Abstract
Quaternary ammonium compounds (QACs) are antimicrobial disinfectants commonly used in commercial and household settings. Extensive use of QACs results in ubiquitous human exposure, yet reproductive toxicity has not been evaluated. Decreased reproductive performance in laboratory mice coincided with the introduction of a disinfectant containing both alkyl dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC). QACs were detected in caging material over a period of several months following cessation of disinfectant use. Breeding pairs exposed for six months to a QAC disinfectant exhibited decreases in fertility and fecundity: increased time to first litter, longer pregnancy intervals, fewer pups per litter and fewer pregnancies. Significant morbidity in near term dams was also observed. In summary, exposure to a common QAC disinfectant mixture significantly impaired reproductive health in mice. This study illustrates the importance of assessing mixture toxicity of commonly used products whose components have only been evaluated individually.
Keywords: Quaternary ammonium compounds, reproductive toxicity, toxicology, fertility, fecundity
1. INTRODUCTION
The impetus for this investigation of the effects of quaternary ammonium compounds (QACs) on reproductive performance in the laboratory mouse was changes in breeding performance in mouse colonies used by the Hunt and Hrubec laboratories. Both groups noted abrupt changes in colony productivity and reductions in maternal and fetal health that coincided with the introduction of disinfectants containing QACs, Alkyl (60% C14, 25% C12, 15% C16) dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC).
QACs are commonly found in cleaning solutions used in residential, commercial and medical settings, as well as in restaurants and food production facilities. The ability to adapt and optimize QAC structure for specific functions has increased the utilization of these compounds in consumer products [1, 2] and, as a result, several generations of QACs exist. The earliest QACs were benzalkonium chloride compounds that were developed as antimicrobial agents. All QACs are permanently charged ions with four alkyl side chains, and biocidal activity is conferred through alkyl chain length [3–5]. Modifications to alkyl chain length have been used to optimize cleaning and antimicrobial properties. Specifically, through substitution of aromatic ring hydrogen with chlorine, methyl, and ethyl groups to increase antimicrobial efficiency and improve detergent strength, different generations of QAC compounds have been generated. Twin-chain or dialkyl quaternary QACs represent the newest generation and exhibit a wide spectrum of activity. These new synthetic polymeric QACs contain multiple positively charged amine centers that confer antimicrobial, anti-static, and surfactant properties in solution.
QACs are often used in shampoos and laundry products to neutralize negative static charges and in cosmetics to preserve products from microbial contamination [6–9]. The increased reliance on QAC mixtures in consumer products has likely resulted in significant human exposure. For instance, products applied directly to and left on the skin, such as body lotions, often contain QACs [10]. In the health care industry QACs have replaced many alcohol-based hand sanitizers, being more effective at reducing bacterial contamination and limiting the spread of nosocomial infections [11]. In addition, elementary students are provided instant hand sanitizers containing QACs to decrease the spread of illness and reduce rates of absenteeism [12]. QACs are being increasingly incorporated into contemporary products that are utilized orally, such as mouth wash, applied to the skin or eyes or administered as a nasal spray [13–20].
QACs have been in use for approximately 50 years and are considered relatively safe. Despite the duration and prevalence of their use in commercial and consumer products, few studies have assessed the toxicity of single QACs. The majority of studies investigating the toxicity of single QACs are unpublished company reports which indicate weight reduction as the main effect in mice [21–23]. Moreover, no peer-reviewed studies have examined the toxicity of newer QAC combinations. Since chemical mixtures can act synergistically to produce greater toxic effects than the sum of the individual components, evaluation of common mixtures is essential in the evaluation of chemical risk [24–27].
Formulation HWS-256 is a commercial mixture containing a combination of two QACs: Alkyl (60% C14, 25% C12, 15% C16) dimethyl benzyl ammonium chloride (ADBAC, benzalkonium chloride) and didecyl dimethyl ammonium chloride (DDAC). Combinations of ADBAC and DDAC are common in disinfectant and cleaning solutions that are widely used in clinical and residential settings. We proposed that a disinfectant solution containing both ADBAC and DDAC caused severe reproductive defects in exposed mice [28]. This assertion is supported by the results of a six-month breeding study, wherein mice exposed to this QAC mixture demonstrated significant declines in fertility and fecundity. Our results show that the ADBAC+DDAC mixture not only significantly impaired reproduction in breeding pairs, but also contributed to dam morbidity.
2. MATERIALS AND METHODS
2.1. Animals and Experimental Design
2.1.1. Case Western Reserve (CWRU) and Washington State University (WSU)
C57BL/6J (Jackson Laboratory, Bar Harbor, ME) mice were housed in ventilated rack caging (Thorin caging (CWRU) or Lab Products (WSU)) in a pathogen-free facility. Breeding stocks were maintained by brother to sister trio mating of two females with one male. To obtain timed pregnancies, six week-old C57BL/6J female pups born in the new facility were placed with adult males, checked each morning for the presence of a copulation plug, and separated from the male on the morning a plug was found. All animal breeding experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at CWRU and WSU; both institutions are fully accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC).
