Four recent publications in Molecular Microbiology have begun to address the importance of a putative protease, BB0104, in the physiology and pathogenesis of Borrelia burgdorferi. BB0104 is a member of a large family of serine proteases designated as the high-temperature requirement (HtrA) protease family (reviewed in Singh et al., 2011; Hansen and Hilgenfeld, 2013). Members of this group have some homology at the amino acid level and similar architecture. All form oligomers (6–24 subunits) and have a central chymotrypsin-like protease domain followed by one or two PDZ (substrate binding and oligomerization) domains. Some HtrA homologues have a N-terminal signal peptide and a trans-membrane domain. In general, these proteases in prokaryotes are responsible for maintaining an operational bacterial proteome by turning over damaged or incorrectly folded proteins generated during abortive cellular localization or under conditions of cell stress (e.g. heat stock, oxidative stress etc.). Additionally, these critical proteases act as chaperones to correctly promote the folding of proteins as they are being translocated from the cytosol to the membranes or to the extracellular milieu. Data also suggest that this chaperone function is required to maintain the correct levels of target proteins in bacterial membranes, thus promoting a bacterial membrane environment that is required for optimal membrane function. This is accomplished by the HtrA binding to specific, target proteins and, if the protein is not able to be inserted in the membrane in a time-dependent fashion, HtrA will transition from chaperone function to protease function and turnover the bound protein. By biochemically ‘sensing’ the integrity of membrane proteins, HtrA proteases provide a monitoring system that allows the cells to adapt readily to changing environmental conditions. As a result of these functions, HtrA proteases play a significant role in the overall physiology of the bacteria.
Recently, it has been shown in different bacterial pathogens that HtrA can play a significant role in the pathology of infections. For example, in Bacillus anthracis, a htrA deletion mutant (ΔhtrA) is dramatically reduced in virulence in guinea pigs (6 orders of magnitude) compared with the wild-type and complemented mutant strains (Chitlaru et al., 2011). This mutant synthesizes normal levels of capsule, lethal toxin and oedema toxin but is more sensitive to increased temperature, reactive oxygen species and osmotic stress (Chitlaru et al., 2011). The decrease in virulence observed in this strain is attributed to the altered export of key proteins involved in protecting B. anthracis from stress-related challenges. Another important pathogen, Mycobacterium tuberculosis, harbours genes encoding three HtrA-like proteases (MtHtrA1, MtHtrA2 and MtHtrA3), which exhibit a variety of functions (Roberts et al., 2013). MtHtrA1, is a membrane bound protease that is apparently essential for bacterial cell survival since numerous attempts to disrupt htrA1 have been unsuccessful (Sassetti et al., 2003; Mohamedmohaideen et al., 2008). Therefore, it has been suggested that MtHtrA1 has protease or chaperone activity for proteins that are essential for the general cell physiology. On the other hand, htrA2 mutants have been isolated that show no obvious defects during in vitro growth. However, these mutants are attenuated in mice suggesting that MtHtrA2 is involved in the processing and localization of key virulence factors important for the pathology of tuberculosis. htrA3 mutants have no detectable phenotype when grown in vitro or when tested for infectivity in mice. Interestingly, while MtHtrA1 localizes exclusively to the membrane fraction of M. tuberculosis, MtHtrA2 and MtHtrA3 localize to the membrane fraction and appear to be secreted (Braunstein et al., 2000). Other bacterial pathogens like Chlamydia trachomatis and Helicobacter pylori have also been shown to export HtrA proteases into the host cell cytosol (C. trachomatis) or extracellularly (H. pylori) (Hoy et al., 2010; Wu et al., 2011). In the case of H. pylori, HpHtrA is proteolytically active against host E-cadherin which is important for epithelial adherence junctions and barrier integrity. It has been proposed that the proteolytic cleavage of E-cadherin by HpHtrA could disrupt the gastric epithelial lining promoting colonization by H. pylori (Hoy et al., 2012). As data accumulate on the HtrA family of proteases, it is becoming very clear that these enzymes are pivotal to the overall physiology and virulence of a variety of bacterial pathogens.
