Abstract
The factor Xa inhibitor apixaban is one of the novel anticoagulants to emerge as alternatives to long-standing standards of care that include low-molecular-weight heparin and warfarin. The development of apixaban reflects a strategy to optimize the clinical pharmacology profile, dosing posology, trial designs, and statistical analyses across multiple indications, and to seek alignment with global health authorities. The primary objective of dose selection was to maintain balance between efficacy and bleeding risk. Twice-daily dosing of apixaban, rather than once daily, was chosen to lower peak concentrations and reduce fluctuations between peak and trough levels. Our discussion here focuses on the use of apixaban for stroke prevention in nonvalvular atrial fibrillation (NVAF). Supporting this indication, a pair of registrational trials was conducted that enrolled the full spectrum of patients who, by guidelines, were eligible for anticoagulation. In the AVERROES study of patients who were unsuitable for warfarin therapy, apixaban was superior to aspirin in reducing the risk of stroke or systemic embolism (SSE), without a significant increase in major bleeding (MB). In the ARISTOTLE (Apixaban for Reduction In STroke and Other ThromboemboLic Events in Atrial Fibrillation) study, apixaban was superior to warfarin on the rates of SSE, MB, and all-cause mortality. Overall, these studies have demonstrated a substantially favorable benefit–risk profile for apixaban over warfarin and aspirin in NVAF.
Keywords: anticoagulation, apixaban, factor Xa inhibition, atrial fibrillation, stroke prevention
Introduction
Apixaban was developed to address an unmet clinical need for a safe, effective, oral anticoagulant drug that would not require routine monitoring for its appropriate use. Before the recent introduction of new oral anticoagulants, vitamin K antagonists (VKAs) were the sole option for chronic oral anticoagulation for over 50 years. Although proven to be efficacious in a number of clinical indications, their use is associated with a high risk of bleeding. Of particular note, clinical trials with VKA treatment in patients with nonvalvular atrial fibrillation (NVAF) completed by the early 1990s demonstrated a 64% reduction in stroke, but at the cost of an increased risk of major hemorrhage.1
Patients with atrial fibrillation (AF) are frequently elderly and thus a challenging population. The risk of hemorrhage increases with age, especially for gastrointestinal bleeding and intracranial hemorrhage. In addition, warfarin and other VKAs have many drug, food, and botanical interactions that are problematic for older patients who are frequently on other medications (often many) for comorbid conditions. Finally, the need for frequent therapeutic monitoring and dose adjustment, as well as interruptions of therapy at the time of procedures, undermines treatment of AF, which must be lifelong in these patients. As a result, by the year 2000, studies indicated that only about half of AF patients at risk for stroke were treated with warfarin (or another VKA).2,3 Thus, there was a clear unmet need for a new oral anticoagulant that would preserve the efficacy benefits of VKAs but without the majority of their liabilities.
Preclinical development of apixaban
Thrombosis and hemostasis are closely related processes. The goal of antithrombotic therapy is to provide the maximal protection from thrombosis that maintains an acceptable level of risk of bleeding. Efforts to discover and develop new antithrombotic agents began with the recognition of characteristics that would improve upon the benefit–risk profile of VKAs. Among these characteristics are pharmacokinetic properties that minimize intra- and interindividual variability, rapid onset and offset of action, a lack of significant interactions with food or other drugs, good oral bioavailability, and a wide therapeutic index. An agent with these properties could be given in fixed doses, eliminating the need for monitoring and dose-titration that are necessary for VKAs.
New oral anticoagulants target competitive inhibition of single enzymes in the coagulation cascade, leading to anticoagulant effects directly related to their concentration. Efforts have mostly focused on enzymes in the final common pathway, that is, factor Xa (FXa) and thrombin.
There is theoretical and preclinical evidence suggesting that inhibition of FXa and its resultant reduction of thrombin generation may have advantages over the direct inhibition of thrombin. Blood coagulation proceeds by a series of sequential, or cascading, reactions, in which it has been estimated that generation of one molecule of FXa results in the production of hundreds of thrombin molecules. More importantly, inhibition of FXa does not interfere with the activity of residual amounts of thrombin that may contribute to maintenance of hemostasis via activation of the high-affinity platelet thrombin receptor. Empirical evidence of a wider therapeutic index for FXa inhibitors over thrombin inhibitors was obtained in several laboratories.4–8 Thus, the program to develop alternatives to warfarin that ultimately led to the development of apixaban was focused on the discovery of direct, selective inhibitors of FXa.
At least as important as the choice of target was the optimization of several compound-specific parameters. A high degree of selectivity for FXa over related enzymes that play important roles in coagulation, fibrinolysis, digestion, and inflammatory responses was a requirement in order to avoid off-target effects that could result in toxicities or adverse events. Low-to-moderate binding to plasma proteins was desired, as this can lead to high intersubject variability and drug–drug interactions. Optimization of pharmacokinetic parameters was a major focus. A high degree of oral bioavailability was preferred because low bioavailability is generally associated with more variable exposures. Efforts targeted a half-life suitable for once- (qd) or twice-daily (bid) dosing, without large differences in peak and trough concentrations. Consideration was also given to identifying a compound with a low intrinsic rate of metabolism; not only would this contribute to a reduced propensity for drug–drug interactions, but it would also enable a suitable half-life without the need for a large volume of distribution. Because a direct FXa inhibitor would have its intended action solely within the blood, a large volume of distribution would only lead to concentrations of the compound in compartments where it was not having its intended action, but could have adverse effects.
The optimization of these characteristics and the initial discovery of apixaban have been summarized elsewhere.9,10 Briefly, efforts beginning at DuPont Pharmaceuticals in 1995 and continuing at Bristol-Myers Squibb following its acquisition of DuPont Pharmaceuticals in 2001 led to the identification of a series of highly potent and selective inhibitors of FXa. Five compounds were advanced to clinical studies, and one compound, previous to apixaban, was evaluated through phase II.11 Since 2007, development of apixaban has been conducted in alliance with Pfizer.
Apixaban was characterized in vitro and in animal models of thrombosis and bleeding.12,13 It was shown to be effective in the prevention of experimental thrombosis at doses that had minimal effect on models of provoked bleeding. In experimental animals, the pharmacokinetic properties of apixaban were found to be consistent with the desired profile described earlier, with good oral bioavailability, small volume of distribution, and low clearance. Pharmacodynamic activity was closely related to concentration.14 Accordingly, apixaban was advanced to clinical studies.
Clinical pharmacology and dose selection for apixaban
Phase I studies of apixaban characterized important parameters of its clinical pharmacology and potential for pharmacokinetic interactions with other drugs (Table1). This profile included no effect of food or gastric pH on absorption, a 12-h half-life, multiple routes of elimination or metabolism, limited renal elimination, and minimal drug–drug interactions, which is well suited especially for elderly patients in need of anticoagulation. Apixaban's favorable profile also made it potentially useful in a wide variety of thromboembolic disorders, including the prevention and treatment of venous thromboembolism (VTE), prevention of recurrent arterial thrombosis, and stroke prevention in AF—the focus of our discussion.
