Abstract
Introduction: In this study we tested the hypothesis that tirasemtiv, a selective fast skeletal muscle troponin activator that sensitizes the sarcomere to calcium, could amplify the response of muscle to neuromuscular input in humans. Methods: Healthy men received tirasemtiv and placebo in a randomized, double-blind, 4-period, crossover design. The deep fibular nerve was stimulated transcutaneously to activate the tibialis anterior muscle and produce dorsiflexion of the foot. The force–frequency relationship of tibialis anterior dorsiflexion was assessed after dosing. Results: Tirasemtiv increased force produced by the tibialis anterior in a dose-, concentration-, and frequency-dependent manner with the largest increases [up to 24.5% (SE 3.1), P < 0.0001] produced at subtetanic nerve stimulation frequencies (10 Hz). Conclusions: The data confirm that tirasemtiv amplifies the response of skeletal muscle to nerve input in humans. This outcome provides support for further studies of tirasemtiv as a potential therapy in conditions marked by diminished neuromuscular input. Muscle Nerve 50: 925–931, 2014
Keywords: fast skeletal muscle troponin activator, force–frequency relationship, Phase 1, skeletal muscle, tirasemtiv
In many diseases, muscle weakness is the result of limited neuromuscular input and can lead to significant disability and increased mortality. Diseases such as amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), and Charcot–Marie–Tooth disease (CMT) cause damage or death to motor neurons that strains the ability of surviving motor neurons to activate muscle to generate force effectively.1–4 In myasthenia gravis (MG), weakness and fatigue result from failure of signal transmission at the neuromuscular junction (NMJ), which limits calcium release and force production.5 Treatment consists of acetylcholinesterase inhibitors and immunosuppression, although weakness and fatigue are still common in these patients.6 Therapeutic options for other neuropathies are limited or nonexistent.
Tirasemtiv (formerly CK-2017357) is an investigational drug that is a highly selective activator of the fast skeletal muscle (type II) troponin complex. It was developed as a means to increase muscle strength by amplifying the response of muscle when neuromuscular input is diminished secondary to a neuromuscular disease.7 Tirasemtiv slows the rate of calcium release from fast skeletal muscle troponin, thus increasing its affinity for calcium and sensitizing muscle to calcium. As a consequence, the force–calcium relationship of fast skeletal muscle fibers shifts leftward, and muscle force increases relative to control at submaximal activation. In preclinical models of nerve–muscle function, tirasemtiv amplified the muscle response to submaximal nerve stimulation but not to tetanic stimulation7; muscle force increased within the operating range of motor unit discharge rates associated with voluntary activation.8 In a preclinical disease model of limited neuromuscular input, namely the passive transfer rat model of MG,9 tirasemtiv increased the force of muscle contraction at submaximal nerve stimulation frequencies, increased grip strength, and decreased muscle fatigability. We next sought to study the unique pharmacological profile of this fast skeletal muscle troponin activator in humans.
Evaluation of skeletal muscle function in humans is complicated by the fact that muscle contraction is under voluntary control, and effort-based tests have substantial variability. Therefore, we assessed the effect of single doses of tirasemtiv on skeletal muscle function in healthy volunteers by measuring the change in the isometric force–frequency response of the tibialis anterior muscle using external electrical stimulation of the deep fibular nerve.10 The primary objective of this study was to determine the change in the force–frequency response and its relationship to tirasemtiv plasma concentrations after oral administration to healthy volunteers. In doing so, we aimed to recapitulate the effects of tirasemtiv on the force–frequency response observed preclinically and establish translation of this novel mechanism of action into humans.