Recent papers published in Molecular Microbiology present data that begin to shed light on the role of an HtrA homolgue, BB0104 (BbHtrA), in the physiology and pathogenesis of the Lyme disease agent, Borrelia burgdorferi (Coleman et al., 2013; Kariu et al., 2013; Russell et al., 2013; Russell and Johnson, 2013). Lyme disease is characterized by initial pathogen colonization of the dermis BbHtrA at the tick bite site, followed by dissemination to secondary tissues and, in some cases, by clinical manifestations such as neuroborreliosis and Lyme arthritis (LA). These reports outline a possible role for BbHtrA in these processes. The first paper, by Kariu et al., suggests a potential role for BbHtrA in the proteolytic processing of BB0323 (Kariu et al., 2013). BB0323 has been shown to be required for normal cell growth and division, and BB0323 mutants are avirulent in mice (Zhang et al., 2009). Ostberg et al. have previously shown that BB0323 is potentially processed by the carboxyl-terminal peptidase CtpA (Ostberg et al., 2004). In that study, analyses of a CtpA mutant indicated that BB0323 is still processed (from ∼46 to ∼32 kDa) but not to the 29 kDa protein observed in wild-type cells (Ostberg et al., 2004). In the more recent publication by Kariu et al., data presented suggest that BB0323 is also processed by purified, recombinant BbHtrA, into fragments of 30 and 15 kDa but not to ∼27 kDa protein detected in wild-type cells. Additionally, they provide data that indicate that the N-terminal domain of BB0323 is required for normal protein function while the C-terminal, LysM domain is required for infectivity in mice (Kariu et al., 2013). Kariu et al. propose a model suggesting that initial processing of BB0323 requires BbHtrA activity while the final processing into enzymatically active polypeptides requires CtpA. However, it was not possible to isolate a BbHtrA mutant to confirm their model.
In a very recent study, Coleman et al. took a biochemical approach to understanding the function of BbHtrA in B. burgdorferi (Coleman et al., 2013). First, they showed that BbHtrA forms a trimer in solution when no substrate is present consistent with structural analyses originally done on the BbHtrA homologue, DegP from E. coli (Hansen and Hilgenfeld, 2013). Second, they were able to demonstrate caseinolytic activity that was both dependent on a conserved catalytic serine (S198) in the protease domain and increased temperature. Third, they present data which show that BbHtrA localizes to the soluble and membrane fractions, and, more importantly, that significant levels of BbHtrA was secreted into the growth media. Previously, several research groups have shown that in Gram-negative bacteria HtrA proteases localize primarily to the periplasmic space where they perform their normal protease and chaperone functions. Yet, in other bacteria, HtrA proteases localize not only to the periplasmic space but also to cell membrane fractions, the cell surface and, in specific cases, also to the extracellular milieu (Roberts et al., 2013). The mechanism by which these proteins are secreted has not been determined and, as pointed out by the authors, B. burgdorferi also has no obvious mechanism or secretory machinery to secrete BbHtrA from the cell. Finally, using BbHtrA antibody, the authors used an immunoprecipitation enrichment to identify proteins from B. burgdorferi that bound to BbHtrA. Several proteins including outer surface protein A (OspA), outer surface protein B (OspB), basic membrane protein D (BmpD), chemotaxis protein X (CheX), flagellar basal body protein (FilL), BB0365 (lpA7) and BB0690 (NapA) were enriched using this technique. Of these, BmpD and CheX were proteolytically degraded by purified BbHtrA. Taken together, these data suggest that BbHtrA functions as a chaperone and protease similar to other members of the HtrA family and that it interacts with a subset of cellular proteins that are essential for chemotaxis and motility. Like Kariu et al. these authors could not isolate an BbHtrA mutant. Considering the putative substrates reported for BbHtrA in these two reports, it seems likely that isolating an BbHtrA mutant is not possible.