Table 1.
Pharmacologic profile of apixaban derived from phase I studies of pharmacokinetics, patient characteristics, and drug interactions
| Pharmacokinetic characteristics | |
| Absorption |
|
| Distribution | Volume of distribution ∼21 L |
| Metabolism |
|
| Elimination |
|
| Patient characteristics | |
| Age | Exposure ↑32% in subjects 65–79 years versus subjects 18–40 years |
| Sex | No significant effect |
| Race | No significant effect |
| Body weight | Compared with subjects weighing 65–85 kg: for weight >120 kg, exposure ↓∼30%; for weight <50 kg, exposure ↑∼30% |
| Renal impairment |
|
| Mild-to-moderate hepatic impairment | No significant effect |
| Drug interactions | |
| Food | No significant effect |
| Strong dual CYP3A4 and P-gp inhibitors | Increase apixaban exposure by ∼2-fold |
| Less potent inhibitors CYP3A4 or P-gp inhibitors | Increase apixaban exposure by ∼50% |
| Strong dual CYP3A4 or P-gp inducers | Decrease apixaban exposure by ∼50% |
| Other agents | Activated charcoal can ↓exposure 27–50% when given within 6 h of apixaban ingestion |
It was not practical to conduct dose-ranging phase II studies for apixaban in all indications. For stroke prevention and risk reduction in AF, for example, event rates are so low that the ability to discriminate between doses would only be possible with studies that are the same size as a typical phase III trial. Dose selection in each of the phase III apixaban trials was intended to optimize the benefit–risk profile for the target patient population. To inform dosing in subsequent trials, a dose-ranging eight-arm phase II study was conducted in patients undergoing elective knee replacement surgery, including three qd apixaban arms, three bid apixaban arms, and two comparator arms: enoxaparin 30 mg subcutaneous bid and warfarin (international normalized ratio (INR) 2–3).15 In a surgical setting, (1) the efficacy end point, a composite of deep vein thrombosis (DVT), pulmonary embolism, or related death, occurs in orthopedic surgery patients with substantially greater frequency than stroke in AF patients, and (2) the frequency of clinically important bleeding is sufficiently high to assess dose dependence. In all six apixaban arms, the efficacy event rate was lower than both comparator arms; and in the 2.5 mg bid and 5 mg qd apixaban arms, the bleeding rate was lower than in either of the comparator arms. For each of three total daily doses of apixaban (5, 10, and 20 mg), the bid dose had a lower efficacy event rate than did the qd dose (Fig.1).15 This observation is supported by an additional exposure–response analysis (Fig. S1) that showed greater avoidance of thrombotic or bleeding events with bid dosing compared with qd dosing.16 On the basis of these studies, an apixaban dose of 2.5 mg bid was selected for phase III studies for the prevention of VTE following elective hip or knee replacement surgery.17–19
Figure 1.
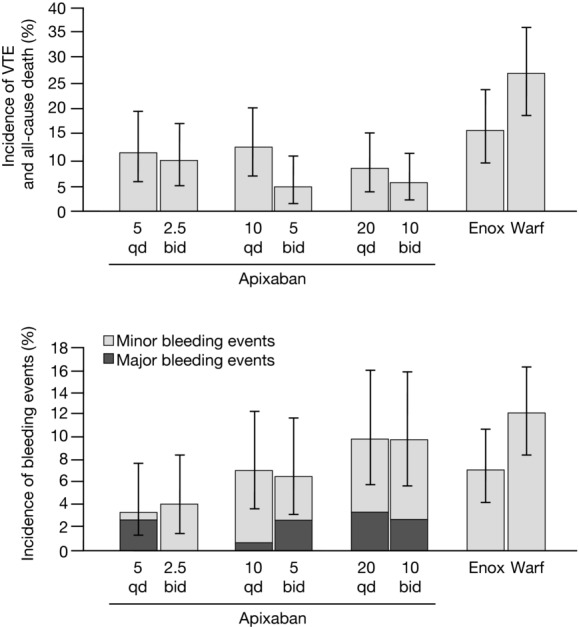
Efficacy and bleeding outcomes in the phase II dose-ranging trial of apixaban for prevention of VTE following elective knee replacement surgery (APROPOS trial). Three total daily dose levels of apixaban were tested (5, 10, 20 mg), administered either qd (5 mg qd, 10 mg qd, 20 mg qd) or in divided doses bid (2.5 mg bid, 5 mg bid, 10 mg bid). Reflecting clinical practice patterns in the United States, two comparators were tested: enoxaparin 30 mg bid (subcutaneous administration) and warfarin (target INR range of 2–3). The phase III dose studied in trials for prevention of VTE was 2.5 mg bid, and the phase III dose studied in trials for stroke prevention in AF was 5 mg bid. Modified, with permission, from Lassen et al.15
Although recognition of postsurgical bleeding risk favored the lowest effective dose of apixaban in an orthopedic population, recognition of the dire consequences of stroke favored a higher dose in an AF population. Important safety information on apixaban at daily doses of 10 or 20 mg, and for a longer treatment duration than had been examined in VTE prevention, was obtained in a phase II study in the treatment of proximal DVT for 3 months.20 The composite of major bleeding (MB) and clinically relevant nonmajor (CRNM) bleeding occurred with similar frequency in all apixaban groups compared with low-molecular-weight heparin/VKA-treated patients.
Apixaban bid dosing was selected for phase III studies across all indications with the expectation that a lower peak–trough ratio of blood levels (i.e., lower peaks and higher troughs), compared with qd, would enable the most favorable composite of stroke prevention and bleeding. Further support for bid dosing came from a phase II trial of dabigatran in which 150 mg bid had a substantially better net clinical outcome compared with 300 mg qd.41 With these considerations, a dose of 5 mg bid was selected as the main dose for pivotal trials in AF.
As it was unlikely that a single apixaban dose would be optimal for all patients with AF, several dose-adjustment schemas were considered. Because renal elimination accounts for only 27% of apixaban elimination, dosage adjustment based solely on renal function was not appropriate. No single factor from phase I studies, such as advanced age, low body weight, or impaired renal function, produced an increase in apixaban concentration greater than 44%, and therefore adjustment of dose based on any one of these factors was not needed. However, if more than one of the factors were present, and considering each factor has been associated with higher bleeding risks in other anticoagulation settings, apixaban concentrations following 5 mg bid may have increased the bleeding risk. Therefore, an empiric formula was derived (and subsequently supported by pharmacokinetic modeling); if at least two of the following three characteristics are present, the apixaban dose should be lowered to 2.5 mg bid: age ≥80 years, body weight ≤60 kg, and serum creatinine ≥1.5 mg/dL (or 133 μmol/L). This dosing algorithm was employed in the phase III AF trials.