METHODS
Study Design
This study was conducted in 2 parts. Part A enrolled 57 healthy men in a double-blind, dose-escalating, placebo-controlled design to establish the maximum tolerated dose (MTD) and determine the pharmacokinetics of tirasemtiv. Each treatment period enrolled a cohort of 8 subjects (randomized to achieve 6 active drug and 2 placebo subjects per treatment period). Each subject was dosed twice; once in each of 2 treatment periods approximately 2 weeks apart. Two cohorts were studied at the same time in an overlapping, leapfrog fashion. Part B enrolled 12 healthy men in a double-blind, randomized dose, placebo-controlled, 4-period, crossover design to assess tirasemtiv pharmacodynamics and their relationship to dose and pharmacokinetics. Healthy men, 18–50 years of age with a body mass index (BMI) of between 18.0 and 30.0 kg/m2, were eligible for enrollment if they were judged to be in good health on the basis of history, physical and laboratory examination, and electrocardiogram (ECG). In Part B, subjects were excluded if they could not tolerate the measurement protocol during a screening exercise.
Standard Protocol Approvals, Registrations, and Patient Consent
The protocol and procedures were approved by the institutional review board governing the Phase I clinical site (Covance, Evansville, Indiana) and were conducted in accordance with the applicable United States Code of Federal Regulations governing the Protection of Human Subjects (21 CFR 50). All subjects provided written informed consent before enrollment.
Procedures
In Part A, active doses consisted of solid tirasemtiv in capsules (Coni-Snap Size 00 Swedish Orange; Capsugel, Inc., Morristown, New Jersey) from 20 to 1250 mg and tirasemtiv in a liquid formulation (suspended in Ora-Blend; Paddock Laboratories, Inc., Minneapolis, Minnesota) from 640 to 2500 mg. Safety evaluations included adverse event (AE) assessments, 12-lead ECGs, vital signs, physical examinations, and laboratory assessments. Neurological examinations, walk tests, cardiac telemetry, and continuous pulse oximetry were also assessed. After each treatment period, progression to the subsequent treatment period was determined based on an interim blinded assessment of safety and available pharmacokinetic (PK) data. Maximum tolerated dose was defined when either the pattern of intolerance clearly distinguished active drug from placebo or the number of intolerant subjects on active drug exceeded intolerant placebo subjects by 2 or more.
In Part B, 12 subjects received placebo and 250-, 500-, and 1000-mg doses of tirasemtiv in the same liquid formulation as in Part A in random order with a minimum washout period of 7 days between each dose. The pharmacodynamic response to tirasemtiv was determined by measuring the force–frequency relationship of tibialis anterior muscle contraction elicited by transcutaneous electrical stimulation of the deep fibular nerve.
To measure tibialis anterior muscle force, 6 adjustable, rigid chair frames with integrated foot-plates incorporating a force sensor were constructed. Each subject was fitted into the chair, and the right foot was strapped firmly to the foot-plate with the lower leg and knee immobilized. The chairs were constructed so that, when seated, the subject's knees were bent approximately 60°, and the ankle angle was fixed at 105° (shin to bottom of foot). A strain-gauge containing a load cell (MLP-75; Transducer Techniques, Temecula, California) coupled to the bottom of the foot-plate was used to measure dorsiflexion force.
An adhesive surface electrode (61-2510; ConMed, USA) fixed to the lateral aspect of the upper leg just below the head of the fibula acted as the cathode and delivered stimulation pulses transcutaneously to the deep fibular nerve. The anode was placed on the medial aspect of the knee. To identify optimal cathode placement, a hand-held, non-adhesive electrode through which low-intensity stimulation pulses were delivered was used to activate the nerve without stimulating antagonistic muscle groups, as determined by palpation. The stimulus intensity was set by slowly increasing the electrical current during each stimulation pulse until the magnitude of the tibialis anterior twitch force and the resulting electromyogram (EMG) signal did not increase in magnitude. The final stimulus current was then set approximately 20% greater to ensure maximal nerve activation throughout the dosing period.
The force–frequency response of each subject was measured at baseline, and at 1, 3, 5, and 7 hours post-dose during each of the 4 dosing periods in Part B. Each stimulation protocol consisted of 5-, 7.5-, 10-, 12.5-, 15-, 17.5-, 25-, and 50-Hz stimulation trains of 0.5-ms pulse width and 800-ms duration. The stimulation frequency was delivered in random order so subjects could not anticipate the intensity of the stimulus with a single stimulus pulse delivered 5 s before and 5 s after each stimulus train to elicit a twitch response. Twitch–train–twitch sequences were separated by 30 s. At each assessment time-point, the stimulation protocol was performed in triplicate, and commensurate blood samples were taken to measure tirasemtiv plasma concentrations.