2.1.2. Virginia Polytechnic Institute (VPI)
All CD-1 mice (Charles River Labs, Raleigh, NC) were bred for two generations in a room free of QAC disinfectants in order to eliminate any potential effects from previous QAC exposure. Mice were housed in disposable caging (Innovive, San Diego, CA). Exposed mice were maintained in a room utilizing an ADBAC+DDAC disinfectant (HWS-256, Sanitation Strategies, Holt, MI) while control mice were in an adjacent room utilizing ethanol as a disinfectant. Both rooms were climate-controlled with a 12-hour light/dark cycle, 20 – 25 °C, and 30 – 60% relative humidity. Ethanol washes were used to remove ADBAC+DDAC contaminants from equipment and personnel prior to entering the room housing the control mice. Personnel also donned hair bonnets, face masks, disposable gowns, gloves, and dedicated footwear prior to entering the control room to reduce potential ADBAC+DDAC contamination. Mice were dosed by adding HWS-256 into Nutra-gel diet (purified dry mix formula, Bio-Serv, Frenchtown, NJ) which was prepared following manufacturer instructions. Doses of ADBAC+DDAC/kg body weight/day were calculated based on the sum of active ingredient in the disinfectant (6.76% ADBAC (60% C-14, 25% C-12, 15% C-16) and 10.1% DDAC), with an average daily food consumption of 28% body weight and provided daily in a 25g Nutra-gel diet cube. Fresh gel cubes were added and food consumption monitored each day. In all experiments, mice were acclimated to the gel diet for one week prior to dosing. A short-term dose finding study was performed in unbred mice to identify the lowest observable adverse effect limit (LOAEL). Gel food was dosed with 0, 60, 120, 240, and 480 mg ADBAC+DDAC disinfectant/kg/day and provided to 5 mice per dose group for two weeks. Mice were monitored daily and evaluated against 16 different health parameters for physical appearance, activity, physiology, and body weight loss. Signs of toxicity, such as inappetance, lethargy, and rough haircoat, were observed in animals receiving the 240 and 480 mg doses; thus, the LOAEL was identified as 240 mg ADBAC+DDAC disinfectant/kg/day. Two dose levels below the LOAEL (i.e., 60 and 120 mg ADBAC+DDAC/kg/day) were selected to evaluate the long-term effects of exposure to QACs. At 5 weeks of age, all mice were provided undosed gel diet. At 6 weeks of age, males and females were combined into breeding pairs and randomly assigned to 0 (control), 60, or 120 mg ADBAC+DDAC/kg body weight/day treatment. Ten dedicated breeding pairs per group were subsequently dosed for a total of six months. Mice were monitored daily and evaluated against 16 different health parameters for physical appearance, activity, physiology and feed consumption. Male mouse weight was recorded weekly. Female body weight was not recorded as body weight fluctuated with their stage of pregnancy. At birth, delivered pups were counted, weighed, evaluated for gross malformations, and then euthanized by IP injection of sodium pentobarbital (0.05 mL/g). This experimental design allowed a multi-tier assessment of chronic toxicity from a QAC mixture directly to adult breeding pairs and indirectly to pups. All animal experiments were approved by the IACUC at the College of Veterinary Medicine at VPI, an AAALAC accredited facility.
2.2. Assessing Cage Contamination
To obtain extracts for chemical analysis, five cages were washed with a small volume of methanol using the following procedure: approximately 40 mL of methanol (JT Baker, Phillipsburg, NJ) was used to thoroughly rinse the inside walls of one polysulfone microisolator cage. The methanol was transferred to a second cage, the rinsing procedure was repeated, and the methanol was transferred to the third cage. This procedure was repeated for two additional cages, thus combining the chemical residue from five cages into one methanol sample. The resultant methanol extracts were collected and stored in glass vials that had been previously tested and demonstrated to be free of exogenous chemical residue (i.e., as for cages, test vials were washed with methanol and extracts run to assess contamination). Analysis of cage extracts was performed with an Agilent 1100 Series HPLC system (Agilent Technologies, Waldbronn, Germany) coupled to an API-4000 triple quadrupole mass spectrometer (Applied Biosystems/MDS SCIEX, Ontario, Canada) operated in positive ionization mode. Instrument control, data acquisition, and initial data analyses were performed by Analyst 1.3.1 software. Only the dimethyl didecyl ammonium (DDA) component of the QAC disinfectant was quantitatively analyzed. Five standard concentrations of dimethyl didecyl ammonium bromide (98%, Aldrich, St. Louis MO) ranging from 3.6 nM to 181 nM were analyzed with each sample batch. Chromatographic separation was carried out using a MM-5-C4W-1000 column (Micro-Tech Scientific Inc., Vista, CA). Mobile phase A was 0.1% acetic acid (J.T. Baker, Phillipsburg NJ), and mobile phase B was acetonitrile (EMD Chemicals Inc., Gibbstown NJ). The column was equilibrated with 70% A for 15 min before injection. The gradient was as follows: 70% A for 5 min, followed by a linear decrease to 37.1% A at 16.5 min; 2% A at 17.5 min; 2% A at 27.5 min; 70% A at 30 min. The injection volume of all standards and samples was 5 uL, and the flow rate was 125 uL min−1. Nitrogen was used as the curtain gas (10 psi) and collision gas, and purified air was used as the source gas. The declustering potential was set at 30V and the collision energy at 40V. Quantitative analyses of DDA were monitored as selected ion reaction m/z pair 326/186.
2.3. Statistics
Time effect was normalized to the standard 20-day reproductive intervals in the mouse (total of 180 days). The first 6 days were discarded to control for initial differences in estrus cycle stage and to ensure that each dam was given sufficient time to enter estrus. Pup number and food consumption were measured cumulatively, as is typical of long-term toxicological studies [29]. Normal probability plots showed that cumulative pup numbers, cumulative amount of feed consumed, male mouse weight, and average weight per pup followed a normal distribution. Time to first litter (defined as the time between initial animal pairing/dosing and the appearance of pups) and average number of pregnancies were skewed. Analysis of the average number of pregnancies was performed over the first 100 days prior to any dam loss (n=10). Effects of ADBAC+DDAC treatment on cumulative pup numbers, cumulative amount of feed consumed, male mouse weight, and average weight per pup were assessed using repeated measures analysis of variance (RM-ANOVA). The linear model specified treatment, 20-day intervals, and the interaction between treatment and 20-day intervals as fixed effects with Kenward-Roger as denominator degrees of freedom. The model also specified that the measurements were repeated over dam identification within treatment with an autoregressive order one (AR1) covariance structure. To specifically examine the effect of treatment at each 20-day interval, the slicediff option of the glimmix procedure was applied followed by Tukey’s procedure for multiple comparisons. Effects of treatment on total number of pups were assessed using one-way analysis of variance (ANOVA) followed by Tukey’s procedure for multiple comparisons. Effects of treatment on time to first litter, average number of pregnancies, and slopes were assessed using the Kruskal-Wallis test (KW) followed by Dunn’s procedure for multiple comparisons. Residual plots for the ANOVA models were inspected to verify model adequacy (i.e., errors followed a normal distribution with constant variance). Statistical significance was set to alpha < 0.05. All analyses were performed using SAS version 9.2 (Cary, NC, USA).