Clinical significance of AF
AF is the most common type of cardiac arrhythmia, accounting for approximately one-third of the hospitalizations attributed to cardiac rhythm disturbances. An estimated 2.6 million people in North America have AF21 and prevalence increases with age.22,23 It is estimated that 3.8% of the population in the United States ≥60 years and 9.0% of the population ≥80 years have AF. As the U.S. population ages, the incidence of AF is projected to increase sharply.
Although AF may cause symptoms of palpitations and light headedness, and while it may impair exercise performance or result in or exacerbate heart failure, it is the strong association of AF with stroke that is a major source of both mortality and major morbidity. The risk of stroke is increased ∼5-fold in patients with AF.24 The attributable risk of stroke with AF increases from 2.8% at ages 60–69 to 9.9% at ages 70–79 and to 23.5% at ages 80–89 years.25 One of every six strokes is the result of AF, and these strokes are more severe than strokes not associated with AF.25 Strokes in patients with AF (or cardioembolic strokes) are associated with a 70% increase in mortality, a 20% increase in the length of hospital stay, and a 40% decrease in the rate of return to home because of more severe functional impairment.26 In addition, the outcome of cardioembolic strokes is poor, with a mortality rate of 25% at 30 days and 50% at 1 year.22 The assessment of the benefit–risk profile of VKA therapy is challenging, as those individuals at greatest risk for stroke will also have a substantial risk for bleeding, whereas those for whom warfarin may be used more safely may benefit less from treatment.
For warfarin to be effective, it must be routinely monitored by means of the INR and maintained in a narrow therapeutic range (2–3) that many patients cannot consistently achieve. Although clinical metrics for the quality of warfarin management have not been established, recent guidelines from the European Society of Cardiology consider warfarin to be well controlled when the time in therapeutic range (TTR) is >70%.27 These challenges, along with several others (e.g., multiple food and drug interactions and unpredictable dosing), make long-term adherence to warfarin therapy problematic, even in high-risk patients with a prior stroke for whom the need for therapy is greatest. For example, Glader et al. reviewed data from the Riks-Stroke, the Swedish Stroke Registry, a national registry covering 80–90% of all stroke events that occurred in Sweden over a period of 1 year;28 of patients found to be in AF at the time of their index event, 89.1% were receiving warfarin during the period 1–4 months after discharge, but by 24 months only 45.0% of survivors were still receiving VKA therapy. Thus, even in those identified with high risk (secondary stroke prevention patients) and managed in a country with an exemplary national anticoagulation system, most patients were unable to remain on warfarin therapy for even 2 years.
Clinical development for NVAF
The clinical development program in NVAF was designed to examine the efficacy and safety of apixaban for the prevention of stroke in this largely elderly patient population. As noted earlier, the rationale for developing an alternative to warfarin in AF patients lay not in concerns over its efficacy, but rather with the important liabilities of warfarin that limit its long-term use, thus creating a large unmet need. A new drug with similar efficacy, but better safety characteristics, tolerability or convenience, would be an advance in addressing this clinical need. These considerations led to the adoption of a noninferiority approach in designing the registrational trial of apixaban versus warfarin. Noninferiority trials compare an investigational therapy with an established one and seek to determine that the former preserves a prespecified proportion of the efficacy of the latter.
The ARISTOTLE trial (Apixaban for Reduction In STroke and Other ThromboemboLic Events in Atrial Fibrillation)29 sought to preserve at least 50% of the benefit of warfarin treatment on the basis of a meta-analysis of six previous warfarin–placebo trials conducted in NVAF patients.30 To do this, the upper limit of the two-sided confidence interval (CI) for the relative risk of apixaban versus warfarin would have to be less than 1.44: termed the noninferiority margin. An event-driven approach was adopted to demonstrate a margin of 1.44 or less at 90% power, requiring that 448 primary efficacy events would have to be collected and adjudicated.
The primary efficacy outcome was the combination of stroke and systemic embolism. Stroke was defined as the onset of a new, nontraumatic focal neurological deficit lasting at least 24 hours. Systemic embolism was judged to occur where there was a clinical history consistent with an acute loss of blood flow to a peripheral artery (or arteries), which was supported by evidence of embolism from surgical specimens, autopsy, angiography, vascular imaging, or other objective testing.29 The primary safety outcome in most AF anticoagulation trials is MB as defined by the International Society of Thrombosis and Hemostasis (ISTH; Table S1). This is a definition that is more conservative than the Thrombolysis in Myocardial Infarction (TIMI) or Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) definitions, and thus more appropriate for assessing bleeding over the long term in elderly patients.29 In addition to ISTH MB, CRNM bleeding events (i.e., nonmajor bleeding that required hospital admission, physician-guided medical or surgical treatment, or that resulted in a change in antithrombotic therapy including prolonged interruption of study drug) were collected, as were TIMI major and GUSTO severe bleeding events. This permitted the trial to compare the risks of apixaban to warfarin at different levels of bleeding severity.29 Another critical element of the trial design is the selection of the patient population. For ARISTOTLE, a global population of warfarin-eligible AF patients would be enrolled, and this included those previously treated with VKA and those naive to such therapy. Moreover, patients with all degrees of CHADS2 risk would be included to assess the benefit–risk profile of apixaban compared with warfarin across a broad patient population.29 Finally, consideration was given to the statistical approaches of interpreting the trial. Clinically important subgroups were identified and prespecified for analysis, including the elderly, those with different CHADS2 risk factors, those with renal insufficiency, and by sex, region, apixaban dose (5.0 mg bid or 2.5 mg bid), and previous VKA use (experienced versus naive). Analysis of these subgroups was conducted for both the primary efficacy and safety outcomes. Finally, a hierarchical, closed testing sequence was prespecified before any interim analyses. In this schema, noninferiority of apixaban to warfarin on stroke or systemic embolism (SSE) would first be tested; if this was satisfied, then superiority on this outcome would be tested; if this was satisfied, superiority of apixaban on ISTH MB would be tested; finally, if this was satisfied, superiority on the outcome of all-cause death would be tested.29 This rigorous procedure was designed to conserve the overall type 1 error and, if successful, to yield robust findings on important clinical outcomes.
The ARISTOTLE trial was a rigorously designed, double-blind global trial that tested whether apixaban was safe and effective for the prevention of stroke in AF patients.29 Nevertheless, the trial would leave several important questions unanswered. First, is apixaban safe and effective in patients who are unsuitable for VKA therapy, and thus not studied in ARISTOTLE? Second, is a single trial sufficient for registration, or should it be replicated? Third, is a noninferiority result sufficient or would a trial yielding superiority of apixaban over its comparator be useful in further defining the benefit–risk profile of apixaban in stroke prevention in AF?