The data acquisition system used to create stimulation pulse trains, amplify the EMG, and measure the strain-gauge output was custom designed. The measurement device and control software were validated to meet 21 CFR 11 regulatory requirements to ensure patient safety, confidentiality, and data integrity for use in a clinical trial.
Statistical Analysis
Version 10.1 or newer of the Medical Dictionary for Regulatory Activities (MedDRA) was used to classify AE by system organ class (SOC) and preferred term. WinNonLin (version 6.1) software was used for pharmacokinetic analysis of plasma concentration–time profiles to determine maximum plasma concentration (Cmax), area under the curve (AUC) using model-independent methods, and other pharmacokinetics parameters.
In Part B, prior to unblinding, force transients and the resulting normalized force–frequency response for each assessment were scored for quality by an expert over-reader based on an assessment of the underlying shape of the force–frequency profile, the variation in replicates, the shape of the stimulus transients, and the lack of volitional response or interference from antagonistic muscles. A rating scale was devised to score each force–frequency profile (Table S1, refer to Supplementary Material available online) on a 1–5 scale, with a score of 1 assigned to continuous, canonical force–frequency profiles and a score of 5 being assigned to an uninterpretable force–frequency profile. Individual dosing periods with assessment scores averaging >2 were rejected. In each dosing period, the baseline assessment was required to have a score of 1 or 2. The over-read data set contained data from each subject and 75% of the overall data collected. In addition, the complete data set was analyzed to ensure consistency of the results between the 2 data sets.
Force transients produced by each stimulation frequency were analyzed with a custom analysis software application to identify the peak force value. To correct for transducer offset, a section of prestimulation baseline was averaged and subtracted from the measured peak force. The peak forces of common stimulation frequencies were averaged. The force–frequency profile was normalized by dividing this average peak force by the average peak force produced by the 50-Hz stimulus, which was interpreted as tetanic (maximum) activation of the muscle.
The data were analyzed in 2 ways. First, the percent change in force from baseline for each assessment time-point in each dosing period was summed over stimulation frequencies from 5 to 25 Hz (%ΣFt) as follows:
| 1 |
where ΣνFt is the sum of the normalized peak force at each stimulation frequency, ν, for a specific assessment time-point, t. Similarly, ΣνFo is the summed baseline (t = 0, pre-dose) response. Statistical analysis of the data was completed using commercial statistics software (SAS version 9.1.3). Data were analyzed with a repeated-measures analysis of covariance (ANCOVA) model that included treatment (dose), sequence, and period as fixed effects; baseline as a covariate; and subject as random effect. Placebo-corrected least-squares mean differences and P-values were reported for each assessment time-point of each dose of tirasemtiv administered. In addition, data were also analyzed with a regression repeated-measures ANCOVA model that included sequence and period as fixed effects, baseline and treatment (dose. in milligrams) as covariates, and subject as random effect.
A second concentration–response analysis examined the effects of tirasemtiv at each stimulation frequency. Here, the percent change in peak force from baseline at each stimulation frequency, %F(ν), was calculated as follows:
| 2 |
where Ft(ν) is the peak force for the stimulation frequency, ν, at the specific assessment time-point t and Fo(ν) is the same for the baseline (pre-dose) response. The analysis assigned each observation into a tirasemtiv concentration bin (i.e., 0–4 μg/ml, 4–6 μg/ml, 6–8 μg/ml, 8–12 μg/ml, and >12 μg/ml) depending on the sampled plasma concentration of tirasemtiv at the time of measurement for all dosing periods in which drug was given, regardless of dose or time-point, and treated the bins as separate groups. All placebo observations were similarly pooled. The repeated-measures ANCOVA model included concentration bin, sequence, and dose period as fixed effects; baseline peak force as a covariate; and subject as a random effect. Least-squares mean differences from placebo and calculated P-values were reported for each stimulation frequency as a function of concentration bin. No adjustments were made for multiple comparisons.
