Abstract
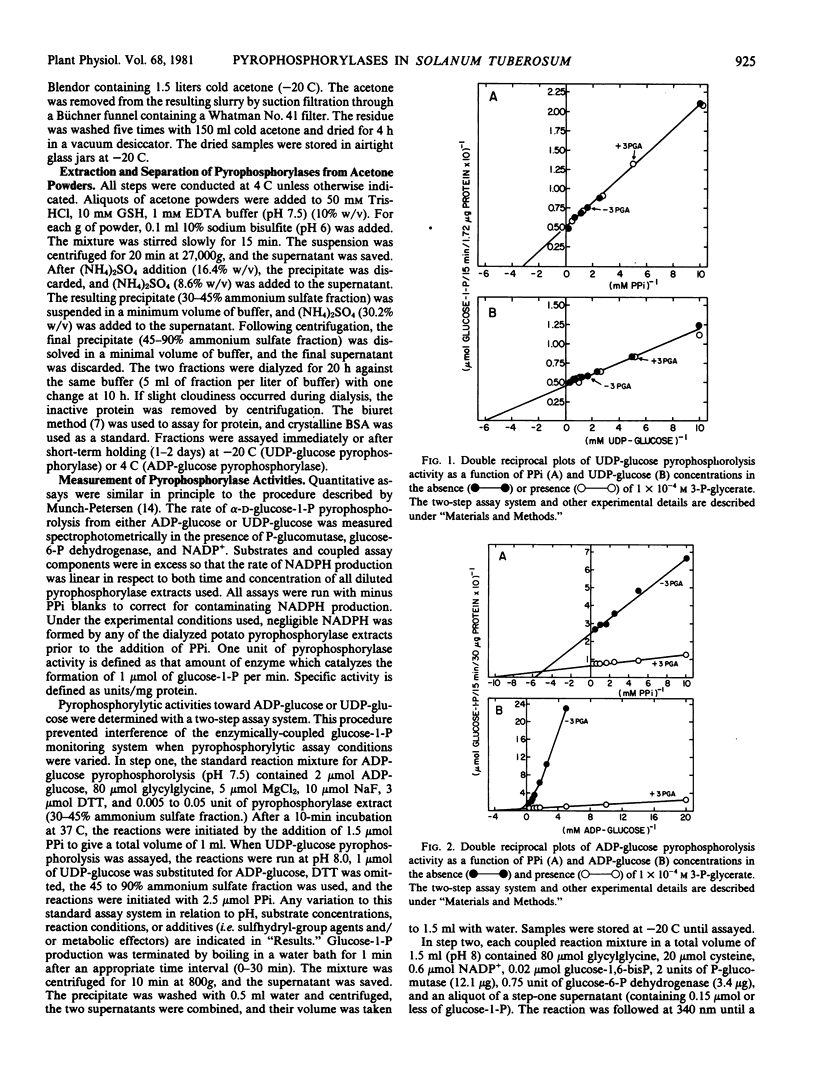
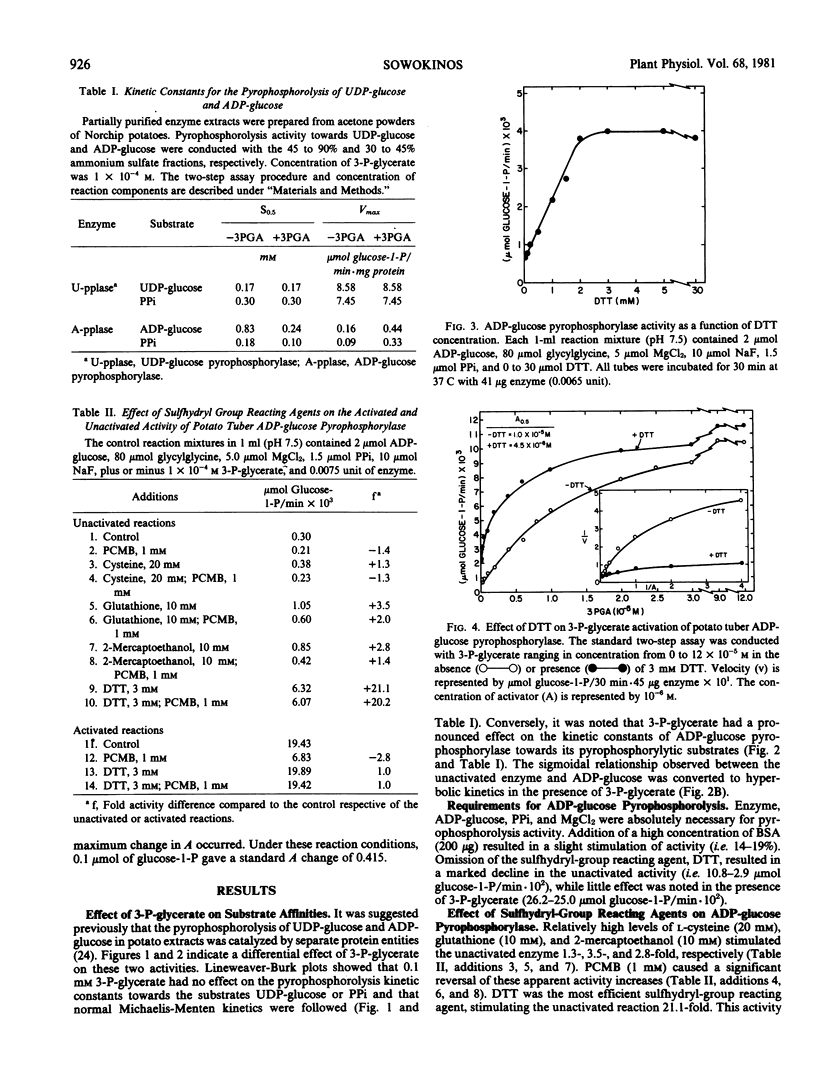
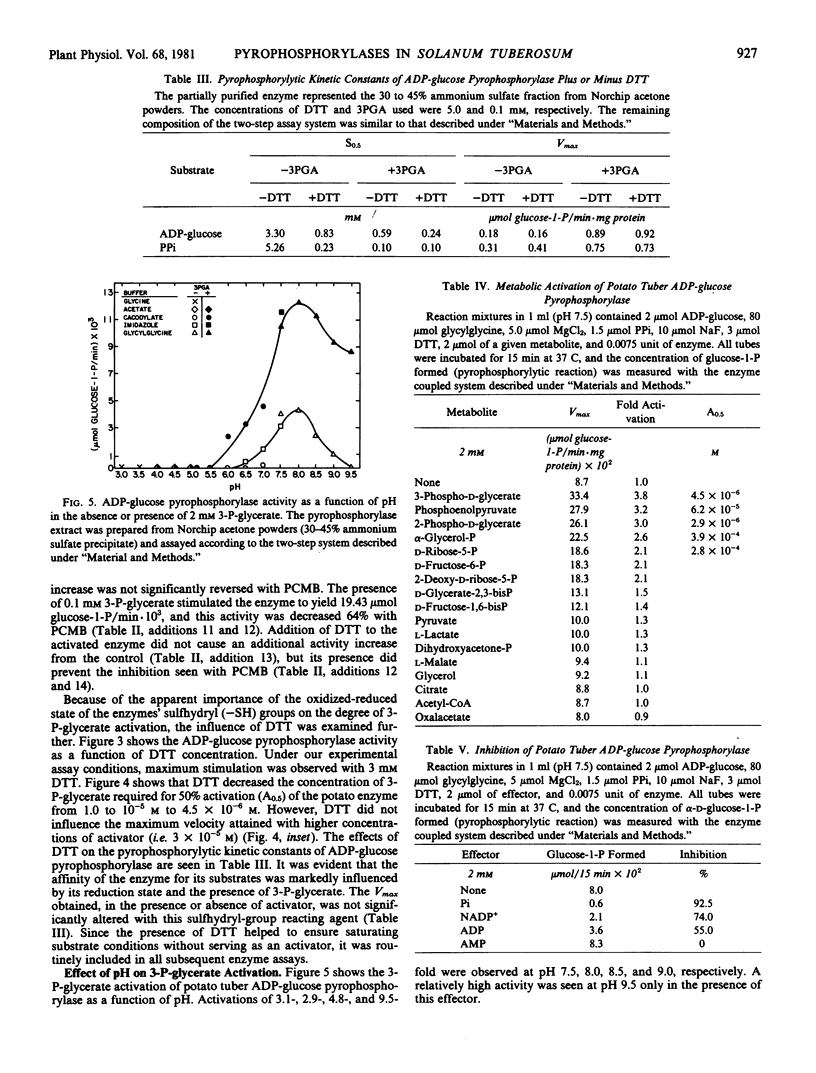
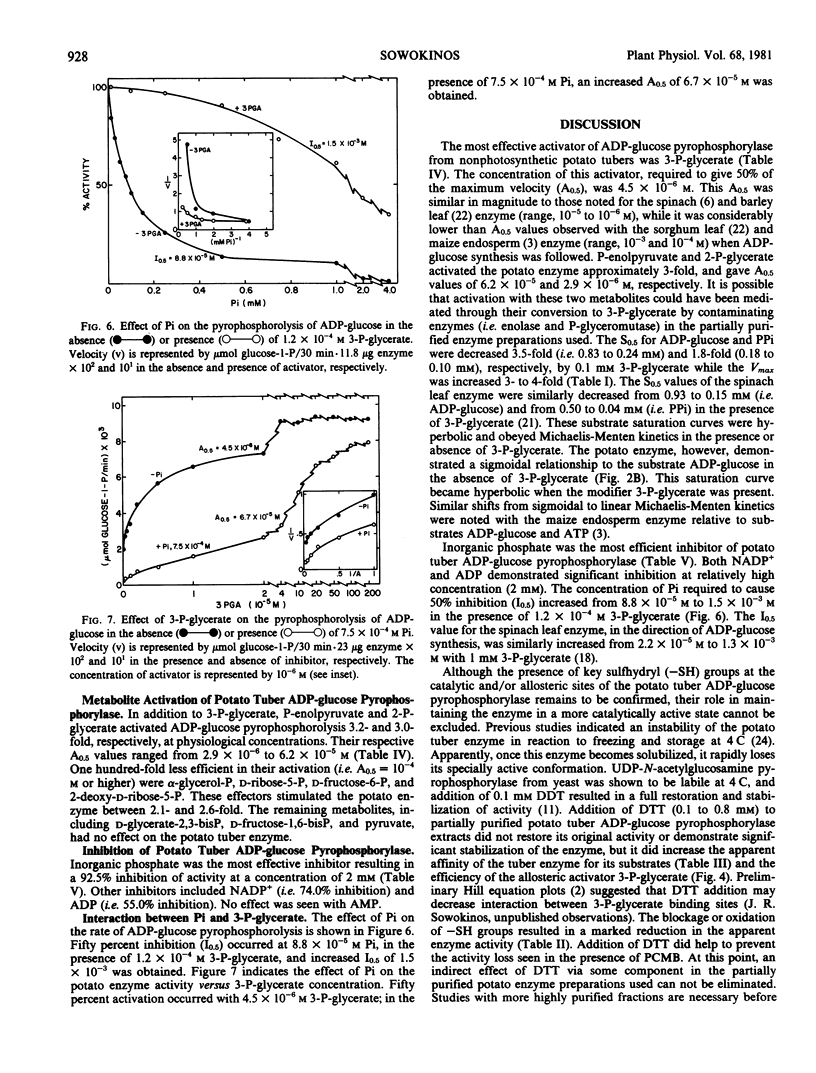
Pyrophosphorylytic kinetic constants (S0.5, Vmax) of partially purified UDP-glucose- and ADP-glucose pyrophosphorylases from potato tubers were determined in the presence of various intermediary metabolites. The S0.5 of UDP-glucose pyrophosphorylase for UDP-glucose (0.17 millimolar) or pyrophosphate (0.30 millimolar) and the Vmax were not influenced by high concentrations (2 millimolar) of these substances. The most efficient activator of ADP-glucose pyrophosphorylase was 3-P-glycerate (A0.5 = 4.5 × 10−6 molar). The S0.5 for ADP-glucose and pyrophosphate was increased 3.5-fold (0.83 to 0.24 millimolar) and 1.8-fold (0.18 to 0.10 millimolar), respectively, with 0.1 millimolar 3-P-glycerate while the Vmax was increased nearly 4-fold. The magnitude of 3-P-glycerate stimulation was dependent upon the integrity of key sulfhydryl groups (−SH) and pH. Oxidation or blockage of −SH groups resulted in a marked reduction