Abstract
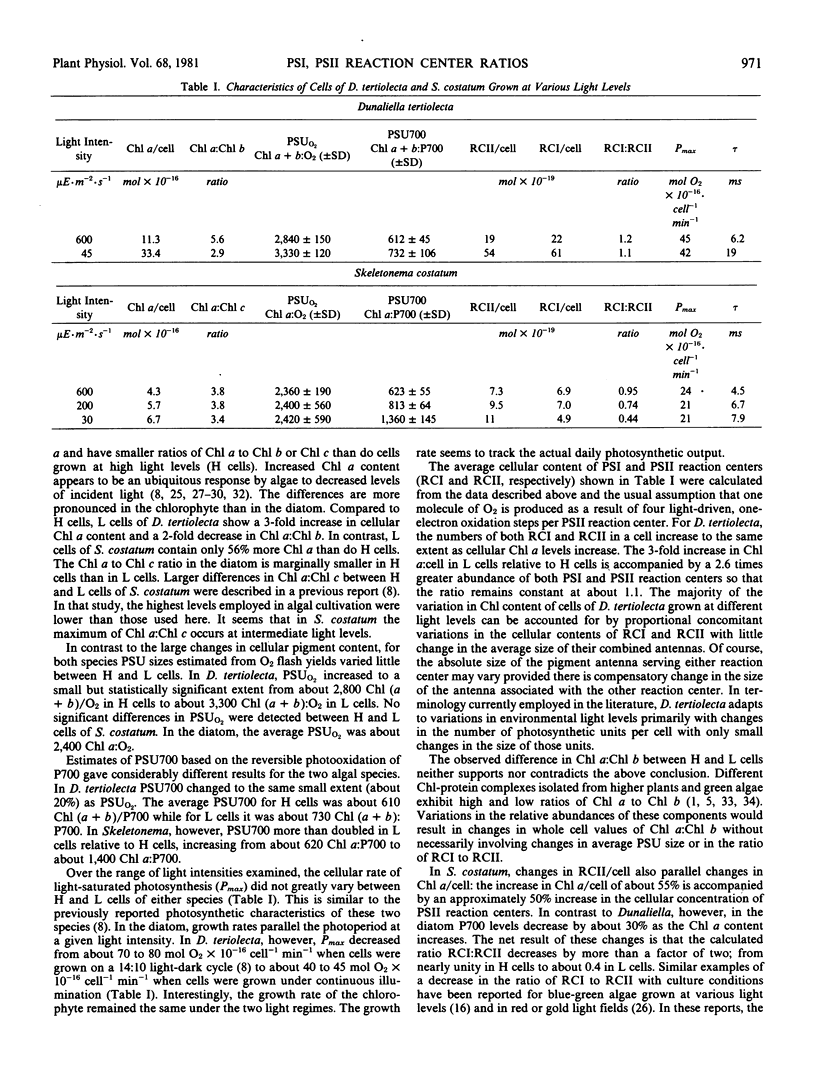
Cells of two species of single-celled marine algae, the diatom Skeletonema costatum (Greve), Cleve, and the chlorophyte Dunaliella tertiolecta Butcher, were cultured in white light of high (500-600 microeinsteins per square meter per second) and low (30 microeinsteins per square meter per second) intensity. For both algal species, cells grown at low light levels contained more chlorophyll a and had a lower ratio of chlorophyll a to chlorophylls b or c than did cells grown at high light levels. When photosynthetic unit sizes were measured on the basis of either oxygen flash yields or P700 photooxidation, different results were obtained with the different species. In the chlorophyte, the cellular content of photosystem I (PSI) and photosystem II (PSII) reaction centers increased in tandem as chlorophyll a content increased so that photosynthetic unit sizes changed only slightly and the ratio PSI:PSII reaction centers remained constant at about 1.1. In the diatom, as the chlorophyll content of the cells increased, the number of PSI reaction centers decreased and the number of PSII reaction centers increased so that the ratio of PSI:PSII reaction centers decreased from about unity to 0.44. In neither organism did photosynthetic capacity correlate with changes in cellular content of PSI or PSII reaction centers. The results are discussed in relationship to the physical and biological significance of the photosynthetic unit concept.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Kohn H. I. THE CHLOROPHYLL UNIT IN PHOTOSYNTHESIS. J Gen Physiol. 1934 Sep 20;18(1):109–112. doi: 10.1085/jgp.18.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B., Mauzerall D. The turnover times of photosynthesis and redox properties of the pool of electron carriers between the photosystems. Biochim Biophys Acta. 1973 May 30;305(2):353–363. doi: 10.1016/0005-2728(73)90181-3. [DOI] [PubMed] [Google Scholar]
- Emerson R., Arnold W. THE PHOTOCHEMICAL REACTION IN PHOTOSYNTHESIS. J Gen Physiol. 1932 Nov 20;16(2):191–205. doi: 10.1085/jgp.16.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Kelly J., Sauer K. Functional photosynthetic unit sizes for each of the two light reactions in spinach chloroplasts. Biochemistry. 1968 Feb;7(2):882–890. doi: 10.1021/bi00842a047. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Thornber J. P., Skrdla M. P. Effect of detergents on the reliability of a chemical assay for P-700. Biochim Biophys Acta. 1980 Jul 8;591(2):391–399. doi: 10.1016/0005-2728(80)90170-x. [DOI] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Mishkind M., Mauzerall D., Beale S. I. Diurnal variation in situ of photosynthetic capacity in ulva is caused by a dark reaction. Plant Physiol. 1979 Nov;64(5):896–899. doi: 10.1104/pp.64.5.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Light Harvesting in Anacystis nidulans Studied in Pigment Mutants. Plant Physiol. 1980 Dec;66(6):1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prézelin B. B., Alberte R. S. Photosynthetic characteristics and organization of chlorophyll in marine dinoflagellates. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1801–1804. doi: 10.1073/pnas.75.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid G. H., Gaffron H. Photosynthetic units. J Gen Physiol. 1968 Aug;52(2):212–239. doi: 10.1085/jgp.52.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Alberte R. S., Hunter F. A., Shiozawa J. A., Kan K. S. The organization of chlorophyll in the plant photosynthetic unit. Brookhaven Symp Biol. 1976 Jun 7;(28):132–148. [PubMed] [Google Scholar]