3. RESULTS
3.1. Preliminary evidence implicated commercial QAC disinfectant mixture as cause of reproductive toxicity
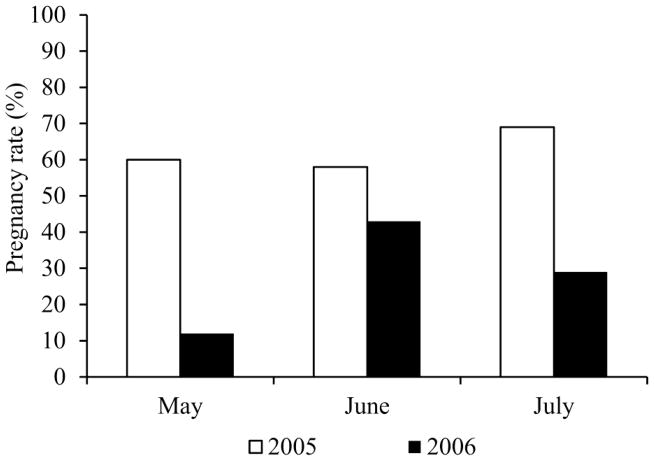
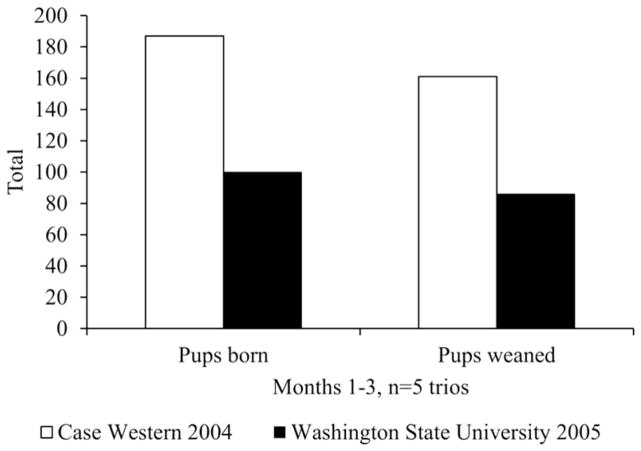
In 2005, the Hunt laboratory relocated to WSU after 14 years at CWRU. The first experimental study at the new institution required timed pregnancies to obtain late gestation fetuses at specified developmental stages. Although mating performance appeared normal during the first four months of the study, only 5 of 46 (10.9%) mated females became pregnant. This was significantly below the 60–70% pregnancy rate expected based on past experience with this strain (Figure 1). In addition, increased rates of dystocia (prolonged or difficult delivery) were noted in the C57BL/6 breeding colony. As shown in Figure 2, a comparison of breeding performance for comparable breeding cages during the same three-month period suggested a significant reduction in productivity in the new vivarium. A variety of environmental variables, including diet, light, and temperature can affect rodent reproductive performance [30]. To improve breeding, environmental variables were systematically modified to replicate as closely as possible the conditions in the previous facility (e.g., changing mouse feed, adjusting light and ambient temperature, and restricting entry and animal handling). When none of these changes eliminated the problems, WSU researchers began to suspect other environmental effects. Three of the five investigators housing animals in the vivarium were conducting reproductive toxicology studies using a variety of chemical compounds, raising concern that the reproductive abnormalities in WSU animals were the result of inadvertent chemical contamination.
Figure 1. Comparison of pregnancy rates for timed matings.
The pregnancy rate for timed matings of C57BL/6 females at CWRU (white bars) and at WSU (black bars). The data represent the pregnancy outcomes during a three-month period of 59 females mated at CWRU in the year preceding the move and 87 females mated at WSU the following year.
Figure 2. Breeding colony productivity.
Total pups born and weaned in the C57BL/6 breeding colony at CWRU (white bars) and at WSU (black bars). The data represent productivity for 5 trio mating’s during the same 3-month period at CWRU in the year preceding the relocation of the laboratory and the following year in the new vivarium at WSU.
3.2. WSU caging material analysis identified QAC contamination
To determine if reproductive abnormalities in WSU animals were the result of inadvertent chemical contamination, caging materials were analyzed using gas chromatograph-mass spectrometry (GC-MS). The inside walls of micro-isolator cages were rinsed with methanol (JT Baker, Phillipsburg, NJ), an effective solvent for organic compounds, and the resultant extract was analyzed via GC-MS. This technique allowed for the detection and potential identification of individual chemicals in a complex mixture. Analysis of the cage extracts revealed a wide array of chemical compounds and, after accounting for components from food and bedding materials, a number of unidentified compounds remained. None of the chemical compounds matched the profiles of the chemicals being used in toxicology studies at WSU; however, a common chemical signature in all extraction experiments was a component of the QAC disinfectant used for sanitation and disinfection in the vivarium.
The QAC disinfectant was suspected as the source of the husbandry issues when a pinworm outbreak elsewhere in the facility required extensive cleaning and fogging of animal rooms with the disinfectant to contain the outbreak. This event coincided with a noticeable reduction in pregnancy rate in the mouse colony. In March 2006, the QAC disinfectant was removed from the facility and replaced with a disinfectant containing sodium chlorite. Levels of QACs were again analyzed in caging materials. To increase precision and accuracy of cage extract analysis, liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used for detection and quantification of a specific ion. As shown in Figure 3, the levels of QACs detected in caging materials uniformly decreased over a period of several months, corresponding with increased breeding performance.
Figure 3. Results of LC-MS/MS cage extract analysis.
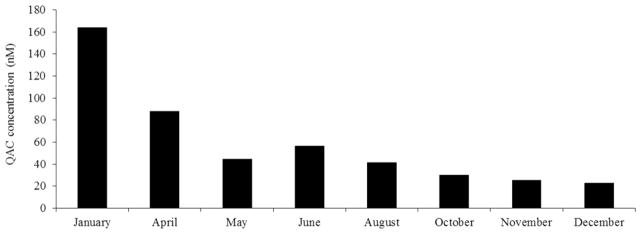
Bars represent QAC concentration in nMs that was detected in cage extracts. Levels of QACs detected in caging materials uniformly decreased over a period of several months.
