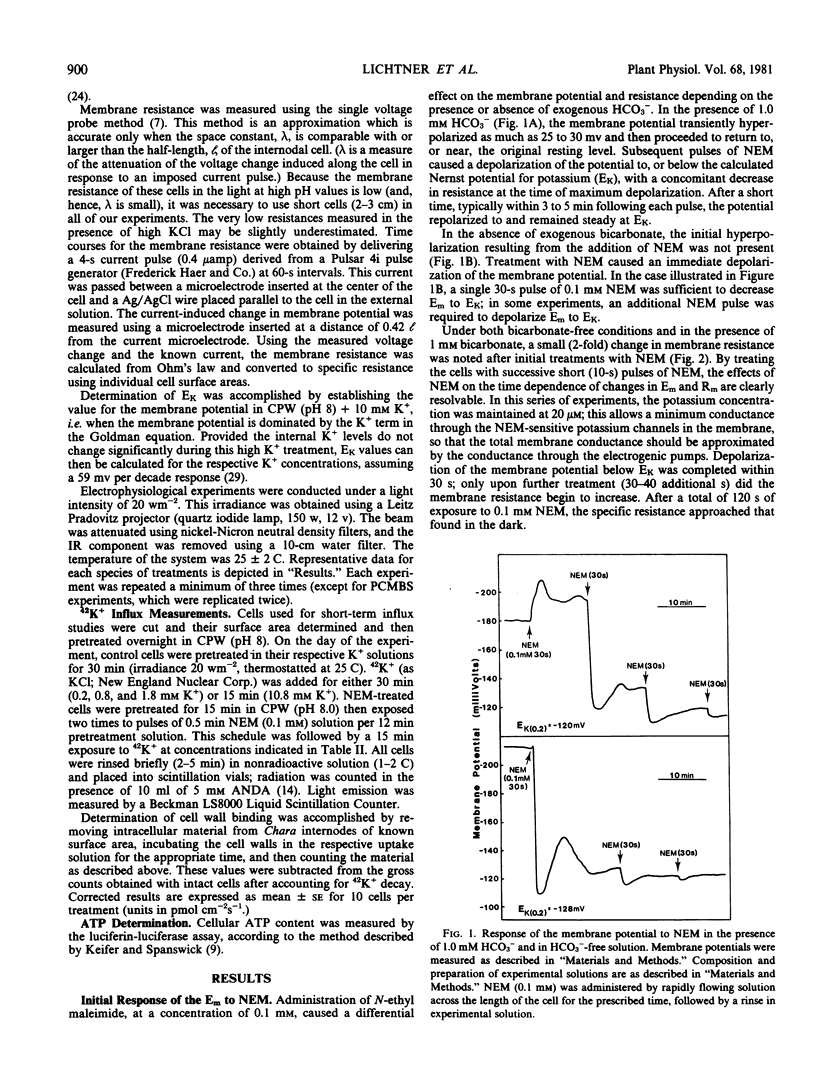
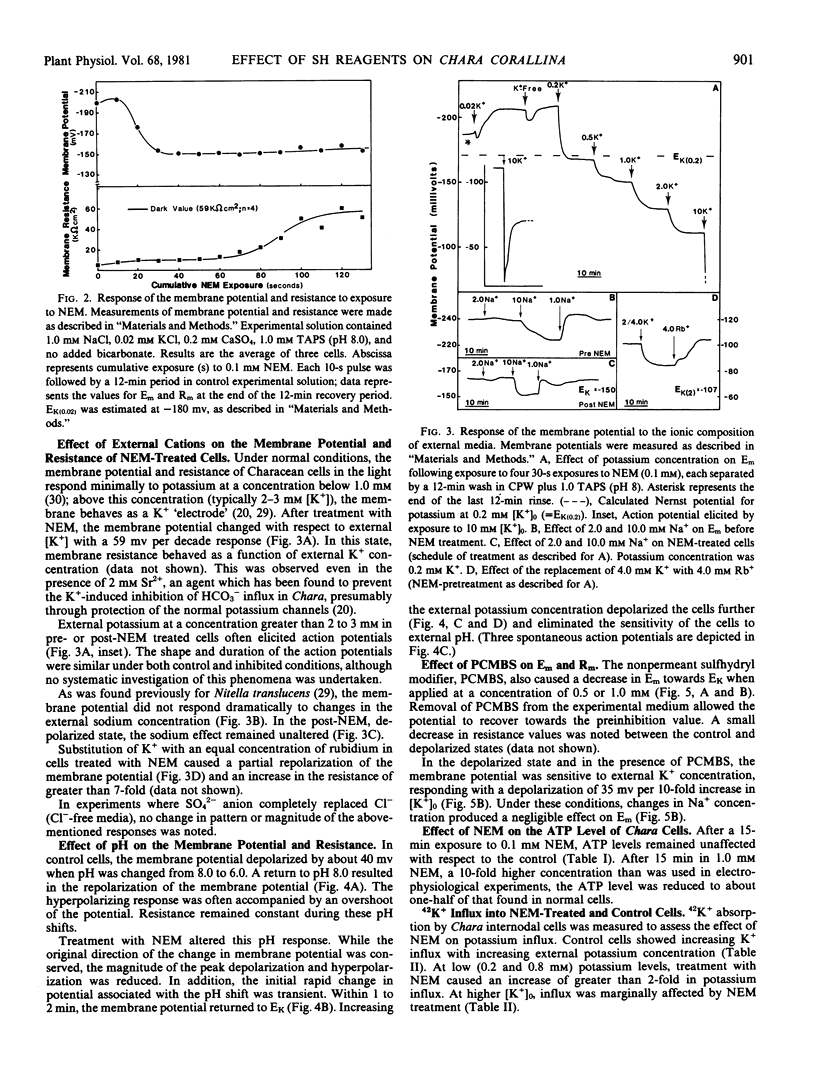
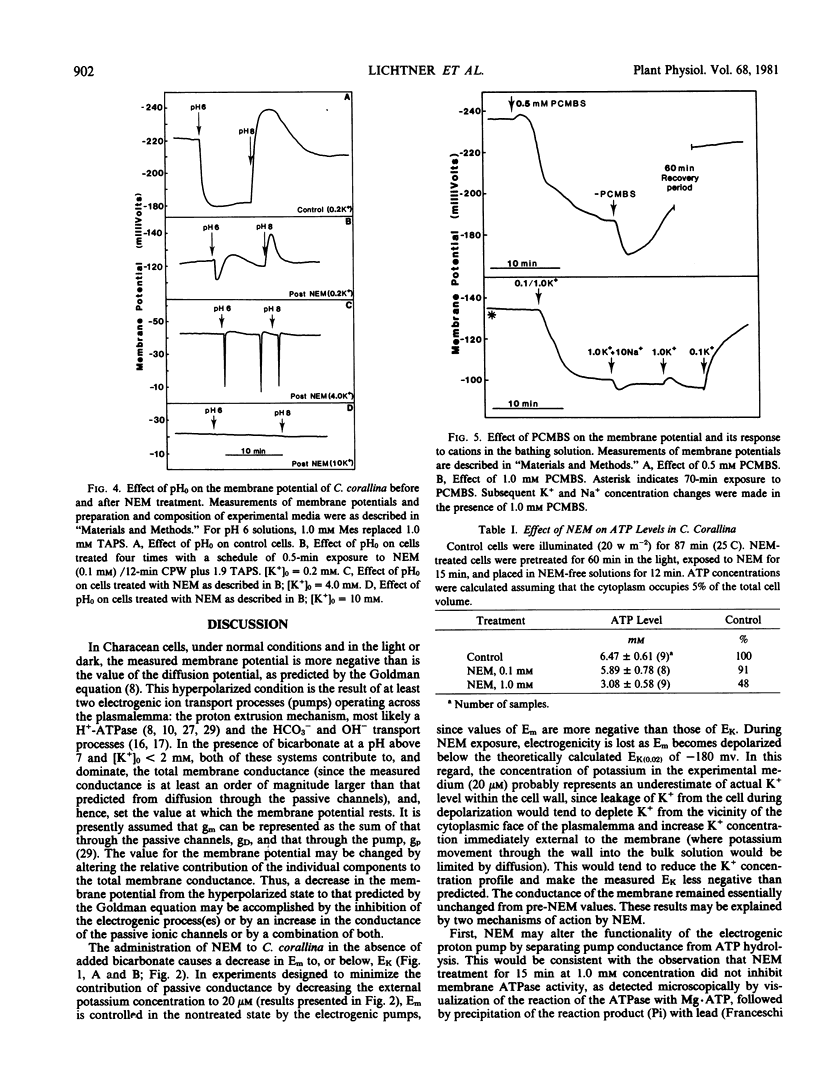
The administration of the sulfhydryl reagent N-ethyl-maleimide (NEM) to internodal cells of Chara corallina caused alterations in the biophysical properties of the plasmalemma, as measured with electrophysiological and radioactive tracer techniques. The membrane potential depolarized to, or near, the calculated Nernst potential for potassium (EK) after 30 seconds' exposure to 0.1 millimolar NEM. During this time, the ATP level did not decrease below the control value, and the specific membrane resistance did not increase; only upon further exposure to NEM did the resistance approach the value observed in the dark. In the depolarized state, the membrane potential responded to changes in the external potassium concentration in the manner of a K+-electrode, but it retained it's relative insensitivity to external sodium.