RESULTS
The goal of Part A was to determine the maximum tolerated dose (MTD) of tirasemtiv. A total of 57 men (47 Caucasian, 5 African American, 2 Asian, 1 American Indian/Alaskan Native, and 2 of other ethnicity), between ages 18 and 49 years [mean (SD): 30.4 (9.4) years], with body mass index (BMI) of 25.3 (2.8) kg/m2, were included and received single oral doses of study medication (Table1). Escalating doses of tirasemtiv ranging from 30 mg to 1250 mg administered as a solid formulation were well tolerated. Because tirasemtiv exposure appeared to plateau without determination of the MTD, a liquid formulation was studied at doses ranging from 640 to 2500 mg in an effort to reach higher exposures. Forty-eight subjects completed 2 periods; the last cohort of 8 subjects only completed dose period 1, because the dose administered (2500 mg) was not tolerated. As dose escalated, AEs (Table S2, see Supplementary Material) of dizziness and euphoric mood increased in frequency and duration; however, at doses of ≤2000 mg, all AEs were characterized as mild in severity. At 2500 mg, moderately severe dizziness and an episode of syncope were reported. No serious AEs occurred. Thus, the MTD was determined to be 2000 mg. There were no clinically significant adverse effects on neurological examination, walk test, laboratory values, vital signs, ECG parameters, pulse oximetry, or cardiovascular telemetry. In terms of pharmacokinetics, tirasemtiv exhibited high systemic exposure and near-dose proportionality in terms of area under the curve (AUC), with relatively low intersubject variability (Table1, and Figs. S1 and S2 in Supplementary Material). The mean terminal t½ ranged from 9.9 to 14.8 hours across the cohorts.
Table 1.
Dosing scheme and pharmacokinetics parameters of tirasemtiv (Part A).
| Treatment periods* | 20 mg† | 40 mg† | 80 mg† | 160 mg† | 320 mg† | 640 mg† | 1000 mg† | 1250 mg† | 640 mg‡ | 1000 mg‡ | 1500 mg‡ | 2000 mg‡ | 2500 mg‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 | X | X | |||||||||||
| Cohort 2 | X | X | |||||||||||
| Cohort 3 | X | X | |||||||||||
| Cohort 4 | X | X | |||||||||||
| Cohort 5 | X | X | |||||||||||
| Cohort 6 | X | X | |||||||||||
| Cohort 7 | X | ||||||||||||
| Pharmacokinetics parameters (mean ± SEM) | |||||||||||||
| Cmax (µg/ml) | 0.55 ± 0.13 | 1.27 ± 0.22 | 1.40 ± 0.23 | 4.08 ± 0.79 | 7.16 ± 2.51 | 7.80 ± 1.54 | 14.1 ± 6.45 | 12.7 ± 1.12 | 14.0 ± 2.68 | 20.7 ± 1.50 | 20.5 ± 2.77 | 29.2 ± 3.79 | 24.6 ± 2.97 |
| AUC24h (h/µg/ml) | 6.26 ± 2.61 | 16.7 ± 2.88 | 20.2 ± 4.52 | 65.7 ± 11.9 | 114 ± 39.9 | 136 ± 24.2 | 237 ± 102 | 232 ± 12.9 | 196 ± 20.7 | 305 ± 29.5 | 309 ± 15.5 | 463 ± 60.1 | 420 ± 30.9 |
| tmax (h) | 4.50 ± 0.54 | 4.83 ± 0.41 | 5.66 ± 2.16 | 5.83 ± 2.04 | 4.50 ± 0.54 | 11.8 ± 6.40 | 12.8 ± 8.73 | 11.5 ± 1.11 | 4.83 ± 0.75 | 4.50 ± 1.51 | 5.33 ± 2.34 | 5.80 ± 2.38 | 6.67 ± 2.58 |
Eight subjects per cohort were assigned randomly to placebo (n = 2) or active (n = 6) treatments; 1 subject in cohort 6 withdrew consent and was replaced. All other subjects completed the indicated dosing period.