of enzyme activity. Stimulations of 3.1-, 2.9-, 4.8-, and 9.5-fold were observed at pH 7.5, 8.0, 8.5, and 9.0, respectively, in the presence of 3-P-glycerate (2 millimolar). The most potent inhibitor of ADP-glucose pyrophosphorylase was orthophosphate (I0.5 = 8.8 × 10−5. molar). This inhibition was reversed with 3-P-glycerate (1.2 × 10−4 molar), resulting in an increased I0.5 value of 1.5 × 10−3 molar. Likewise, orthophosphate (7.5 × 10−4 molar) caused a decrease in the activation efficiency of 3-P-glycerate (A0.5 from 4.5 × 10−6 molar to 6.7 × 10−5 molar). The significance of 3-P-glycerate activation and orthophosphate inhibition in the regulation of α-glucan biosynthesis in Solanum tuberosum is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir J., Cherry J. H. Purification and properties of adenosine diphosphoglucose pyrophosphorylase from sweet corn. Plant Physiol. 1972 Jun;49(6):893–897. doi: 10.1104/pp.49.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. ADP glucose pyrophosphorylase from maize endosperm. Arch Biochem Biophys. 1969 Mar;130(1):119–128. doi: 10.1016/0003-9861(69)90017-4. [DOI] [PubMed] [Google Scholar]