To address these concerns, the sponsors undertook a second trial in warfarin-unsuitable patients. The AVERROES trial (apixaban versus acetylsalicylic acid (ASA) to prevent stroke in AF patients who have failed or are unsuitable for VKA treatment) was performed in patients who were either demonstrated to be, or expected to be, unsuitable for VKA therapy.31 The study was double-blind and used aspirin (81–324 mg, dose chosen by investigator at the time of randomization) as a comparator. The dose of apixaban was either 5 mg or 2.5 mg bid, employing the same dose reduction algorithm as in ARISTOTLE. Similarly, patients had to have documented AF and CHADS2 risk factors. AVERROES was designed as a superiority study; in addition, it would replicate the findings of ARISTOTLE, provide additional insight into the benefit–risk profile in the broadest possible AF patient population (i.e., those who were unsuitable for VKA, as well as those able to be treated with warfarin), and provide a superiority finding to reinforce a noninferiority outcome from ARISTOTLE. Importantly, the trial was carefully designed to include planned interim analyses, looking not only at safety but also for superiority of apixaban over aspirin, assuming that apixaban had a treatment effect similar to warfarin.31
Trial results
AVERROES
AVERROES randomized 5598 patients, completing its enrollment in December 2009. After 6 months, in May 2010, the Data Monitoring Committee recommended stopping the trial owing to a treatment benefit of apixaban that exceeded the prespecified modified Haybittle–Peto boundary by four standard deviations.31
There were 51 primary outcome events (1.6% per year) in the apixaban arm and 113 (3.7% per year) in those assigned to aspirin (hazard ratio (HR) 0.45; 95% CI 0.32–0.62; P < 0.001). The rates of death were 3.5% per year in the apixaban group and 4.4% per year in the aspirin group (HR 0.79; 95% CI 0.62–1.02; P = 0.07). Importantly, there were 45 cases of MB (1.4% per year) in the apixaban group and 29 (0.9% per year) in the aspirin group (HR 1.54; 95% CI 0.96–2.45; P = 0.07); there were five cases of fatal bleeding and 11 cases of intracranial bleeding in each treatment arm.31
The efficacy findings confirmed that apixaban had a much more potent treatment effect than aspirin in preventing stroke, as might be anticipated from a drug designed to replace warfarin. In addition, apixaban exhibited a bleeding profile statistically similar to an aspirin arm in which 91% were assigned a dose of 162 mg/day or less, that is, much less bleeding on apixaban than one might expect from a VKA relative to aspirin.31
ARISTOTLE
The ARISTOTLE study achieved database lock in June 2011 after randomizing 18,201 patients. The mean CHADS2 score was 2.1, the mean age 70 years, and 43% of patients were warfarin naive. Study drug discontinuation was significantly less frequent in apixaban-treated than warfarin-treated patients (25.3% vs. 27.5%; P = 0.001) over a mean exposure of 1.7 years. Apixaban achieved superiority on SSE compared with warfarin (1.27% vs. 1.60% per year; HR 0.79; 95% CI 0.66–0.95; P = 0.01). The Kaplan–Meier plots of the primary efficacy outcome (Fig.2) show early and continued separation of the event curves, and correspond to a 21% relative risk reduction in SSE. The fact that the upper bound of the 95% CI was below both the prespecified noninferiority margin and less than 1 meant that the first two steps of the hierarchical testing sequence were satisfied in the intent-to-treat population: apixaban was not only noninferior, it was also superior to warfarin in the prevention of SSE.29
Figure 2.
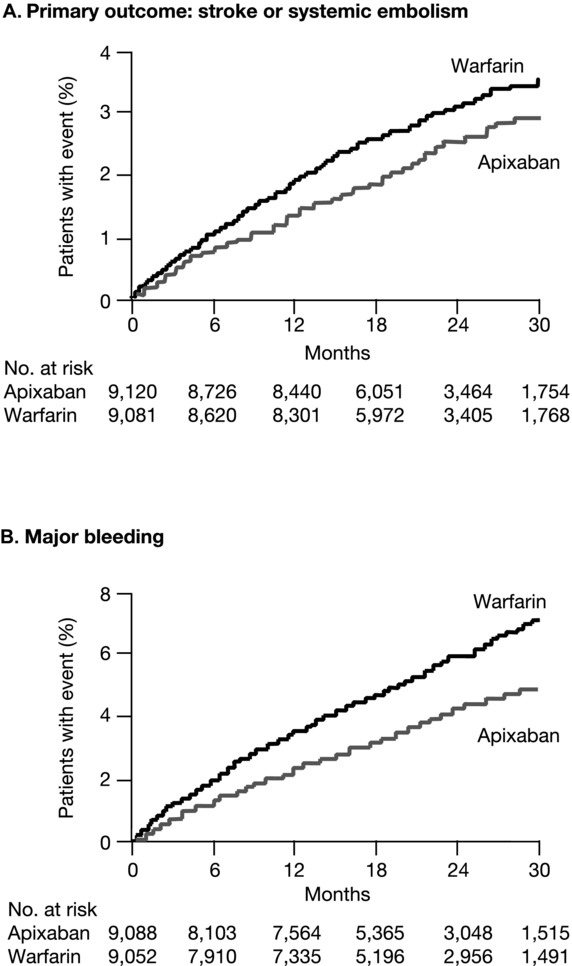
Kaplan–Meier curves for the primary efficacy and safety outcomes in the phase III warfarin-controlled trial of apixaban for stroke prevention in atrial prevention (ARISTOTLE trial). The primary efficacy outcome (Panel A) was stroke or systemic embolism. The primary safety outcome (Panel B) was major bleeding, as defined according to the criteria of the ISTH. Modified, with permission, from Granger et al.29
With regard to ISTH MB, the primary safety end point and the third step in the testing sequence, apixaban resulted in a 31% reduction in such bleeding compared with warfarin (2.13% vs. 3.09% per year; HR 0.69; 95% CI 0.60–0.80; P < 0.001). Again, the Kaplan–Meier plots of this outcome show an early and continued separation over time. The most feared bleeding complication—intracranial hemorrhage—was significantly reduced in apixaban-treated patients (0.33% vs. 0.80% per year; HR 0.42; 95% CI 0.30–0.58; P < 0.001). Gastrointestinal hemorrhage was numerically less frequent with apixaban than with warfarin (0.76% vs. 0.86% per year). The safety benefit of apixaban over warfarin was clear; there was a consistent, substantial reduction in bleeding that was more marked as the severity of the bleeding definition increased (Table2) from ISTH MB to TIMI MB and to GUSTO severe bleeding.29
Table 2.