Doses were administered as solid in capsule with a matched placebo.
Doses were administered as a suspension in OraBlend with a matched placebo.
In Part B, the primary goal was to measure the response of the tibialis anterior muscle to nerve stimulation at 3 doses of tirasemtiv and placebo. Twelve healthy men (9 Caucasian, 2 black or African American, 1 Native American), between 21 and 41 years of age [30.4 (7.0) years] and with a BMI of 26.5 (2.7) kg/m2, were enrolled and completed the study.
An example of forces elicited with stimulus frequencies from 5 to 50 Hz and the resulting force–frequency relationship from a subject after placebo treatment is shown in Figure 1. Stimulation frequencies ranged from 5 Hz, where there was little or no summation of individual muscle twitches, up to 50 Hz, where the maximum force was observed. The force–frequency response was steepest around 10 Hz, which is close to the minimal discharge rate of single motor units in this muscle.8,11,12
FIGURE 1.
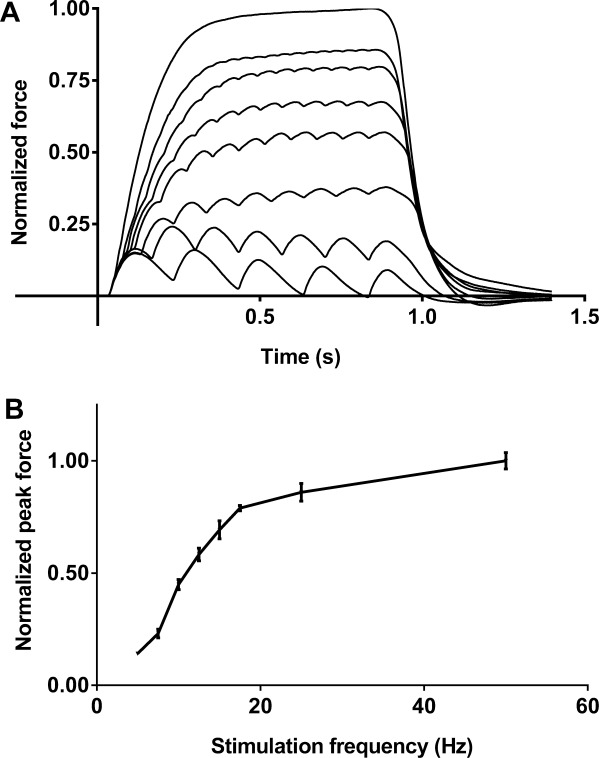
Example force profiles and force–frequency response. (A) One set (of 3) normalized force profiles from a stimulation protocol. The forces (lowest to highest) were elicited with 800-ms stimulus trains of 5, 7.5, 10, 12.5, 15, 17.5, 25, and 50 Hz. The forces were normalized to the peak force elicited with 50-Hz stimulation. (B) An example of the normalized force–frequency response at a single time-point (5 hours). Peak forces (mean ± SEM) from each of the 3 replicates at each stimulation frequency were normalized to the 50-Hz average peak force.
Tirasemtiv increased the placebo-corrected summed percent change from baseline of peak force (ΣF) in a dose-dependent manner (Fig. 2A). Statistically significant increases in force relative to pre-dose baseline values when compared with placebo occurred at doses of 500 and 1000 mg at all time-points and for all comparisons between 250 and 1000 mg (P < 0.05). Although comparisons of intermediate dose steps (i.e., 250–500 or 500–1000 mg) did not generally reach statistical significance, the overall dose dependence was strongly significant statistically at all time-points (P < 0.002). Forces increased between the 1- and 3-hour time-points commensurate with the rise in tirasemtiv plasma concentrations; the increases in force were maintained out to the last time-point measured at 7 hours, as were tirasemtiv plasma concentrations.
FIGURE 2.