3.3. Exposure experiments at WSU were impeded by QAC contamination
To verify the effect of QAC exposure in a controlled experiment, several breeding cages from WSU were separated from the original colony. These animals were exposed to aerosolized QAC disinfectant three times weekly while an equal number of control breeders were exposed to water aerosolized in the same manner. Breeding performance, dystocia, and gestational day 21 pup weights were monitored. Cages housing QAC-exposed animals were washed and autoclaved separately and washing was followed by an additional purge cycle to remove contaminated water from the cage washer. Within weeks of initiating the experiment, levels of QACs detected during random monitoring of caging materials increased, suggesting that the limited use of QAC in the exposure study was sufficient to introduce generalized contamination. At this stage, the QAC-exposure study was terminated and all QAC-containing compounds were removed from the facility. Although the inability to obtain control data precluded publication of the findings, these experiences were published in interview format in Nature [28].
3.4. QAC-induced reproductive decline at VPI provided the impetus for further studies
In 2008, the Hrubec laboratory at VPI began to notice a decline in breeding performance in their mouse colony. Around this time, staff began using an ADBAC+DDAC containing disinfectant to clean the floors, walls, and equipment in the animal facility. Additionally, rodent handling procedures often required wetting gloves with disinfectant before touching the animals. The liberal application of disinfectant in the rodent facility suggested it might be responsible for the decline in reproduction. An interview with Dr. Hunt that was published in Nature related exposure to the same QAC disinfectant compounds to reproductive defects in mice. In accordance with Dr. Hunt’s observations, VPI colony productivity improved within several generations after discontinuing use of QAC disinfectants in the facility. To directly test the effects of ADBAC+DDAC disinfectants on reproduction, a controlled breeding study was conducted. In the short-term dose finding study, unbred mice were dosed at 0, 60, 120, 240, and 480 mg ADBAC+DDAC/kg/day for two weeks. Mice in the 240 and 480 mg doses mice became inappetent, lethargic, and developed a rough haircoat. The LOAEL was identified as 240 mg/kg/day and two dose levels below this (i.e., 60 and 120 mg/kg/day) were selected to evaluate the long-term reproductive effects of exposure to QACs. Three groups of breeding pairs were provided 0, 60 or 120 mg ADBAC+DDAC disinfectant/kg/day in a gel food for 180 days. In order to avoid QAC contamination issues experienced by the Hunt lab, mice were housed in disposable caging at VPI and control mice were reared in a separate “QAC- free” room.
3.5. Daily QAC treatment in mice did not affect food consumption but significantly reduced survival
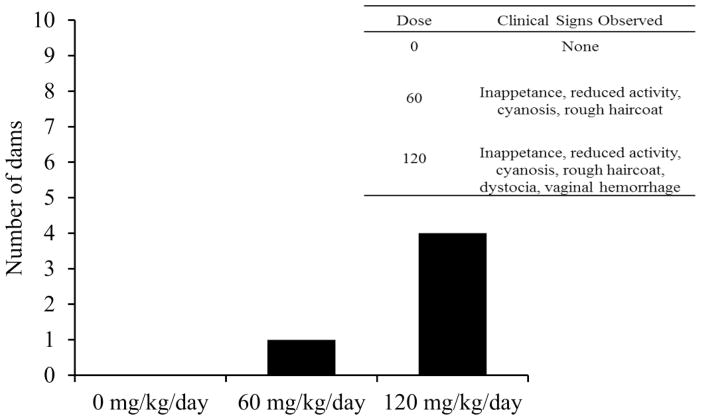
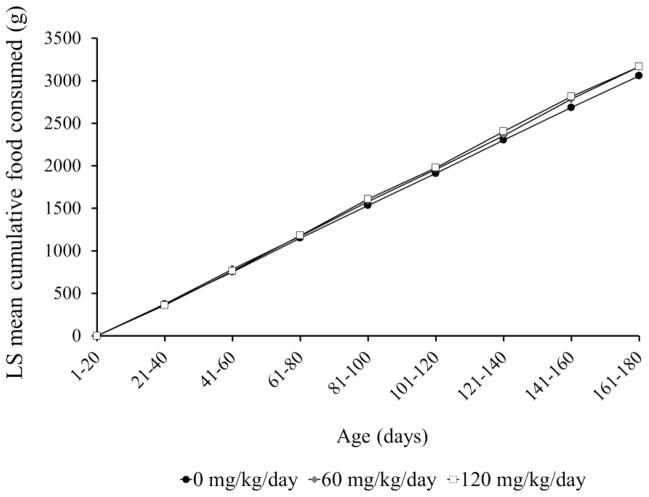
Mice were monitored daily and evaluated against 16 different health parameters for physical appearance, activity, physiology, and body weight loss. Clinical signs initiating euthanasia included: partial closure of eyelids (squinting), significantly reduced activity, ataxia, kyphosis, hypothermia, rapid breathing and dyspnea, and cyanosis. All males and females receiving 0 mg ADBAC+DDAC/kg/day appeared normal and healthy throughout the duration of the study. Clinical signs necessitating euthanasia of pregnant dams were observed in the 60 and 120 dose groups; 1 of the 10 females in the 60 mg/kg/day and 4 of the 10 females in the 120 mg/kg/day dose groups required euthanasia (Figure 4). Clinical signs were only observed in late pregnancy or during delivery and included inappetence, lethargy, ataxia, kyphosis, labored breathing, cyanosis, vaginal hemorrhage, and dystocia. Differences in least squares means for food consumption among treatment groups were not significantly different, indicating that animals were receiving the proper dose of ADBAC+DDAC (RM-ANOVA, p>0.05; Figure 5). Male mouse weights did not differ between groups (data not shown, ANOVA, p=0.81).
Figure 4. Number of females in each ADBAC+DDAC dose group exhibiting clinical signs necessitating euthanasia.
The 120 mg/kg/day dose group experienced the highest female morbidity rate with 4 dams out of 10 euthanized, while in the lower 60 mg/kg/day dose 1 dam of 10 was euthanized. No morbidity was observed in the 0 mg/kg/day group, or in any of the dosed males.
Figure 5. Least squares mean cumulative food intake among in exposed and control mice.
Three ADBAC+DDAC disinfectant dose groups of 10 co-housed male and female pairs were provided 25g of dosed gel food daily. Food weights were taken daily and cumulated for each interval. No significant differences were observed in food consumption means among treatment groups (RM-ANOVA, p>0.05).
