Abstract
Background
Chronic alcohol impairs gut barrier function and induces inflammatory cytokines. The effects of acute alcohol binge on the gut are partially understood. Micro-RNA-155 (miR-155), a modulator of cytokine and T-cell immune response in the gut, stabilizes tumor necrosis factor-α (TNFα) mRNA. Here, we investigated the role of the inflammation modulator miR-155 as well as the effects of acute binge and chronic alcohol feeding in the small bowel (SB) in mice.
Methods
For the acute alcohol binge, wild-type (WT) mice received 5 g/kg 50% alcohol/d or equal amount of water oral gavage for 3 days. WT and miR-155-deficient (miR-155-knockout [KO]) mice received ethanol containing Lieber-DeCarli or isocaloric control diet for 5 weeks. MiR-155, antimicrobial peptide, regenerating islet-derived 3-beta (Reg3b), inflammation markers, Src homology 2-containing inositol phosphatase-1 (SHIP1), TNFα, and nuclear factor-κB (NF-κB) were measured in proximal intestinal tissue. Endotoxin was measured in the serum.
Results
Acute alcohol binge enhanced, whereas chronic alcohol feeding decreased, Reg3b mRNA and protein levels in the SB. Both acute binge and chronic alcohol feeding increased serum endotoxin levels, intestinal NF-κB activation and TNFα mRNA levels. However, TNFα protein and miR-155 were increased only after chronic alcohol feeding in the SB. Furthermore, miR-155-KO mice were protected from chronic alcohol-induced increase in serum endotoxin, intestinal TNFα, and NF-κB activation. Also, alcohol-fed miR-155-KO mice had no decrease of Reg3b and SHIP1 levels.
Conclusions
These results demonstrate that both acute binge and chronic ethanol administration result in increased serum-endotoxin levels. Our study identifies a novel role for miR-155 in chronic alcohol-induced intestinal inflammation and barrier dysfunction.
Keywords: Binge Drinking, Ethanol, Proximal Intestine, Tumor Necrosis Factor-α, Gut Barrier
Ethanol impairs gastrointestinal barrier functions via epithelial cell apoptosis, necrosis, and inflammation (Beck and Dinda, 1981; Bhonchal et al., 2008; Bode and Bode, 2003; Kirpich et al., 2013; Tamai et al., 2000). Chronic alcohol consumption causes bacterial overgrowth and impaired epithelial tight junctions in the proximal small bowel (SB) (Hauge et al., 1997), allowing systemic translocation of pathogen- or danger-associated molecular patterns (PAMPs or DAMPs) (Menu and Vince, 2011). PAMPs and DAMPs then activate pro-inflammatory pathways via pattern-recognition receptors (Menu and Vince, 2011). During alcohol ingestion the SB is exposed to high concentrations of alcohol that may affect gut microbiome and barrier functions (Bode and Bode, 2003). Endotoxin is suggestive of impaired gut barrier function and elevated endotoxin levels were observed in animal models of alcoholism indicating increased gut permeability (Mathurin et al., 2000). Moreover, patients with chronic alcohol use have increased serum-endotoxin levels likely related to disruption of the intestinal barrier (Bode et al., 1987).
Antimicrobial peptides, such as regenerating islet-derived 3-beta (Reg3b), are part of the host defense mechanism against pathogens in the gut enhancing the barrier function (Everard et al., 2013). Decreased Reg3g and Reg3b expression has been observed in an animal model of alcoholism in SB and alcoholic patients in the duodenum (Yan et al., 2011), suggesting the decreased ability to eliminate bacteria and therefore contributing to bacterial overgrowth. After the loss of the barrier integrity of the SB, inflammation might occur in the wall of the SB which can contribute to further loss of function. Furthermore, alcoholic patients have duodenal inflammation (Bhonchal et al., 2008). Tumor necrosis factor-α (TNFα) production as well as nuclear factor-κB (NF-κB) activation has been suggested to play a role in the pathogenesis of intestinal inflammatory processes (Atreya et al., 2008; Suenaert et al., 2002).