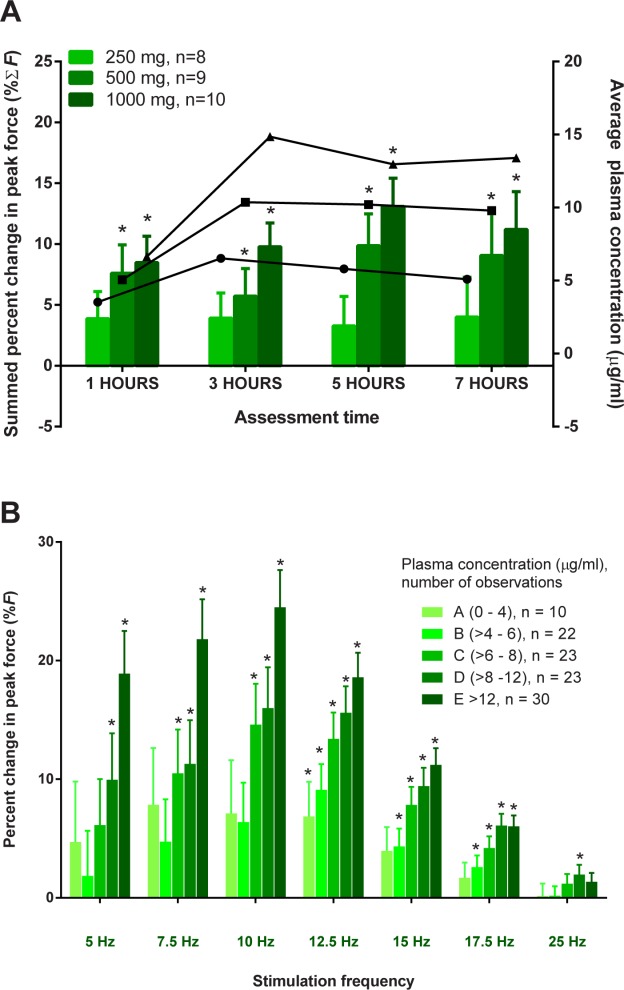
Tirasemtiv increases the response of muscle to neuromuscular activation in a dose- and concentration-related manner. (A) The placebo-subtracted summed mean percent change from baseline of peak force (%ΣF) is plotted in (A) (± SEM) from each assessment time-point for 250-, 500-, and 1000-mg doses. Asterisks denote statistical significance (P < 0.05) compared with placebo. The lines with circles, squares, and triangles are the average plasma concentrations for each population for the 250-, 500-, and 1000-mg doses, respectively. (B) The placebo-subtracted percent change in peak force (%F) is plotted. Mean ± SEM data for each tirasemtiv plasma level concentration bin are plotted for each stimulus frequency. Asterisks denote statistical significance (P < 0.05).
The concentration–response of tirasemtiv (Fig. 2B) was explored as a function of stimulus frequency. In this analysis, the placebo-corrected percent changes in peak force (%F) at each frequency were binned and averaged together by the coincident tirasemtiv plasma concentration obtained at the time of the measurement. These increases can be interpreted directly as the increase in force at each stimulus frequency. For example, the largest response corresponds to a 24.5% (3.1%) [least-squares mean (SE)] increase in peak force at 10 Hz in the highest compound plasma level bin of >12 μg/ml. Statistically significant increases in peak force were generally seen at plasma concentrations >6 μg/ml; the magnitude peaked at ∽10 Hz and then declined gradually as stimulation frequencies approached 25 Hz, when the muscle approached its maximum force. Pharmacokinetic parameters for each dose of tirasemtiv are listed in Table2.
Table 2.
Dosing scheme and pharmacokinetic parameters of tirasemtiv (Part B).
| Dose (mg) | Cmax (µg/ml) | C24h (µg/ml) | AUC24h (h/µg/ml) | tmax (h) |
|---|---|---|---|---|
| 250 | 7.11 ± 1.71 | 2.43 ± 1.26 | 98.7 ± 36.1 | 3.50 ± 0.91 |
| 500 | 10.6 ± 2.85 | 4.86 ± 2.22 | 170 ± 53.5 | 4.08 ± 2.07 |
| 1000 | 16.0 ± 4.14 | 9.54 ± 3.26 | 282 ± 83.6 | 5.25 ± 3.11 |
Data expressed as mean ± SEM.