3.6. Daily QAC exposure significantly reduced fertility
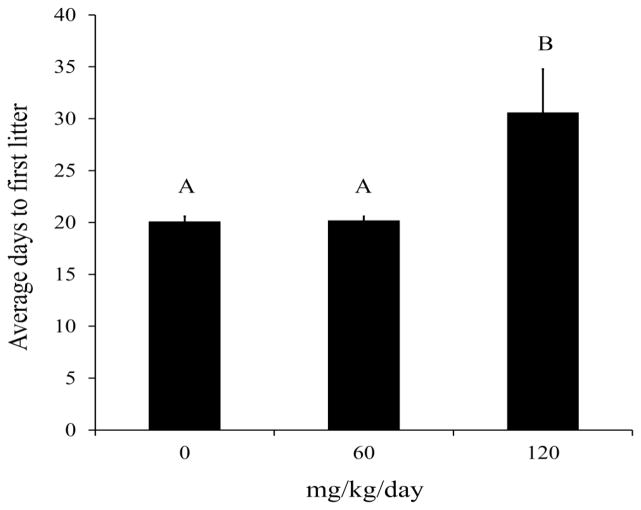
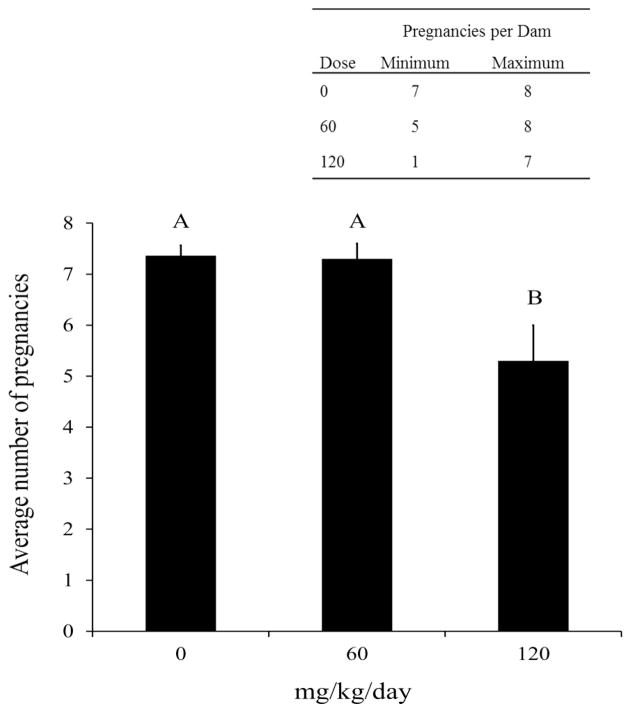
Fertility was assessed by measuring time to first litter and the total number of litters per dam for each treatment group over the duration of during the 180 day exposure period. Time to first litter represents the time between the initial pairing and dosing of mice to the appearance of pups. This metric incorporates time to pregnancy and also gestation length and can be affected by either factor. Time to first litter was significantly longer in breeding pairs exposed to 120 mg ADBAC+DDAC disinfectant/kg/day compared to the 0 and 60 treatment groups (p=0.01; Figure 6). First litters were delivered after an average of 20 days of treatment in the 0 and 60 mg/kg/day dose groups, while the 120 dose group delivered after an average of 31 days. Not surprisingly, this contributed to a significant reduction in the average number of pregnancies in the 120 mg/kg/day dose group by comparison with the 0 and 60 treatment groups (p=0.005; Figure 7).
Figure 6. The effect of ADBAC+DDAC disinfectant on time to first litter.
Time to first litter includes both time to pregnancy and gestation length and reflects changes in either. Dams in the 120 mg/kg/day treatment group took significantly longer to deliver their first litter compared to 0 (p=0.01) and 60 mg/kg/day (p=0.01) treatment groups (KW followed by Dunn’s procedure for multiple comparisons, p≤0.05).
Figure 7. Average number of pregnancies per dam in the first 100 days of exposure before any dam loss.
Significantly fewer pregnancies per dam were observed in the 120 mg/kg/day group (p=0.005) compared to 0 and 60 mg/kg/day dose groups (n=10 for all dose groups; KW followed by Dunn’s procedure for multiple comparisons, p≤0.05).
3.7. Daily QAC exposure significantly reduced fecundity
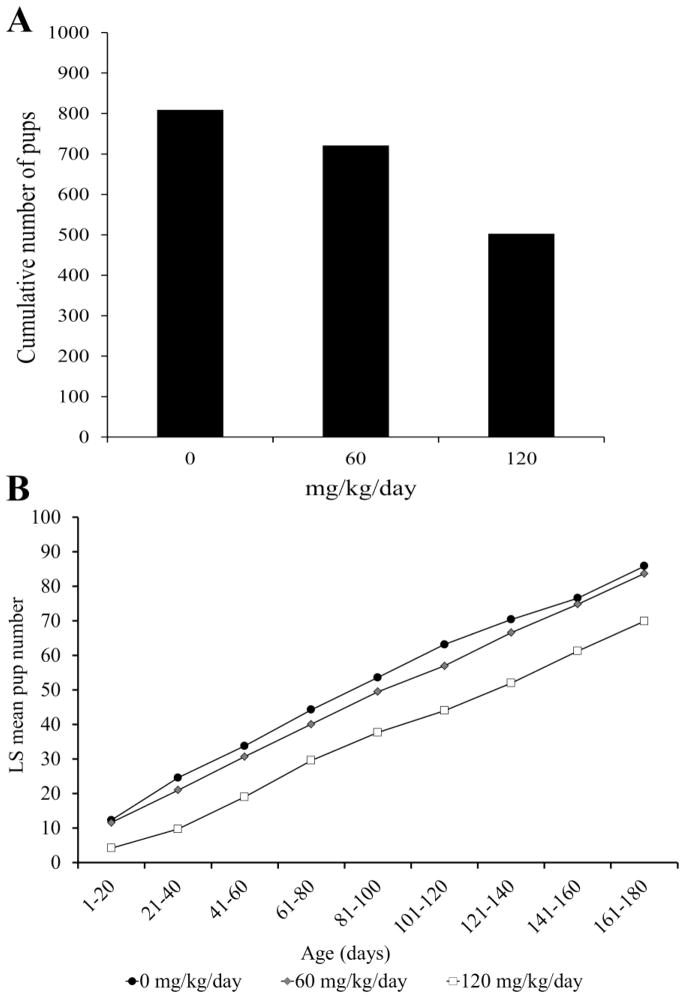
Dam morbidity was a confounding factor that precluded a simple comparison of total pups produced between treatment groups during the 180 day exposure period. An analysis of cumulative pup numbers during the first 100 days of the study prior to loss of any breeding females, demonstrated significantly reduced cumulative pup numbers in the 120 mg/kg/day exposure group compared to 0 and 60 (Figure 8a, RM-ANOVA, p≤0.05). An analysis of pups produced during the entire 180 day treatment using least squares mean estimates to account for dam morbidity, demonstrated a similar reduction of pups in the 120 mg/kg/day group in comparison to 0 and 60 dose groups (p=0.0002 and p=0.004, respectively; Figure 8b, RM-ANOVA, p≤0.05). Starting at days 21–40, significantly fewer pups were delivered in the 120 mg group compared to the 0 and 60 dose groups. Although the number of pups was consistently lower in the 60 dose group than in controls, the difference was not significant during any point of the 180 day exposure period. Finally, a comparison of pup weights at birth revealed no obvious treatment-related pattern, and apparent differences (e.g., during days 1–20 and 41–60 between 0 and 120 mg/kg/day dose groups, p=0.04 and 0.02, respectively; Figure 9) are likely a reflection of other factors such as litter size.
