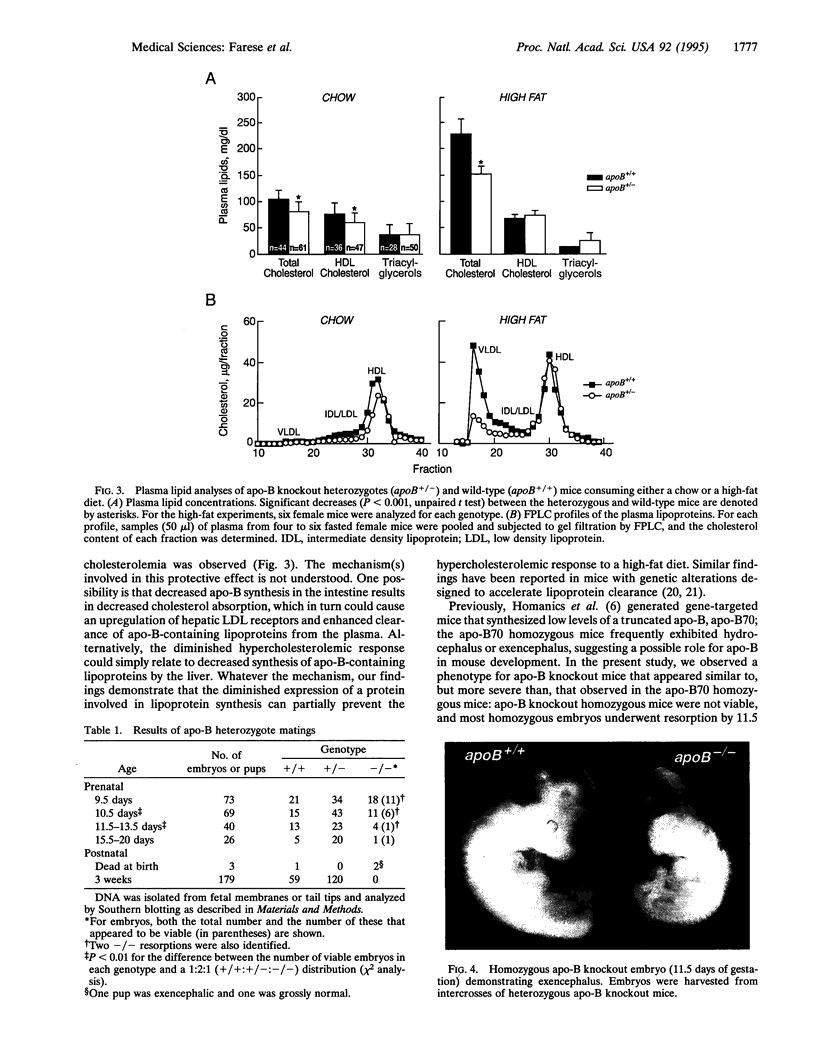
Abstract
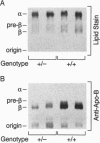
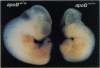
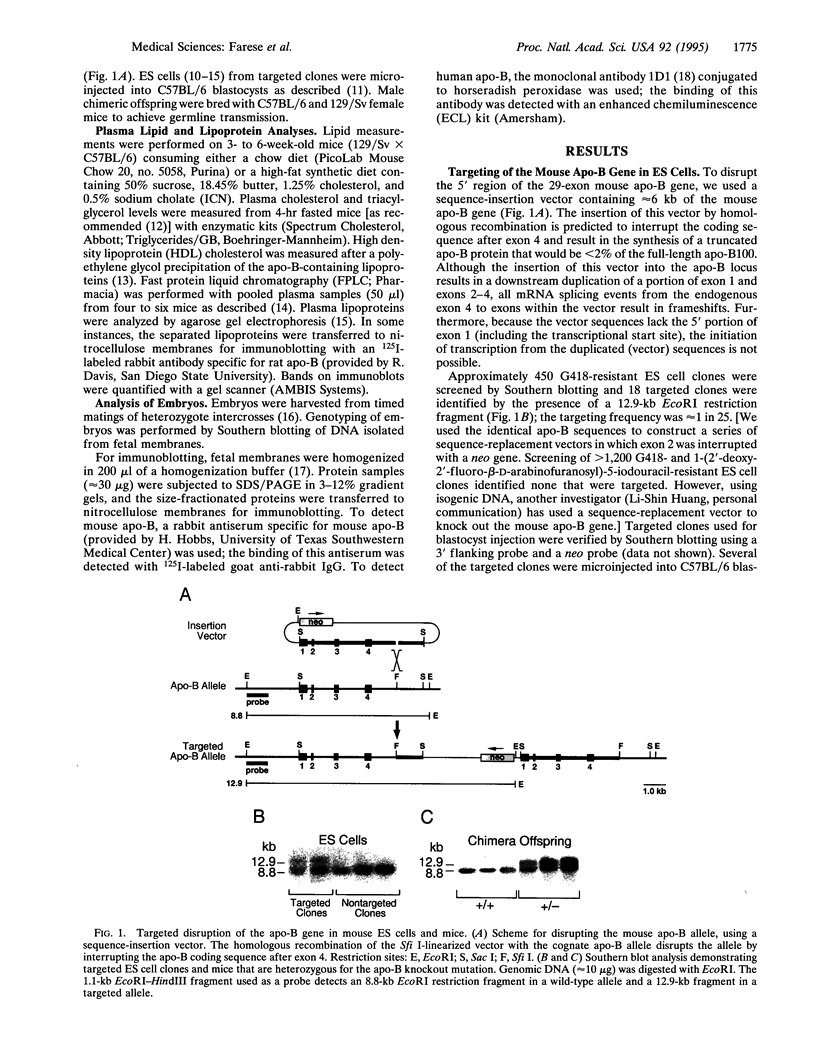
Apolipoprotein B is synthesized by the intestine and the liver in mammals, where it serves as the main structural component in the formation of chylomicrons and very low density lipoproteins, respectively. Apolipoprotein B is also expressed in mammalian fetal membranes. To examine the consequences of apolipoprotein B deficiency in mice, we used gene targeting in mouse embryonic stem cells to generate mice containing an insertional disruption of the 5' region of the apolipoprotein B gene. Mice that were heterozygous for the disrupted apolipoprotein B allele had an approximately 20% reduction in plasma cholesterol levels, markedly reduced plasma concentrations of the pre-beta and beta-migrating lipoproteins, and an approximately 70% reduction in plasma apolipoprotein B levels. When fed a diet rich in fat and cholesterol, heterozygous mice were protected from diet-induced hypercholesterolemia; these mice, which constitute an animal model for hypobetalipoproteinemia, should be useful for studying the effects of decreased apolipoprotein B expression on atherogenesis. The breeding of heterozygous mice yielded no viable homozygous apolipoprotein B knockout mice. Most homozygous embryos were resorbed by midgestation (before gestational day 11.5); several embryos that survived until later in gestation exhibited exencephalus. The embryonic lethal phenotype was rescued by complementation with a human apolipoprotein B transgene--i.e., human apolipoprotein B transgenic mice that were homozygous for the murine apolipoprotein B knockout mutation were viable. Our findings indicate that apolipoprotein B plays an essential role in mouse embryonic development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHENG D. W., CHANG L. F., BAIRNSON T. A. Gross observations on developing abnormal embryos induced by maternal vitamin E deficiency. Anat Rec. 1957 Oct;129(2):167–185. doi: 10.1002/ar.1091290204. [DOI] [PubMed] [Google Scholar]