Bleeding outcomes from the ARISTOTLE study for different bleeding definitions
| Bleeding outcomes in ARISTOTLE | |||||
|---|---|---|---|---|---|
| Type of | Apixaban event | Warfarin event | HR (apixaban/) | ||
| bleeding | rate (% per year) | rate (% per year) | (warfarin) | 95% CI | P value |
| ISTH major | 2.13 | 3.09 | 0.69 | (0.60–0.80) | <0.0001 |
| ISTH major/CRNM | 4.07 | 6.01 | 0.68 | (0.61–0.75) | <0.0001 |
| TIMI major | 0.96 | 1.69 | 0.57 | (0.46–0.70) | <0.0001 |
| GUSTO severe | 0.52 | 1.13 | 0.46 | (0.35–0.60) | <0.0001 |
| Intracranial | 0.33 | 0.80 | 0.42 | (0.30–0.58) | <0.0001 |
| Fatal | 0.06 | 0.24 | 0.27 | (0.13–0.53) | 0.0002 |
Note that as the severity of the bleeding outcome increases, the hazard ratio of apixaban to warfarin decreases, indicating a favorable trend for safety.29
All-cause mortality was the fourth and final step in the testing sequence. Once again apixaban proved superior to warfarin (3.52% vs. 3.94% per year; HR 0.89; 95% CI 0.80–0.998; P = 0.047).29 Although a 11% reduction in mortality might appear modest, it is important to recognize that warfarin itself has an established mortality benefit compared with placebo, so the reduction seen with apixaban is in addition to the effect of warfarin. In an imputed placebo analysis for death with the ARISTOTLE data using a meta-analysis of the historical warfarin trials,1 apixaban was associated with a 34% reduction in mortality compared with placebo (HR 0.66; 95% CI 0.50–0.88; P = 0.004).32 A similar analysis performed using the AVERROES data and a previous aspirin meta-analysis1 yielded a reduction in mortality of 33% (HR 0.67; 95% CI 0.48–0.94; P = 0.02). The two trials replicate the finding and show a consistent and marked effect.
Given the superiority findings noted above—on SSE, MB, and mortality—one might well ask if there are any groups that did not benefit from apixaban. The forest plots of the prespecified subgroup analyses appear in Figures S2 and S3.29,31 With respect to efficacy, the pattern of treatment effect across these major subgroups—age, sex, weight, risk factors, renal function, and region—all tend to favor apixaban, and do not reveal evidence of a treatment interaction. Only 1 of the 31 groups (age <65 years) had a point estimate favoring warfarin, and the few events and wide CIs suggest that this is likely a chance finding. A review of these same subgroups with respect to ISTH MB also shows a pattern favoring apixaban, with many CIs that exclude unity; in none of these subgroups do the point estimates favor warfarin. These findings are especially important for vulnerable patients; for example, for patients ≥75 years, there was a 29% reduction in SSE with apixaban compared with warfarin (HR 0.71; 95% CI 0.53–0.95); and there was a 35% reduction in ISTH MB (HR 0.65; 95% CI 0.55–0.76). Thus, in the elderly—a group that defines the greatest unmet need for therapy—apixaban demonstrated benefit over warfarin for both SSE and MB with effect sizes that were greater than in the overall population. A similar case could be made for the high-risk patient groups (CHADS2 score ≥3): there was a substantial reduction in both SSE (HR 0.70; 95% CI 0.54–0.91) and ISTH MB (HR 0.70; 95% CI 0.56–0.88). That the effect sizes are similar or larger than in the overall population is important given that high-risk patients not only suffer the greatest risk of stroke but they are also most likely to bleed, and thus to have their antithrombotic therapy interrupted or discontinued, which increases their vulnerability to stroke.
Another group to consider that might benefit from warfarin instead of apixaban is composed of patients who have done well on warfarin previously or who have achieved very good INR control while on warfarin. A review of the forest plots (Fig. S2) reveals that warfarin-experienced patients did significantly better on apixaban than warfarin for both efficacy (HR 0.73; 95% CI 0.57–0.95) and MB (HR 0.66; 95% CI 0.55–0.80).29 Good INR control is essential in an active comparator trial utilizing warfarin for a fair comparison to be made. In ARISTOTLE, the median TTR using the method of Rosendaal was 66%,29 which is better than that attained in clinical practice in North America and many other regions of the world.33–36
One way to assess the effect of INR control on outcomes is to analyze the results based upon the INRs achieved in the warfarin-treated patients at each site. Since the apixaban-treated patients cannot use INRs for comparison, a site (center)-based method was employed. Each center's average TTR (cTTR) was estimated with the use of a linear mixed model for TTR in warfarin-treated patients, including a fixed effect for country and random effect for center.37 Subjects were weighted according to the number of INR values that contributed to their TTR to reflect the greater variability that is likely to occur for patients (and sites) with fewer measurements. The results were divided into quartiles containing nearly equal numbers of patients, and a comparison of the effects of INR control (worst quartile to best quartile) on results was made. A forest plot of these findings is included in Figure S4, which shows that the benefit of apixaban on safety and efficacy across the quartiles is preserved, even when INR control is exceptionally good, including the quartile with the best INR control (TTR >71%).37
Another way to analyze this relationship is as a continuous curve rather than in quartiles. The HR and 95% CI can be plotted for key outcomes across a range of INR control by analyzing the results of subjects from sites where TTR exceeds the specified cutoff. The curves for both SSE and ISTH MB appear in Figure3.37 In both cases, the estimate for the HR and 95% CI were favorable for apixaban, with stability across a wide range of TTRs up to about 80% TTR. Above that value, the very few numbers of events and small numbers of patients in this group (only 9.3% of treated patients achieved a TTR of ≥80%) cause the analysis to break down. These findings provide further evidence that even across a wide range of very good INR control, apixaban is superior to warfarin.
Figure 3.