Similar to Part A, treatment with single doses of tirasemtiv appeared well-tolerated in this group of healthy men (Table3). The majority of AEs were mild (46 of 47) in severity; 1 subject had moderately severe pharyngitis during the study. No serious AEs occurred. As seen in Part A, the most commonly reported AEs were headache, dizziness, somnolence, and euphoric mood. In Part B, no clinically significant changes or findings were noted from clinical laboratory evaluations, vital sign measurements, physical examinations, or 12-lead ECGs.
Table 3.
Reported adverse events (Part B).
| Distinct subjects [n (%)] | |||||
|---|---|---|---|---|---|
| Adverse event | Placebo (n = 12) | 250 mg (n = 12) | 500 mg (n = 12) | 1000 mg (n = 12) | Overall (n = 12) |
| Euphoric mood | 0 (0.0%) | 3 (25.0%) | 6 (50.0%) | 7 (58.3%) | 10 (83.3%) |
| Dizziness | 1 (8.3%) | 1 (8.3%) | 4 (33.3%) | 6 (50.0%) | 9 (75.0%) |
| Somnolence | 2 (16.7%) | 3 (25.0%) | 3 (25.0%) | 3 (25.0%) | 6 (50.0%) |
| Headache | 2 (16.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (16.7%) |
| Nausea | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 1 (8.3%) |
| Pharyngitis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 1 (8.3%) |
| Muscle spasms | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 1 (8.3%) |
| Rhinorrhea | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 0 (0.0%) | 1 (8.3%) |
| Contact dermatitis | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 1 (8.3%) | 1 (8.3%) |
DISCUSSION
This initial study in humans of the fast skeletal muscle troponin activator tirasemtiv was conducted in 2 parts. The first part established the maximum tolerated dose of tirasemtiv and characterized its safety, tolerability, and pharmacokinetics. In the second part, we explored the effect of tirasemtiv on the response of muscle to nerve activation. Here, tirasemtiv produced significant increases in force elicited in the tibialis anterior muscle by electrical stimulation of the deep fibular nerve, consistent with an amplification of the response of muscle to nerve input. Clear correlations with dose and plasma levels of tirasemtiv as well as stimulation frequency were found. The change in the force–frequency response was most pronounced around the typical discharge rate of motor units during physical activity and diminished at higher stimulus frequencies. These observations are consistent with the change in the force–frequency relationship produced by tirasemtiv preclinically in the rat.7 Overall, tirasemtiv was well tolerated at doses up to 2000 mg with a dose–limiting effect that is likely secondary to an off-target effect in the central nervous system given the constellation of symptoms observed. In this study we have provided clinical evidence for the translation into humans of a novel mechanism to directly improve skeletal muscle function, fast skeletal muscle troponin activation.
The magnitude of force increases should be considered in light of the selectivity of tirasemtiv for fast skeletal muscle. One study of autopsy samples from young men who died suddenly found type II fiber content in the deep tibialis anterior averaging 27.3% (95% CI 21.9–32.8).13 Given the selectivity of tirasemtiv for activation of fast skeletal muscle troponin,7 an increase in force of 25% at the 10-Hz stimulation frequency for plasma levels >12 mg/ml would imply that the force per type II fiber increased by substantially more than 25%, given the preponderance of type I fibers in this particular muscle.14
The therapeutic applications for this mechanism of action have potential significance for patients with neuromuscular diseases where deficits in muscle strength are attributable to diminished nerve input to the muscle. This study has demonstrated that tirasemtiv amplifies the response of muscle to nerve activation, albeit in the absence of voluntary control of muscle. The effects of tirasemtiv on voluntary muscle function have been examined in studies of tirasemtiv in patients with amyotrophic lateral sclerosis15 and myasthenia gravis,16 which have demonstrated potential improvements in muscle function after single doses of drug. Activation of the sarcomere via fast skeletal muscle troponin could offset the reduced neuromuscular activation seen in many disease conditions and improve muscle function in patients with neuromuscular diseases.