- Castle C. K., Colca J. R., Melchior G. W. Lipoprotein profile characterization of the KKA(y) mouse, a rodent model of type II diabetes, before and after treatment with the insulin-sensitizing agent pioglitazone. Arterioscler Thromb. 1993 Feb;13(2):302–309. doi: 10.1161/01.atv.13.2.302. [DOI] [PubMed] [Google Scholar]
- Chiesa G., Hobbs H. H., Koschinsky M. L., Lawn R. M., Maika S. D., Hammer R. E. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a). J Biol Chem. 1992 Dec 5;267(34):24369–24374. [PubMed] [Google Scholar]
- Demmer L. A., Levin M. S., Elovson J., Reuben M. A., Lusis A. J., Gordon J. I. Tissue-specific expression and developmental regulation of the rat apolipoprotein B gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8102–8106. doi: 10.1073/pnas.83.21.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio S., Lee Y. L., Ji Z. S., Rall S. C., Jr Type III hyperlipoproteinemic phenotype in transgenic mice expressing dysfunctional apolipoprotein E. J Clin Invest. 1993 Sep;92(3):1497–1503. doi: 10.1172/JCI116728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeve J., Altkemper I., Dieterich J. H., Greten H., Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993 Aug;34(8):1367–1383. [PubMed] [Google Scholar]
- Homanics G. E., Smith T. J., Zhang S. H., Lee D., Young S. G., Maeda N. Targeted modification of the apolipoprotein B gene results in hypobetalipoproteinemia and developmental abnormalities in mice. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2389–2393. doi: 10.1073/pnas.90.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins B., Sharpe C. R., Baralle F. E., Graham C. F. Organ distribution of apolipoprotein gene transcripts in 6-12 week postfertilization human embryos. J Embryol Exp Morphol. 1986 Sep;97:177–187. [PubMed] [Google Scholar]
- Horie Y., Fazio S., Westerlund J. R., Weisgraber K. H., Rall S. C., Jr The functional characteristics of a human apolipoprotein E variant (cysteine at residue 142) may explain its association with dominant expression of type III hyperlipoproteinemia. J Biol Chem. 1992 Jan 25;267(3):1962–1968. [PubMed] [Google Scholar]