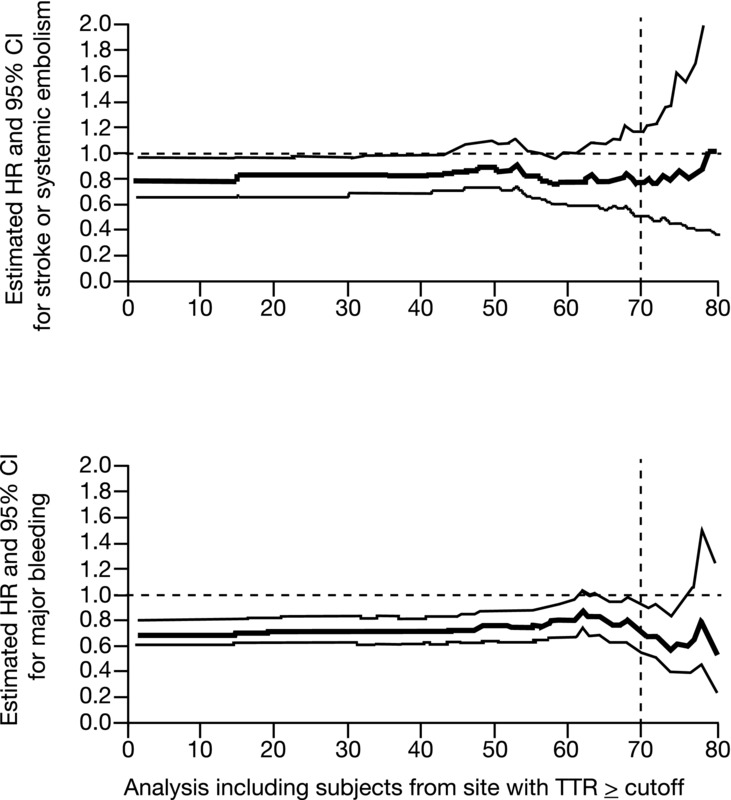
Relative efficacy and safety of apixaban compared with warfarin as a function of center-based time-in-therapeutic range (TTR) in the phase III trial for stroke prevention in AF (ARISTOTLE trial). As previously described, a center-based TTR was calculated for each investigative site in the trial and comparisons of outcomes on apixaban and warfarin were made at each site.37 For each level of center-based TTR from 1% to 80% on the y-axis, an HR for apixaban versus warfarin was calculated from all sites with a TTR greater than or equal to each value on the y-axis. The bolded curves are the HRs for the efficacy and safety outcomes on apixaban versus warfarin at each level of TTR; also shown are the 95% CIs. The estimated HR remains below unity for both efficacy and bleeding; this suggests a persistent benefit of apixaban over warfarin over a wide range of TTR values. The dashed vertical line at a TTR = 70% indicates the site level mean TTR above which European Society of Cardiology guidelines27,37 consider warfarin to be well controlled. Overall in ARISTOTLE, 75% of sites had a TTR >60%, 50% of sites had a TTR ≥66%, and 25% of sites had a TTR ≥71%. At sites in the United States, the overall TTR was 72%.37
Regulatory strategies to enable global registration
A key aspect of the regulatory strategy for the development of apixaban was consistent engagement with global health authorities (HAs), particularly in North America, Europe, and Japan, to facilitate a streamlined global development program, regulatory review, and approval. In early discussions with the U.S. Food and Drug Administration (FDA) and European Medicines Agency, alignment was achieved to waive a phase II AF for apixaban study on the basis of the rationale that such a study would not be meaningful owing to low event rates. Before finalization of the phase III AVERROES and ARISTOTLE protocols, global HA interactions were undertaken to align on program adequacy and rigor to meet evidentiary standards and key labeling objectives. Strategies for expediting global submissions and review were identified, enabling simultaneous approvals in the United States, European Union (EU), Canada, and Japan within a period of 6 weeks. Several other global HA approvals followed shortly thereafter.
Summary and conclusions
One difference between VKAs and apixaban was that the original VKA was discovered, whereas apixaban was developed. In the case of apixaban, factor X was targeted because of its key position in the clotting cascade. Thousands of potential compounds were synthesized and then further modified to enhance key properties of the molecule. Of the several promising agents refined by this process, apixaban had the best overall profile. Extensive pharmacokinetic and pharmacodynamic testing was performed, first in healthy human volunteers (phase I) and then in patients (phase II) to determine the optimal dose and dosing interval. A key principle in this stage of development was the need to individualize dosing to reflect the risk–benefit profile of a particular indication and patient population; one size was not assumed to fit all. Dosing twice daily appeared to confer benefits on both efficacy and safety (bleeding) across the range of indications, perhaps by minimizing the peak to trough levels. In addition, a reduced dose algorithm was developed for vulnerable patients with the aim of matching exposure between this group and the general study population.
How did these early development decisions play out in the later (phase III) development in NVAF? The AF program was very large (23,800 patients), with two registrational trials to test the drug and assess its risk–benefit profile in the broadest possible population (CHADS2 risks of 1–6; both VKA suitable and VKA unsuitable). The trials were double-blind, with hard end points relevant to the disease (including stroke, systemic embolism, MB, and death) and prespecified statistical analysis plans. Both trials yielded superiority in efficacy outcomes. In AVERROES, apixaban was superior to aspirin for the prevention of SSE, with a bleeding rate that was numerically, but not significantly, higher.31 In ARISTOTLE, apixaban was superior to warfarin for the prevention of SSE risk reduction, caused significantly fewer MB events than warfarin, and conferred a mortality benefit over warfarin.29 In both trials, the overall results were preserved across important subgroups: age, sex, weight, renal function, degree and type of risk factors, and geographic region. In both trials, other than bleeding, no significant safety issues were uncovered.
For more than 50 years, warfarin and VKAs have been the only therapeutic options for chronic (oral) anticoagulation. In the past 5 years, a number of new agents have been developed, tested, and approved for this purpose. These development programs took place contemporaneously, were conducted in similar (although not always identical) populations, and used the same outcomes (e.g., SSE for efficacy, ISTH MB for safety). Although there were other differences in the trials (e.g., most, but not all, were double-blind; most used VKA as a comparator, AVERROES used aspirin), the similarities invite a brief comparison. Although one should avoid making cross-trial comparisons in the absence of head-to-head data, one might ask how the new agents compare with warfarin, the standard. Figure4 shows such a comparison, for stroke prevention in AF with the new agents compared to VKA.29,38,39 Each drug (or dose of a drug) is represented with the results for both efficacy and safety (HR and 95% CI).
Figure 4.
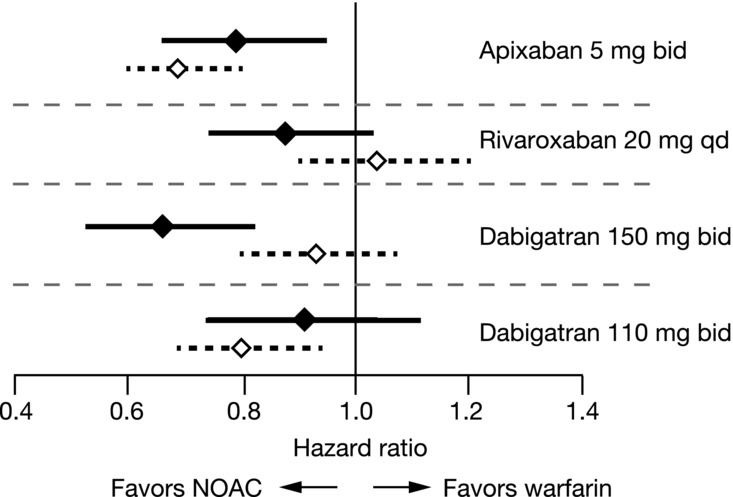
Relative efficacy and safety of novel oral anticoagulants in AF. HRs and 95% CIs are shown for the primary efficacy end point of stroke or systemic embolism (solid symbols) and for ISTH major bleeding (open symbols) in the phase III warfarin-controlled registrational trials for apixaban, rivaroxaban, and dabigatran.29,38,39
Dabigatran (Pradaxa®, Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT), a factor II (thrombin) inhibitor, was studied at two doses in the RE-LY trial (150 mg bid and 110 mg bid).39 The study was open label and included patients of all different risks (CHADS2 score 1–6). The 150-mg bid dose of dabigatran achieved superiority over VKA for SSE, but not for bleeding. Bleeding at this dose of dabigatran increased with age, and exceeded that of warfarin for patients >75 years.40 The 110-mg dose of dabigatran was noninferior to VKA for the prevention of SSE, but superior on MB. Although this dose of the drug was not approved by the FDA for use in the United States, it has been approved in other countries.