Figure 8. The effects of ADBAC+DDAC disinfectant on fecundity of breeding pairs.
A. Cumulative number of pups born per treatment group in the first 100 days of exposure prior to any dam loss. Cumulative pup number was significantly lower in the 120 exposure group even before dam morbidity occurred (RM-ANOVA, p≤0.05). B. Cumulative least squares means estimate of pup number over the full 180 days. Least squares means were calculated to account for dam morbidity and approximate cumulative pup number over 180 days of treatment. Significantly fewer pups overall were delivered by dams in the 120 group compared to 0 (p=0.002) and 60 mg/kg/day (p=0.008) treatment groups (RM-ANOVA, p≤0.05).
Figure 9. Average weight per pup (± SEM) among breeding pairs exposed to ADBAC+DDAC disinfectant for 180-days.
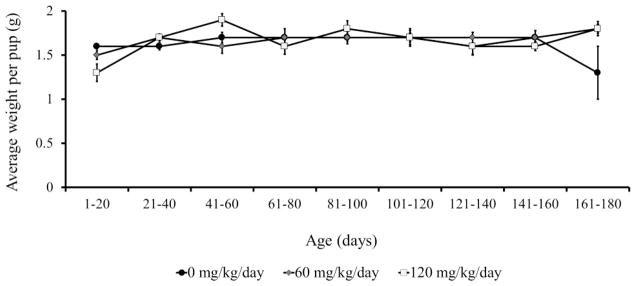
Average weight per pup was significantly different during days 1–20 and days 41–60 between 0 and 120 mg/kg/day dose groups, p=0.04 and 0.02, respectively (RM-ANOVA, p≤0.05). No pattern of significance was observed.
4. DISCUSSION
4.1. Impetus and experimental design to test QAC reproductive toxicity
The 1976 Toxic Substances Control Act (TSCA) created a system to oversee the production and use of chemicals in the United States. The original act, however, has never been amended and does not mandate safety evaluations for all chemicals in active commerce. Consequently, it is possible for chemicals to become widely used in the U.S., despite the fact that their potential reproductive effects have not been directly assessed. QAC disinfectants are one such class of chemicals. As disinfectants, they are extremely effective and have several highly desirable attributes: they are odorless, leave no visible residue, and are non-corrosive. As a result, their use and application has grown beyond simple disinfectants and they have become additives in a wide range of personal care products, including shampoos, cosmetics, and even baby wipes. The only studies of their biological impact have been those conducted by the manufacturers, with the only adverse effect reported being a slight reduction in weight in exposed offspring [21–23].
The independent realization that the introduction of QAC-containing disinfectants coincided with noticeable declines in fertility in both WSU and VPI mouse breeder colonies prompted the present studies. The first episode correlated with the relocation of the Hunt laboratory to WSU. Although all evidence suggested that the QAC disinfectant was to blame for poor pregnancy rates and increased dystocia due to late fetal demise, efforts to provide experimental evidence were thwarted when a small controlled study resulted in generalized QAC contamination throughout the facility. Nevertheless, the WSU experience provided important preliminary data that was briefly discussed in a published interview [28]. Specifically, data from random cage monitoring during QAC use and in the months after use of QAC disinfectants was discontinued, provided evidence that these chemicals persist in the environment (Figure 3). Further, despite careful care and washing of caging materials during an attempted controlled study, the reintroduction of QAC contamination suggested that QACs can be easily spread.
Several years later, the Hrubec laboratory at VPI encountered similar breeding problems suggesting the possibility of an environmental exposure. Because reproductive changes appeared to coincide with the introduction of the QAC disinfectant, attempts to investigate the relationship between QACs and fertility led to the article describing the experience in the Hunt laboratory [28]. Subsequently, the decision to directly test the effects of QACs on reproduction was made. A controlled breeding study was designed and conducted, using disposable cages and oral dosing via food (vs. inhalation) to reduce the possibility of cross contamination. The present results provide compelling evidence that QACs adversely affect rodent fertility and fecundity.