- Kayden H. J., Traber M. G. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res. 1993 Mar;34(3):343–358. [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton M. F., Farese R. V., Jr, Young S. G. Familial hypobetalipoproteinemia. J Lipid Res. 1993 Apr;34(4):521–541. [PubMed] [Google Scholar]
- Milne R., Théolis R., Jr, Maurice R., Pease R. J., Weech P. K., Rassart E., Fruchart J. C., Scott J., Marcel Y. L. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J Biol Chem. 1989 Nov 25;264(33):19754–19760. [PubMed] [Google Scholar]
- Ramírez-Solis R., Davis A. C., Bradley A. Gene targeting in embryonic stem cells. Methods Enzymol. 1993;225:855–878. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Katsuki M., Shimada M., Gotoda T., Harada K., Murase T., Fukazawa C., Takaku F., Yazaki Y. Overexpression of apolipoprotein E in transgenic mice: marked reduction in plasma lipoproteins except high density lipoprotein and resistance against diet-induced hypercholesterolemia. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1750–1754. doi: 10.1073/pnas.89.5.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Deng C., Capecchi M. R. High-fidelity gene targeting in embryonic stem cells by using sequence replacement vectors. Mol Cell Biol. 1992 Jul;12(7):2919–2923. doi: 10.1128/mcb.12.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K., Wei King D. Disorders of the developing nervous system of vitamin E-deficient rats. Acta Anat (Basel) 1967;67(4):623–635. doi: 10.1159/000143009. [DOI] [PubMed] [Google Scholar]
- Yokode M., Hammer R. E., Ishibashi S., Brown M. S., Goldstein J. L. Diet-induced hypercholesterolemia in mice: prevention by overexpression of LDL receptors. Science. 1990 Nov 30;250(4985):1273–1275. doi: 10.1126/science.2244210. [DOI] [PubMed] [Google Scholar]
- Young S. G. Recent progress in understanding apolipoprotein B. Circulation. 1990 Nov;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]