Rivaroxaban (Xarelto®, Janssen Pharmaceuticals, Inc., Titusville, NJ), an FXa inhibitor, was studied in the Rivaroxaban Once-daily Oral Direct FXa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial at a dose of 20 mg qd.39 The trial was double-blind, used warfarin as a comparator, and studied high-risk patients (CHADS2 score ≥2, mean 3.5). The results revealed that rivaroxaban was noninferior to warfarin for the prevention of SSE, and noninferior on MB. Of note, concern was expressed over INR management during the trial, given the relatively low TTR (mean 55%).
Apixaban, as described earlier, achieved superiority versus warfarin on the primary efficacy outcome (SSE), on the primary safety outcome (ISTH MB), as well as a statistically significant reduction in all-cause mortality. These results were maintained across a broad array of important subgroups (the elderly, high-risk patients, and those with renal impairment (for example, serum creatinine ≥1.5 mg/dL)), whether suitable or unsuitable for VKA therapy and across different levels of INR control. Apixaban was approved in 2012 for stroke prevention in AF as Eliquis® (Bristol-Myers Squibb Company, Princeton, NJ).
With the development of these novel oral anticoagulation agents, physicians and patients now have a choice of drugs with profiles that differ from the traditional VKA agent. The ability to choose an agent on the basis of its efficacy and safety profile, and to match it to a patient and an indication, is a marked advance in our ability to address an important unmet need and prevent mortality and serious morbidity in patients at risk for thromboembolic disease.
Acknowledgments
The development of apixaban is sponsored by Bristol-Myers Squibb and Pfizer. The authors greatly appreciate the substantial input, guidance, and leadership provided by our academic partners throughout the design and execution of the AF trials, especially Christopher Granger (Duke Clinical Research Institute, Durham, NC), Lars Wallentin (Uppsala Clinical Research, Uppsala, Sweden), Stuart Connolly (Population Health Research Institute, McMaster University, Hamilton, ON, Canada), and Salim Yusuf (Population Health Research Institute, McMaster University, Hamilton, ON, Canada). The authors thank Margarida Geraldes (formerly Global Biostatistics, Bristol-Myers Squibb Co.) for her substantial contributions as biostatistics lead during the development of apixaban. We also thank Junyuan Wang (Global Biostatistics, Bristol-Myers Squibb Co.) who performed the analysis of outcomes versus continuous TTR in Figure3. Editorial assistance was provided by Dana Fox, CMPP at Caudex Medical, and funded by Bristol-Myers Squibb and Pfizer, Inc.
Conflicts of interest
The authors were or are employees of Bristol-Myers Squibb Company during the development and execution of the apixaban clinical program.
Supporting Information
Additional supporting information may be found in the online version of this article.
Therapeutic utility index (TUI) as a function of daily steady-state apixaban exposure (AUCss) and regimen in the phase II trial of apixaban for prevention of VTE following elective knee replacement surgery (APROPOS trial). The TUI was used to integrate efficacy and safety outcomes to quantify apixaban's efficacy/safety balance and provides a measure of clinical events avoided; hence, a higher TUI is associated with a more favorable benefit–risk profile. Of the apixaban dosage regimens tested in phase II, the 2.5 mg bid regimen had the highest TUI (86.2%). This was also higher than the TUI for either 30 mg bid enoxaparin (82.5%) or for warfarin (71.8%). TUI was higher for bid dosing than for qd dosing across the full range of apixaban exposures. The boxes at the bottom of the figure represent the distribution of apixaban exposures for the doses indicated. Exposure distributions are shown for total daily dose (TDD) because the distributions of AUCss should be the same for bid and qd regimens for the same TDD. Modified, with permission, from Leil et al.16
Forest plots from ARISTOTLE for apixaban versus warfarin for the primary efficacy outcome (stroke or systemic embolism) and the primary safety outcome (ISTH major bleeding). Modified, with permission, from Granger et al.29
Forest plots from AVERROES for apixaban versus warfarin for the primary efficacy outcome (stroke or systemic embolism) and the primary safety outcome (ISTH major bleeding). Modified, with permission, from Connolly et al.31
Outcomes with apixaban versus warfarin in relation to quartiles of predicted cTTR. The interaction test was based on the continuous cTTR. The primary efficacy outcome was stroke (ischemic, hemorrhagic, or unspecified) or systemic embolism (SSE). ISTH major bleeding was the primary safety outcome. Net clinical benefit was the composite of SSE, all-cause death, and ISTH major bleeding. Modified, with permission, from Wallentin et al.37
Definition of ISTH major bleeding.
References
- 1.Hart RG, Pearce LA. Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Baker WL, Cios DA, Sander SD, et al. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J. Manag. Care. Pharm. 2009;15:244–252. doi: 10.18553/jmcp.2009.15.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer DE, Albers GW. Dalen JE American College of Chest Physicians. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 4.Harker LA, Hanson SR. Kelly AB. Antithrombotic benefits and hemorrhagic risks of direct thrombin antagonists. Thromb. Haemost. 1995;74:464–472. [PubMed] [Google Scholar]
- 5.Morishima Y, Tanabe K, Terada Y, et al. Antithrombotic and hemorrhagic effects of DX-9065a, a direct and selective factor Xa inhibitor: comparison with a direct thrombin inhibitor and antithrombin III-dependent anticoagulants. Thromb. Haemost. 1997;78:1366–1371. [PubMed] [Google Scholar]
- 6.Sato K, Kawasaki T, Taniuchi Y, et al. YM-60828, a novel factor Xa inhibitor: separation of its antithrombotic effects from its prolongation of bleeding time. Eur. J. Pharmacol. 1997;339:141–146. doi: 10.1016/s0014-2999(97)01389-7. [DOI] [PubMed] [Google Scholar]
- 7.Wong PC, Crain EJ, Watson CA, et al. Nonpeptide factor Xa inhibitors III: effects of DPC423, an orally-active pyrazole antithrombotic agent, on arterial thrombosis in rabbits. J. Pharmacol. Exp. Ther. 2002;303:993–1000. doi: 10.1124/jpet.102.040089. [DOI] [PubMed] [Google Scholar]
- 8.Wong PC, Crain EJ, Watson CA, et al. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J. Thromb. Haemost. 2009;7:1313–1320. doi: 10.1111/j.1538-7836.2009.03503.x. [DOI] [PubMed] [Google Scholar]
- 9.Pinto DJP, Wong PC, Knabb RM. Case history: eliquis (apixaban), a potent and selective inhibitor of coagulation factor Xa for the prevention and treatment of thrombotic diseases. In: Desai MC, et al., editors. Annual Reports in Medicinal Chemistry. Vol. 47. Burlington: Academic Press; 2012. pp. 123–141. [Google Scholar]
- 10.Pinto DJ, Smallheer JM, Cheney DL, et al. Factor Xa inhibitors: next-generation antithrombotic agents. J. Med. Chem. 2010;53:6243–6274. doi: 10.1021/jm100146h. [DOI] [PubMed] [Google Scholar]