Two new research articles published in this issue of Molecular Microbiology by Russell and Johnson, and Russell et al. provide data that indicate that BbHtrA degrades an interesting variety of host proteoglycans (Russell et al., 2013; Russell and Johnson, 2013). In their first paper, Russell and Johnson describe the binding and cleavage of aggrecan, the most abundant proteoglycan in the extracellular matrix (ECM) of joint and connective tissues. They first were able to demonstrate binding of aggrecan to intact B. burgdorferi cells and, subsequently targeting two specific proteins that were significantly enriched using aggrecan-affinity chromatography and mass spectrometry. One protein, identified was BB0588 (a glycosaminoglycan binding protein designated Bgp), which has been previously described (Parveen and Leong, 2000; Parveen et al., 2003). The second protein was identified as BbHtrA (BB0104). Russell and Johnson then demonstrated caseinolytic activity for BbHtrA similar to that described by Coleman et al. Recombinant BbHtrA and BbHtrA S226A [the same serine residue describe above (S198) but numbered based upon the full length protein] were purified and used in protease assays against purified aggrecan. Like the human proteases (HtrA1, AT5 and MMP2) that have been shown to degrade aggrecan, BbHtrA was able to proteolytically cleave recombinant human and natural bovine aggrecan into three fragments with molecular masses similar to those generated by the other proteases tested (Russell and Johnson, 2013). This suggested that BbHtrA yielded products from specific cleavage sites within aggrecan near those previously described for the human proteases. To determine the cleavage sites, Russell and Johnson isolated proteolytic fragments generated from the incubation of purified BbHtrA with aggrecan and the putative sites were mapped using monoclonal antibodies targeting the newly generated, exposed aggrecan neoepitopes and identified by protein sequencing. These data indicate that BbHtrA cleaved aggrecan within a small region of the interglobular domain at or near arginine-374. Importantly, Hu et al. had previously detected fragments of aggrecan, which had been cleaved at this same site in the synovial fluid of patients with LA (Hu et al., 2001). Hu et al. and Behera et al. were also able to identify similar fragments generated by incubating B. burgdorferi cells with human cartilage explants and show that this degradation was not generated by human aggrecanases/proteases which proteolytically attack aggrecan during inflammatory arthritis (Hu et al., 2001; Behera et al., 2006). As demonstrated by Coleman et al., Russell and Johnson show that antibodies to BbHtrA are commonly detected in patients with Lyme disease. These results indicate an important role for BbHtrA in the pathogenesis of LA.
In their second paper, Russell et al. expand their characterization of BbHtrA by examining in-depth the interaction of the protease with ECM components. Because of the homology of BbHtrA with human HtrA1, the authors speculate that BbHtrA might have enzymatic activity against other important structural components of the ECM in addition to aggrecan. In vitro enzyme assays using purified BbHtrA provided direct evidence that proteoglycans biglycan, decorin, neuorcan, brevican and versican were degraded by purified BbHtrA (Russell et al., 2013). These proteoglycans are widely distributed in ECMs present in various tissues, joints and the CNS (Wu et al., 2005; Frischknecht and Seidenbecher, 2008). Compromising the structural integrity of the ECM at various sites would facilitate penetration and colonization by B. burgdorferi. Additionally, BbHtrA was able to degrade E-cadherin, an important glycoprotein that promotes efficient epithelial cell adhesion. Hoy et al. demonstrated that some bacterial pathogens are able to degrade E-cadherin and they suggest that this degradative process promotes dissemination (Hoy et al., 2012). Another glycoprotein which was susceptible to degradation by BbHtrA was fibronectin. The degradation of fibronectin by purified BbHtrA yielded peptide fragments which harboured fibronectin type III repeats 13 and 14 (FnIII13–14) and a N-terminal fragment designated Fn-f 29. FnIII 13–14 have been shown to cause damage to connective tissue by inducing human MMP protease and aggrecanases (Sofat et al., 2011) while Fn-f29 is pro-inflammatory (Su et al., 2005). Previous reports have shown that fibronectin fragments generated by HtrA1 stimulated the production of numerous cytokines and chemokines (Grau et al., 2005; Pulai et al., 2005; Austin et al., 2009; Tiaden et al., 2012). Therefore, it seemed plausible to the authors that BbHtrA generated fragments of fibronectin might be able to stimulate a similar response. Therefore purified BbHtrA was incubated with ECM producing, cultured chondrocytes and indeed culture supernatant contained increased levels of chemokines (CXCL1, CCL1, CCL2, CCL5 and IL-8) and cytokines (IL-6 and slCAM-1). Chemokine and cytokine levels have been shown to be elevated in the epidermis of patients with erythema migrans, in the cerebrospinal fluid patients with neuroborreliosis and in the synovial fluid of patients with LA (Grygorczuk et al., 2005; Mullegger et al., 2007; Zhao et al., 2007; Strle et al., 2009). These results thus suggest that BbHtrA, along with B. burgdorferi lipoproteins, contribute to the pathology of neuroborreliosis and LA. However, these data should not be over interpreted before BbHtrA's role in these manifestations of Lyme disease are confirmed in vivo.