- FRYDMAN R. B. STARCH SYNTHETASE OF POTATOES AND WAXY MAIZE. Arch Biochem Biophys. 1963 Aug;102:242–248. doi: 10.1016/0003-9861(63)90177-2. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Cardini C. E. Studies on the biosynthesis of starch. I. Isolation and properties of the soluble adenosine diphosphate glucose: starch glucosyltransferase of Solanum tuberosum. Arch Biochem Biophys. 1966 Sep 26;116(1):9–18. doi: 10.1016/0003-9861(66)90005-1. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Levi C., Preiss J. Regulatory Properties of the ADP-Glucose Pyrophosphorylase of the Blue-Green Bacterium Synechococcus 6301. Plant Physiol. 1976 Dec;58(6):753–756. doi: 10.1104/pp.58.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Greenberg E., Lammel C., Preiss J. Starch Synthetase, Phosphorylase, ADPglucose Pyrophosphorylase, and UDPglucose Pyrophosphorylase in Developing Maize Kernels. Plant Physiol. 1973 Jan;51(1):1–5. doi: 10.1104/pp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Lammel C., Sabraw A. A unique adenosine diphosphoglucose pyrophosphorylase associated with maize embryo tissue. Plant Physiol. 1971 Jan;47(1):104–108. doi: 10.1104/pp.47.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Greenberg E., Hardie J., Cameron E. C., Preiss J. Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADPglucose pyrophosphorylase. Plant Physiol. 1968 Mar;43(3):417–427. doi: 10.1104/pp.43.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Preiss J. Biosynthesis of starch in Chlorella pyrenoidosa. II. Regulation of ATP: alpha-D-glucose 1-phosphate adenyl transferase (ADP-glucose pyrophosphorylase) by inorganic phosphate and 3-phosphoglycerate. Arch Biochem Biophys. 1967 Mar;119(1):454–469. doi: 10.1016/0003-9861(67)90477-8. [DOI] [PubMed] [Google Scholar]
- Sowokinos J. R. Pyrophosphorylases in Solanum tuberosum: I. Changes in ADP-Glucose and UDP-Glucose Pyrophosphorylase Activities Associated with Starch Biosynthesis during Tuberization, Maturation, and Storage of Potatoes. Plant Physiol. 1976 Jan;57(1):63–68. doi: 10.1104/pp.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]