Acknowledgments
These findings were presented as a poster at the 40th annual meeting of the Society for Neuroscience, San Diego, California, November 2010.
Glossary
- AE
adverse event
- ALS
amyotrophic lateral sclerosis
- ANCOVA
analysis of covariance
- AUC
area under the plasma concentration–time curve
- AUC24
area under the plasma concentration–time curve from 0 to 24 hours
- BMI
body mass index
- CFR
Code of Federal Regulations
- Cmax
maximum plasma concentration
- CMT
Charcot–Marie–Tooth
- ECG
electrocardiogram
- EMG
electromyogram
- MedDRA
Medical Dictionary for Regulatory Activities
- MG
myasthenia gravis
- MTD
maximum tolerated dose
- NMJ
neuromuscular junction
- PK
pharmacokinetics
- SMA
spinal muscular atrophy
- SOC
system organ class
- t
assessment time-point
- t½
half-life
- tmax
time post-dose to reach Cmax
Supporting Information
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Sharma KR, Miller RG. Electrical and mechanical properties of skeletal muscle underlying increased fatigue in patients with amyotrophic lateral sclerosis. Muscle Nerve. 1996;19:1391–1400. doi: 10.1002/(SICI)1097-4598(199611)19:11<1391::AID-MUS3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Kent-Braun JA, Miller RG. Central fatigue during isometric exercise in amyotrophic lateral sclerosis. Muscle Nerve. 2000;23:909–914. doi: 10.1002/(sici)1097-4598(200006)23:6<909::aid-mus10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Patzkó A, Shy ME. Update on Charcot–Marie–Tooth disease. Curr Neurol Neurosci Rep. 2011;11:78–88. doi: 10.1007/s11910-010-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monani UR. Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron. 2005;48:885–896. doi: 10.1016/j.neuron.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 6.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116:2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, Driscoll L, et al. Activation of fast skeletal muscle troponin as a potential therapeutic approach for neuromuscular diseases. Nat Med. 2012;18:452–455. doi: 10.1038/nm.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jesunathadas M, Klass M, Duchateau J, Enoka RM. Discharge properties of motor units during steady isometric contractions performed with the dorsiflexor muscles. J Appl Physiol. 2012;112:1897–1905. doi: 10.1152/japplphysiol.01372.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindstrom JM, Engel AG, Seybold ME, Lennon VA, Lambert EH. Pathological mechanisms in experimental autoimmune myasthenia gravis in rats with anti-acetylcholine receptor antibodies. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine receptor antibodies. J Exp Med. 1976;144:739–753. doi: 10.1084/jem.144.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baudry S, Klass M, Duchateau J. Postactivation potentiation of short tetanic contractions is differently influenced by stimulation frequency in young and elderly adults. Eur J Appl Physiol. 2008;103:449–459. doi: 10.1007/s00421-008-0739-1. [DOI] [PubMed] [Google Scholar]
- 11.Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977;264:673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquet B, Carpentier A, Duchateau J. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. J Neurophysiol. 2005;94:3126–3133. doi: 10.1152/jn.00537.2005. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MA, Polgar J, Weightman D. Appleton D. Data on the distribution of fibre types in thirty-six human muscles: an autopsy study. J Neural Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 14.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force–velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shefner J, Cedarbaum JM, Cudkowicz ME, Maragakis N, Lee J, Jones D, et al. Safety, tolerability and pharmacodynamics of a skeletal muscle activator in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13:430–438. doi: 10.3109/17482968.2012.684214. [DOI] [PubMed] [Google Scholar]
- 16.Sanders DB, Rosenfeld J, Dimachkie M, Meng L, Malik FI. A study to evaluate efficacy, safety, and tolerability of single doses of tirasemtiv in patients with myasthenia gravis. Neurology. 2013;80:e203. doi: 10.1007/s13311-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.