The accumulating data published in Molecular Microbiology regarding BbHtrA demonstrate the importance of this protein in the physiology and pathogenesis of B. burgdorferi ( Fig. 1). The protein is reported to be involved in the localization and processing of putative virulence factors, such as BB0323 and BmpD (Coleman et al., 2013; Kariu et al., 2013). Additionally, proteolytic activity of BbHtrA against aggrecan, a key structural proteoglycan present in connective tissue and joints, suggests a direct role in LA has been shown (Russell and Johnson, 2013). More importantly, Russell et al. provide convincing data that HtrA is able to degrade fibronectin and various proteoglycans found in joints, epidermis and neurological tissues. Most significant, purified recombinant BbHtrA interacted with chondrocytes triggering the release of chemokines and pro-inflammatory cytokines (Russell et al., 2013). These data indicate that HtrA is potentially important in cell physiology (motility, chemotaxis), protein processing (BB0323), bacterial dissemination (e.g. degradation of E-cadherin and aggrecan) and actively contributes to release of inflammatory cytokines (IL-6 and slCAM-1) and chemokines (e.g. CXCL1, CCL1, etc.) from cultured chondrocytes. It seems very clear from these data that BbHtrA is critical in the physiology and pathogenesis of B. burgdorferi.
Fig 1.
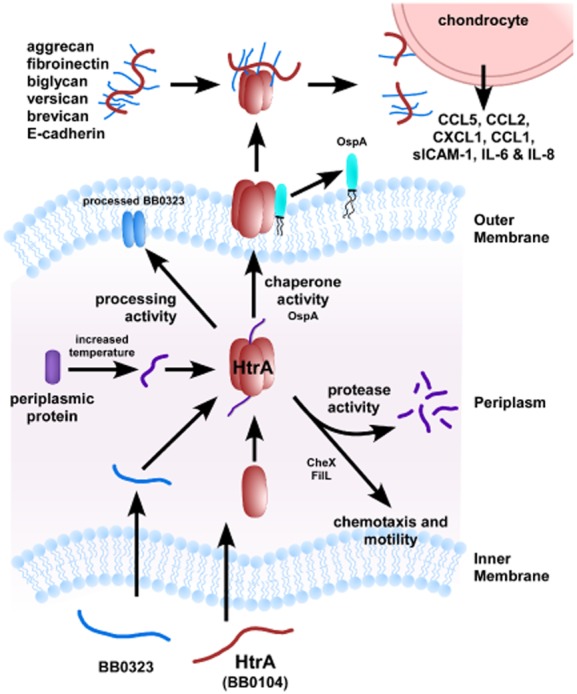
A model for the different activities linked to BbHtrA.
References
- Austin BA, Liu B, Li Z. Nussenblatt RB. Biologically active fibronectin fragments stimulate release of MCP-1 and catabolic cytokines from murine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:2896–2902. doi: 10.1167/iovs.08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera AK, Hildebrand E, Szafranski J, Hung HH, Grodzinsky AJ, Lafyatis R, et al. Role of aggrecanase 1 in Lyme arthritis. Arthritis Rheum. 2006;54:3319–3329. doi: 10.1002/art.22128. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Griffin TI, Kriakov JI, Friedman ST, Grindley ND. Jacobs WR. Identification of genes encoding exported Mycobacterium tuberculosis proteins using a Tn552′phoA in vitro transposition system. J Bacteriol. 2000;182:2732–2740. doi: 10.1128/jb.182.10.2732-2740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitlaru T, Zaide G, Ehrlich S, Inbar I, Cohen O. Shafferman A. HtrA is a major virulence determinant of Bacillus anthracis. Mol Microbiol. 2011;81:1542–1559. doi: 10.1111/j.1365-2958.2011.07790.x. [DOI] [PubMed] [Google Scholar]
- Coleman JL, Crowley JT, Toledo AM. Benach JL. The HtrA protease of Borrelia burgdorferi degrades outer membrane protein BmpD and chemotaxis phosphatase CheX. Mol Microbiol. 2013;88:619–633. doi: 10.1111/mmi.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R. Seidenbecher CI. The crosstalk of hyaluronan-based extracellular matrix and synapses. Neuron Glia Biol. 2008;4:249–257. doi: 10.1017/S1740925X09990226. [DOI] [PubMed] [Google Scholar]
- Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, et al. The role of human HtrA1 in arthritic disease. J Biol Chem. 2005;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- Grygorczuk S, Zajkowska J, Swierzbinska R, Pancewicz S, Kondrusik M. Hermanowska-Szpakowicz T. Concentration of interferon-inducible T cell chemoattractant and monocyte chemotactic protein-1 in serum and cerebrospinal fluid of patients with Lyme borreliosis. Rocz Akad Med Bialymst. 2005;50:173–178. [PubMed] [Google Scholar]
- Hansen G. Hilgenfeld R. Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response. Cell Mol Life Sci. 2013;70:761–775. doi: 10.1007/s00018-012-1076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy B, Lower M, Weydig C, Carra G, Tegtmeyer N, Geppert T, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO J. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy B, Geppert T, Boehm M, Reisen F, Plattner P, Gadermaier G, et al. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J Biol Chem. 2012;287:10115–10120. doi: 10.1074/jbc.C111.333419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Eskildsen MA, Masgala C, Steere AC, Arner EC, Pratta MA, et al. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 2001;44:1401–1410. doi: 10.1002/1529-0131(200106)44:6<1401::AID-ART234>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kariu T, Yang X, Marks CB, Zhang X. Pal U. Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi. Mol Microbiol. 2013;88:510–522. doi: 10.1111/mmi.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamedmohaideen NN, Palaninathan SK, Morin PM, Williams BJ, Braunstein M, Tichy SE, et al. Structure and function of the virulence-associated high-temperature requirement A of Mycobacterium tuberculosis. Biochemistry. 2008;47:6092–6102. doi: 10.1021/bi701929m. [DOI] [PubMed] [Google Scholar]