Although preliminary findings from both laboratories suggested that QAC exposure adversely affects reproduction, they did not provide insight to the route of exposure. That is, cage contamination could result in transdermal exposure from contaminated bedding, oral exposure from consumption of contaminated food and water, or even respiratory exposure from aerosolization of the disinfectant used during animal handling and cage change. Because oral dosing via food provided a simple exposure method with little risk of cross contamination, we chose this exposure route for our initial experimental studies. The doses elected were based on toxicity data from a preliminary dose-finding study (see methodology). Mice in the QAC disinfectant room potentially received additional exposure due to routine husbandry practices. The disinfectant was reconstituted to the manufacturer’s suggested concentration and used to disinfect walls, floors, animal racks and caging material; footwear and equipment were also disinfected upon entry and exit of the mouse room. Unfortunately, the influence of this additional exposure cannot be estimated; disinfectant use could potentially have exposed the mice via inhalation, dermal and oral routes. Specific toxicity from the different routes of exposure is not known and likely varied with the amount of disinfectant applied by individual animal care staff. The ambient exposure, however, would have applied equally to both 60 and 120 mg/kg/day dose groups. The significant decrease in productivity in the highest exposure group and increased dam loss in both the high and low dose groups confirmed the preliminary fertility findings from both laboratories. Importantly, because no differences in food consumption or male body weight were evident among groups, these differences in reproductive endpoints cannot be ascribed to nutritional deficits [32].
4.2. QACs decrease murine fertility and fecundity
Even after accounting for maternal morbidity, dams exposed to 120 mg ADBAC+DDAC disinfectant/kg/day produced significantly fewer offspring than dams in the 0 and 60 dose groups. Further, in addition to producing fewer pups per litter (an average of 8/litter for females receiving the 120 treatment compared to 10–12/litter for the 0 and 60 dose groups), high dose females had fewer pregnancies and longer pregnancy intervals; thus, in addition to confirming the preliminary findings from both laboratories, these results provide the first experimental evidence that QAC exposure decreases fecundity in mice.
4.3. QAC exposure increases dam mortality
Although the doses were chosen because they did not elicit signs of toxicity in the initial study, exposure to ADBAC+DDAC disinfectant resulted in an increase in late gestation maternal morbidity in both the 60 and 120 exposure groups. No adverse clinical signs or morbidity were observed in control females or males of any dose group. Both late term abortion and dystocias were observed in dosed females and contributed to dam morbidity. Similarly, late fetal demise and dystocia were observed previously with QAC exposure in the Hunt laboratory (data not shown). Because the endpoint for these studies was live-birth, it could not be determined if fetal death always preceded onset of maternal delivery; therefore, it is not clear from these studies if the maternal distress that necessitated euthanasia was due to maternal or fetal toxicity.
Although these results clearly demonstrate that exposure to a common QAC mixture affects reproduction in the laboratory mouse, the design of this study does not allow the distinction between toxic effects on the dam, sire, fetus or a combination of effects to be made. Nevertheless, consistent with the preliminary findings from both laboratories, the results of prospective studies suggest that QACs affect both the maternal ability to achieve and sustain pregnancy and the developing fetus. An essential next step is to establish dose response relationships, determine the effects of different routes of exposure, and define critical development windows during which exposure elicits effects.
Although QACs are commonly used in combination to enhance bactericidal efficiency of cleaning products, to date, risk assessments have only tested the effects of QACs individually [33]. Indeed, by themselves, ADBAC and DDAC, the two QACs that make up HWS-256, are considered safe [34]. For this reason, it remains possible that these findings are the result of synergistic effects that are greater than the effects of the individual components. In future studies, assessing the effects of individual QACs as well as QAC mixtures will be essential.
5. CONCLUSIONS
Lastly, although QACs are used in a wide variety of consumer products, current levels of human exposure remain unknown. The relevance of these findings to humans remains to be determined; however, given their widespread use and persistence in the environment, determining the levels of human exposure and whether QACs affect human reproduction is critically important. Indeed, the extensive use of QACs in industrial and clinical settings suggests that some individuals are likely chronically exposed to levels that are similar to those that elicited the preliminary effects in the laboratory rodents. Consequently, epidemiological studies of individuals in specific work environments where QAC disinfects are heavily used (e.g., custodial workers and individuals in clinical settings) may be particularly informative.
Research Highlights.
Tested the reproductive toxicity of a common disinfectant used in many commercial and residential products
Disinfectant contained the active ingredients: alkyl dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC)
Mouse breeding pairs were dosed with ADBAC+DDAC at 0, 60 or 120 mg/kg/day in the feed for 6 months
Long term exposure decreased fertility and fecundity and caused dam mortality in a dose dependent manner
This study highlights the importance of testing the toxicity of mixtures over individual compounds
Acknowledgments
The authors gratefully acknowledge the technical support of Jodi Jackson. These studies were supported by NIH grant R01 HD37502 (PH) and the Passport Foundation (TH).
Abbreviations
- QAC
quaternary ammonium compound
- ADBAC
alkyl dimethyl benzyl ammonium chloride
- DDAC
didecyl dimethyl ammonium chloride
- DDA
dimethyl didecyl ammonium
- HWS-256
disinfectant mixture of alkyl (60% C14, 25% C12, 15% C16) dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC)
- GC-MS
gas chromatography-mass spectrometry
- HPLC
high-performance liquid chromatography
- LC-MS
liquid chromatography-mass spectrometry
- CWRU
Case Western Reserve University
- WSU
Washington State University
- VPI
Virginia Polytechnic Institute and State University
Footnotes