- 11.Quan ML, Lam PY, Han Q, et al. Discovery of 1-(3’-aminobenzisoxazol-5’-yl)-3-trifluoromethyl-N-[2-fluoro-4- [(2’-dimethylaminomethyl)imidazol-1-yl]phenyl]-1H-pyrazole-5-carboxyamide hydrochloride (razaxaban), a highly potent, selective, and orally bioavailable factor Xa inhibitor. J. Med. Chem. 2005;48:1729–1744. doi: 10.1021/jm0497949. [DOI] [PubMed] [Google Scholar]
- 12.Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J. Thromb. Haemost. 2008;6:820–829. doi: 10.1111/j.1538-7836.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher WA, Bostwick JS, Stewart AB, et al. Effect of the direct factor Xa inhibitor apixaban in rat models of thrombosis and hemostasis. J. Cardiovasc. Pharmacol. 2010;55:609–616. doi: 10.1097/FJC.0b013e3181daded3. [DOI] [PubMed] [Google Scholar]
- 14.He K, Luettgen JM, Zhang D, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur. J. Drug. Metab. Pharmacokinet. 2011;36:129–139. doi: 10.1007/s13318-011-0037-x. [DOI] [PubMed] [Google Scholar]
- 15.Lassen MR, Davidson BL, Gallus A, et al. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J. Thromb. Haemost. 2007;5:2368–2375. doi: 10.1111/j.1538-7836.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 16.Leil TA, Feng Y, Zhang L, et al. Quantification of apixaban's therapeutic utility in prevention of venous thromboembolism: selection of phase III trial dose. Clin. Pharmacol. Ther. 2010;88:375–382. doi: 10.1038/clpt.2010.106. [DOI] [PubMed] [Google Scholar]
- 17.Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N. Engl J. Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 18.Lassen MR, Raskob GE, Gallus A, et al. ADVANCE-2 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 19.Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N. Engl. J. Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 20.Buller H, Deitchman D, Prins M, et al. Botticelli Investigators, Writing Committee. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep-vein thrombosis. The Botticelli DVT dose-ranging study. J. Thromb. Haemost. 2008;6:1313–1318. doi: 10.1111/j.1538-7836.2008.03054.x. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. J. Am. Med. Assoc. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 22.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 23.Heeringa J, Kuip D, Hofman A, et al. Prevalence, incidence, and lifetime risk of atrial fibrillation: the Rotterdam study. Eur. Heart. J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 25.Wolf PA, Abbott RD. Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 26.Marini C, de Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36:1115–1119. doi: 10.1161/01.STR.0000166053.83476.4a. [DOI] [PubMed] [Google Scholar]
- 27.Camm AJ, Lip GHY, De Caterina R, et al. Focused update of the ESC guidelines for the management of AF. Eur. Heart. J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 28.Glader EL, Sjölander M. Eriksson M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41:397–401. doi: 10.1161/STROKEAHA.109.566950. [DOI] [PubMed] [Google Scholar]
- 29.Granger CB, Alexander JH, McMurray JJV, et al. for the ARISTOTLE committees and investigators. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 30.Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 31.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 32.McMurray JJV, Connolly SJ, Hart R, et al. Effect of apixaban on all-cause mortality in atrial fibrillation: an imputed placebo analysis. Eur. Heart. J. 2012;33(1 Suppl):519. [Google Scholar]
- 33.Pengo V, Pegoraro C, Cucchini U, et al. Worldwide management of oral anticoagulant therapy: the ISAM study. J. Thromb. Thrombolysis. 2006;21:73–77. doi: 10.1007/s11239-006-5580-y. [DOI] [PubMed] [Google Scholar]
- 34.van Walraven C, Jennings A, Oake N, et al. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155–1166. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 35.McBride D, Bruggenjurgen B, Roll S, et al. Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice. J. Thromb. Thrombolysis. 2007;24:65–72. doi: 10.1007/s11239-006-0002-8. [DOI] [PubMed] [Google Scholar]
- 36.Rose AJ, Hylek EM, Ozonoff A, et al. Risk-adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the veterans affairs study to improve anticoagulation (VARIA) Circ. Cardiovasc. Qual. Outcomes. 2011;4:22–29. doi: 10.1161/CIRCOUTCOMES.110.957738. [DOI] [PubMed] [Google Scholar]
- 37.Wallentin L, Lopes RD, Hanna M, et al. ARISTOTLE Investigators. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127:2166–2176. doi: 10.1161/CIRCULATIONAHA.112.142158. [DOI] [PubMed] [Google Scholar]
- 38.Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 39.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 40.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 41.Nagarakanti R, Ezekowitz MD, Parcham-Azad K, et al. Abstract 4629: long-term open label extension of the prevention of embolic and thrombotic events on dabigatran in atrial fibrillation (PETRO- Ex study) Circulation. 2008;118:S_922. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Therapeutic utility index (TUI) as a function of daily steady-state apixaban exposure (AUCss) and regimen in the phase II trial of apixaban for prevention of VTE following elective knee replacement surgery (APROPOS trial). The TUI was used to integrate efficacy and safety outcomes to quantify apixaban's efficacy/safety balance and provides a measure of clinical events avoided; hence, a higher TUI is associated with a more favorable benefit–risk profile. Of the apixaban dosage regimens tested in phase II, the 2.5 mg bid regimen had the highest TUI (86.2%). This was also higher than the TUI for either 30 mg bid enoxaparin (82.5%) or for warfarin (71.8%). TUI was higher for bid dosing than for qd dosing across the full range of apixaban exposures. The boxes at the bottom of the figure represent the distribution of apixaban exposures for the doses indicated. Exposure distributions are shown for total daily dose (TDD) because the distributions of AUCss should be the same for bid and qd regimens for the same TDD. Modified, with permission, from Leil et al.16
Forest plots from ARISTOTLE for apixaban versus warfarin for the primary efficacy outcome (stroke or systemic embolism) and the primary safety outcome (ISTH major bleeding). Modified, with permission, from Granger et al.29
Forest plots from AVERROES for apixaban versus warfarin for the primary efficacy outcome (stroke or systemic embolism) and the primary safety outcome (ISTH major bleeding). Modified, with permission, from Connolly et al.31
Outcomes with apixaban versus warfarin in relation to quartiles of predicted cTTR. The interaction test was based on the continuous cTTR. The primary efficacy outcome was stroke (ischemic, hemorrhagic, or unspecified) or systemic embolism (SSE). ISTH major bleeding was the primary safety outcome. Net clinical benefit was the composite of SSE, all-cause death, and ISTH major bleeding. Modified, with permission, from Wallentin et al.37
Definition of ISTH major bleeding.