- Mullegger RR, Means TK, Shin JJ, Lee M, Jones KL, Glickstein LJ, et al. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–4628. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostberg Y, Carroll JA, Pinne M, Krum JG, Rosa P. Bergstrom S. Pleiotropic effects of inactivating a carboxyl-terminal protease, CtpA, in Borrelia burgdorferi. J Bacteriol. 2004;186:2074–2084. doi: 10.1128/JB.186.7.2074-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen N. Leong JM. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–1234. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- Parveen N, Caimano M, Radolf JD. Leong JM. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol Microbiol. 2003;47:1433–1444. doi: 10.1046/j.1365-2958.2003.03388.x. [DOI] [PubMed] [Google Scholar]
- Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS. Loeser RF. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Personne Y, Ollinger J. Parish T. Proteases in Mycobacterium tuberculosis pathogenesis: potential as drug targets. Future Microbiol. 2013;8:621–631. doi: 10.2217/fmb.13.25. [DOI] [PubMed] [Google Scholar]
- Russell TM. Johnson BJ. Lyme disease spirochaetes possess an aggrecan-binding protease with aggrecanase activity. Mol Microbiol. 2013;90:288–240. doi: 10.1111/mmi.12276. [DOI] [PubMed] [Google Scholar]
- Russell TM, DeLorey MJ. Johnson BJB. Borrelia burgdorferi BbHtrA degrades host ECM proteins and stimulates release of inflammatory cytokines in vitro. Mol Microbiol. 2013;90:241–251. doi: 10.1111/mmi.12377. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH. Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Singh N, Kuppili RR. Bose K. The structural basis of mode of activation and functional diversity: a case study with HtrA family of serine proteases. Arch Biochem Biophys. 2011;516:85–96. doi: 10.1016/j.abb.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Sofat N, Robertson SD. Wait R. Fibronectin III 13–14 domains induce joint damage via Toll-like receptor 4 activation and synergize with interleukin-1 and tumour necrosis factor. J Innate Immun. 2011;4:69–79. doi: 10.1159/000329632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Drouin EE, Shen S, Khoury JE, McHugh G, Ruzic-Sabljic E, et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis. 2009;200:1936–1943. doi: 10.1086/648091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SL, Tsai CD, Lee CH, Salter DM. Lee HS. Expression and regulation of Toll-like receptor 2 by IL-1beta and fibronectin fragments in human articular chondrocytes. Osteoarthritis Cartilage. 2005;13:879–886. doi: 10.1016/j.joca.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Tiaden AN, Klawitter M, Lux V, Mirsaidi A, Bahrenberg G, Glanz S, et al. Detrimental role for human high temperature requirement serine protease A1 (HTRA1) in the pathogenesis of intervertebral disc (IVD) degeneration. J Biol Chem. 2012;287:21335–21345. doi: 10.1074/jbc.M112.341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Lei L, Gong S, Chen D, Flores R. Zhong G. The chlamydial periplasmic stress response serine protease cHtrA is secreted into host cell cytosol. BMC Microbiol. 2011;11:87. doi: 10.1186/1471-2180-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ. Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang X, Kumar M. Pal U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J Infect Dis. 2009;200:1318–1330. doi: 10.1086/605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fleming R, McCloud B. Klempner MS. CD14 mediates cross talk between mononuclear cells and fibroblasts for upregulation of matrix metalloproteinase 9 by Borrelia burgdorferi. Infect Immun. 2007;75:3062–3069. doi: 10.1128/IAI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