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carson RT, Larson E, Levy SB, Marshall BM, Aiello AE. Use of antibacterial consumer products containing quaternary ammonium compounds and drug resistance in the community. The Journal of antimicrobial chemotherapy. 2008;62:1160–2. doi: 10.1093/jac/dkn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microbial drug resistance. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- 3.Pavlikova-Moricka M, Lacko I, Devinsky F, Masarova L, Milynarcik D. Quantitative relationships between structure and antimicrobial activity of new “soft” bisquaternary ammonium salts. Folia microbiologica. 1994;39:176–80. doi: 10.1007/BF02814644. [DOI] [PubMed] [Google Scholar]
- 4.Ahlstrom B, Thompson RA, Edebo L. The effect of hydrocarbon chain length, pH, and temperature on the binding and bactericidal effect of amphiphilic betaine esters on Salmonella typhimurium. APMIS. 1999;107:318–24. doi: 10.1111/j.1699-0463.1999.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert P, McBain AJ. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clinical microbiology reviews. 2003;16:189–208. doi: 10.1128/CMR.16.2.189-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrington JK, Martz EL, Wells SJ, Ennis CC, Holder J, Levchuk JW, et al. Ability of laboratory methods to predict in-use efficacy of antimicrobial preservatives in an experimental cosmetic. Applied and environmental microbiology. 1994;60:4553–8. doi: 10.1128/aem.60.12.4553-4558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schorr WF. Cosmetic allergy. A comprehensive study of the many groups of chemical antimicrobial agents. Archives of dermatology. 1971;104:459–66. doi: 10.1001/archderm.104.5.459. [DOI] [PubMed] [Google Scholar]
- 8.Gao T. Evaluation of hair humidity resistance/moisturization from hair elasticity. Journal of cosmetic science. 2007;58:393–404. [PubMed] [Google Scholar]
- 9.Blenman TV, Morrison KL, Tsau GJ, Medina AL, Gerlach RW. Practice implications with an alcohol-free, 0.07% cetylpyridinium chloride mouthrinse. American journal of dentistry. 2005;18(Spec No):29A–34A. [PubMed] [Google Scholar]
- 10.Loretz LJ, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, et al. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43:279–91. doi: 10.1016/j.fct.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Dyer DL, Gerenraich KB, Wadhams PS. Testing a new alcohol-free hand sanitizer to combat infection. AORN journal. 1998;68:239–41. 43–4, 47–51. doi: 10.1016/s0001-2092(06)62517-9. [DOI] [PubMed] [Google Scholar]
- 12.White CG, Shinder FS, Shinder AL, Dyer DL. Reduction of illness absenteeism in elementary schools using an alcohol-free instant hand sanitizer. The Journal of school nursing: the official publication of the National Association of School Nurses. 2001;17:258–65. [PubMed] [Google Scholar]
- 13.Compton FH, Beagrie GS. Inhibitory effect of benzethonium and zinc chloride mouthrinses on human dental plaque and gingivitis. Journal of clinical periodontology. 1975;2:33–43. doi: 10.1111/j.1600-051x.1975.tb01724.x. [DOI] [PubMed] [Google Scholar]
- 14.Hallen H, Graf P. Benzalkonium chloride in nasal decongestive sprays has a long-lasting adverse effect on the nasal mucosa of healthy volunteers. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 1995;25:401–5. doi: 10.1111/j.1365-2222.1995.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 15.de Groot ACJWW, Nater JP. Unwanted Effects of Cosmetics and Drugs used in Dermatology. 3. Elsevier Science B.V; 1993. [Google Scholar]
- 16.Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Progress in retinal and eye research. 2010;29:312–34. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Flyvholm MA. Preservatives in registered chemical products. Contact dermatitis. 2005;53:27–32. doi: 10.1111/j.0105-1873.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 18.Lebe E, Baka M, Yavasoglu A, Aktug H, Ates U, Uyanikgil Y. Effects of preservatives in nasal formulations on the mucosal integrity: an electron microscopic study. Pharmacology. 2004;72:113–20. doi: 10.1159/000080183. [DOI] [PubMed] [Google Scholar]
- 19.Kamysz W, Turecka K. Antimicrobial preservative effectiveness of natural peptide antibiotics. Acta poloniae pharmaceutica. 2005;62:341–4. [PubMed] [Google Scholar]
- 20.Muszynski Z, Dlugaszewska J. Antimicrobial conservation of some cosmetics. Medycyna doswiadczalna i mikrobiologia. 1994;46:87–90. [PubMed] [Google Scholar]
- 21.Gill MW, Hermansky SJ, Wagner CL. Chronic Dietary Oncogenicity Study with Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) in Mice Unpublished report number 53-515. Bushy Run Research Center; Export, PA, U. S: 1991. [Google Scholar]
- 22.Weaver EWaJPVM. Unpublished report number 51-514. Bushy Run Research Center; Export, PA, U. S: 1988. Two-Week Dose Range Finding Screen with Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) in Mice. [Google Scholar]
- 23.Van Miller JPaEVW. Unpublished report number 51-504. Bushy Run Research Center; Export, PA, U. S: 1988. Ninety-Day Dose Range-Finding Study with Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) in Mice. [Google Scholar]
- 24.Hermens J, Leeuwangh P, Musch A. Joint toxicity of mixtures of groups of organic aquatic pollutants to the guppy (Poecilia reticulata) Ecotoxicology and environmental safety. 1985;9:321–6. doi: 10.1016/0147-6513(85)90049-1. [DOI] [PubMed] [Google Scholar]
- 25.Faust M, Altenburger R, Backhaus T, Blanck H, Boedeker W, Gramatica P, et al. Joint algal toxicity of 16 dissimilarly acting chemicals is predictable by the concept of independent action. Aquatic toxicology. 2003;63:43–63. doi: 10.1016/s0166-445x(02)00133-9. [DOI] [PubMed] [Google Scholar]
- 26.Narotsky MG, Best DS, McDonald A, Godin EA, Hunter ES, 3rd, Simmons JE. Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids. Reproductive toxicology. 2011;31:59–65. doi: 10.1016/j.reprotox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Lin GH, Hemming M. Ocular and dermal irritation studies of some quaternary ammonium compounds. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1996;34:177–82. doi: 10.1016/0278-6915(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 28.Hunt P. Lab disinfectant harms mouse fertility. Patricia Hunt interviewed by Brendan Maher. Nature. 2008;453:964. doi: 10.1038/453964a. [DOI] [PubMed] [Google Scholar]
- 29.Tennant DR. Estimating acute dietary intakes of chemicals. Regulatory toxicology and pharmacology: RTP. 1999;30:S99–102. doi: 10.1006/rtph.1999.1333. [DOI] [PubMed] [Google Scholar]
- 30.Murray KA, Parker NJ. Breeding genetically modified rodents: tips for tracking and troubleshooting reproductive performance. Lab animal. 2005;34:36–41. doi: 10.1038/laban0405-36. [DOI] [PubMed] [Google Scholar]
- 31.EPA US. Reregistration Eligibility Decision for Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) 2006. [Google Scholar]
- 32.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. American journal of physiology Regulatory, integrative and comparative physiology. 2004;287:R1277–96. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 33.Jeffrey DJ. Chemicals used as disinfectants: active ingredients and enhancing additives. Revue scientifique et technique. 1995;14:57–74. doi: 10.20506/rst.14.1.828. [DOI] [PubMed] [Google Scholar]
- 34.EPA US. Reregistration Eligibility Decision for Aliphatic Alkyl Quaternaries (DDAC) 2006. [Google Scholar]