These results are interpreted in the following manner: NEM causes a) an increase in the membrane permeability to K+ (i.e. an increase in K+ conductance); and b) perturbation of the electrogenic transport system(s) of the plasma membrane. The latter effect is manifested in a manner that is not consistent with an inhibition of ATP catalysis by a voltage-dependent ATPase possessing conductance. The nonpermeant sulfhydryl modifier, p-chloromercuribenzenesulfonic acid, appeared to affect membrane properties in a similar, but reversible, way.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biber J., Hauser H. The role of SH-groups in the concentrative transport of D-glucose into brush border membrane vesicles. FEBS Lett. 1979 Dec 15;108(2):451–456. doi: 10.1016/0014-5793(79)80586-4. [DOI] [PubMed] [Google Scholar]
- Bogucka K., Wojtczak L. On the mechanism of mercurial-induced permeability of the mitochondrial membrane to K+. FEBS Lett. 1979 Apr 15;100(2):301–304. doi: 10.1016/0014-5793(79)80356-7. [DOI] [PubMed] [Google Scholar]
- Etherton B. Comparison of three methods for measuring electrical resistances of plant cell membranes. Plant Physiol. 1977 Nov;60(5):684–688. doi: 10.1104/pp.60.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg J., Williams E. J., Johnston R. J. A simplified method for measuring membrane resistances in Nitella translucens. Biochim Biophys Acta. 1968 Apr 29;150(3):518–520. doi: 10.1016/0005-2736(68)90152-1. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Activity of the Electrogenic Pump in Chara corallina as Inferred from Measurements of the Membrane Potential, Conductance, and Potassium Permeability. Plant Physiol. 1978 Oct;62(4):653–661. doi: 10.1104/pp.62.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto U., Kami-ike N., Takeuchi Y. The role of electrogenic pump in Chara corallina. J Membr Biol. 1980 Jul 15;55(2):149–156. doi: 10.1007/BF01871157. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Weber H., Tanner W. Essential Sulfhydryl Group in the Transport-catalyzing Protein of the Hexose-Proton Cotransport System of Chlorella. Plant Physiol. 1978 May;61(5):785–786. doi: 10.1104/pp.61.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., Silverman M. P. Gating effects in Halobacterium halobium membrane transport. J Biol Chem. 1979 Jun 10;254(11):4750–4755. [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981 Apr;67(4):869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Alexander J. M. Sulfhydryl Group Involvement in Plasmalemma Transport of HCO(3) and OH in Chara corallina. Plant Physiol. 1980 Feb;65(2):274–280. doi: 10.1104/pp.65.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J. HCO(3) Influx across the Plasmalemma of Chara corallina: Physiological and Biophysical Influence of 10 mm K. Plant Physiol. 1978 Apr;61(4):487–493. doi: 10.1104/pp.61.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E. A. The active transport of ions in plant cells. Q Rev Biophys. 1970 Aug;3(3):251–294. doi: 10.1017/s0033583500004741. [DOI] [PubMed] [Google Scholar]
- Moroney J. V., Andreo C. S., Vallejos R. H., McCarty R. E. Uncoupling and energy transfer inhibition of photophosphorylation by sulfhydryl reagents. J Biol Chem. 1980 Jul 25;255(14):6670–6674. [PubMed] [Google Scholar]
- Okada Y., Inouye A. Tip potential and fixed charges on the glass wall of microelectrode. Experientia. 1975 May 15;31(5):545–546. doi: 10.1007/BF01932449. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]
- Underwood C., Gould J. M. Proton efflux through the chloroplast ATP synthase (CF0 . CF1) in the presence of sulfhydryl-modifying agents. Biochim Biophys Acta. 1980 Feb 8;589(2):287–298. doi: 10.1016/0005-2728(80)90045-6. [DOI] [PubMed] [Google Scholar]
- Will P. C., Hopfer U. Apparent inhibition of active non-electrolyte transport by an increased sodium permeability of the plasma membrane. Mechanism of action of p-chloromercuribenzene sulfonate. J Biol Chem. 1979 May 25;254(10):3806–3811. [PubMed] [Google Scholar]
- Willard J. M., Davis J. J., Wood H. G. Phosphoenolpyruvate carboxytransphosphorylase. IV. Requirement for metal cations. Biochemistry. 1969 Aug;8(8):3137–3144. doi: 10.1021/bi00836a002. [DOI] [PubMed] [Google Scholar]