Micro-RNAs are small noncoding RNAs that can regulate many pivotal biological functions including inflammation (Ambros, 2003; Liu and Abraham, 2013). Each micro-RNA can target multiple messenger RNAs to exert their regulatory effects and each mRNA can be regulated by different micro-RNAs (Cardoso et al., 2012; Szabo et al., 2012). Micro-RNA-155 (miR-155) is a master regulator of inflammation and it has been shown to negatively regulate proteins involved in suppressing inflammation (Cardoso et al., 2012). We have recently demonstrated that miR-155 is increased by chronic alcohol in the liver and it promoted TNFα production by increasing the half-life of TNFα in Kupffer cells in alcoholic liver disease in mice (Bala et al., 2011).
Here, we investigated the impact of acute binge (3-day alcohol gavage) and chronic alcohol feeding (5 weeks) on inflammation in murine proximal SB. Our data show that both acute binge and chronic alcohol feeding increases serum endotoxin levels, which were transient after acute alcohol binge. Acute alcohol gavage resulted in up-regulation of the antimicrobial protein, Reg3b, whereas Reg3b was reduced in chronic alcohol feeding in murine SB. In contrast to acute alcohol binge, miR-155 was only up-regulated after chronic alcohol feeding in murine SB. Furthermore, we found NF-κB activation and increased TNFα production in the SB of chronic alcohol-fed mice that was absent in miR-155-deficient (miR-155-knockout [KO]) mice. Our results suggest that miR-155 contributes to the different effect of acute binge and chronic alcohol feeding on murine SB and that miR-155 contributes to gut inflammation.
MATERIALS AND METHODS
Animals
This study was conducted according to the regulations of the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School (UMASS; Worcester, MA). Six- to 8-week-old female C57BL/6J wild-type (WT) or miR-155-KO mice were employed. MiR-155-KO mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and further inbreed in the Animal Facility at UMASS. The animals on chronic ethanol (EtOH)-regime received 5% (v/v) ethanol (36% ethanol-derived calories) containing Lieber-DeCarli diet or pair-fed diet with an equal amount of calories where the alcohol-derived calories were substituted with dextrane-maltose (Bio-Serv, Frenchtown, NJ) for 5 weeks (Lippai et al., 2013). The amount of alcohol diet consumed by WT and miR-155-KO mice was comparable. The animals on acute alcohol-regime received 5 g/kg 50% (v/v) alcohol diluted in water or an equal amount of water via oral gavage for 3 consecutive days. For the alcohol metabolism study, mice received a single oral gavage of 5 g/kg 50% (v/v) alcohol diluted in water.
Sample Collection
Across the study animals were sacrificed in the morning following cheek bleeding. In the acute model, this was 6 or 12 hours after the last gavage and in the chronic alcohol model at 12 hours after the start of the last feeding as indicated in the individual figures. The sample collection after chronic feeding in the morning was approximately 3 to 5 hours following the actual end of the final feeding. Serum was isolated from blood by centrifugation. The proximal third of the small intestine was immediately isolated and kept in 2% fetal bovine serum containing phosphate buffered saline (PBS) on ice for further processing. The intestines were flushed with cold PBS, dissected longitudinally, washed further in PBS, until intestinal content was removed. Peyer patches were dissected and discarded. The first and the third to sixth cm of the proximal intestine were snap frozen for protein evaluation. The second cm was incubated in RNAlater (Qiagen, Hilden, Germany) at 4° overnight. All samples were stored at −80°.
Polymerase Chain Reaction
RNA was extracted from the second cm of proximal small intestine using RNeasy kit (Qiagen, Hilden, Germany). Optical density (260/280 and 260/230 ratios) was measured to check RNA quality. cDNA was transcribed from 1 µg of total RNA by using Reverse Transcription System (Promega Corp., Madison, WI) in a final volume of 30 µl. SYBR-Green-based real-time quantitative polymerase chain reaction (PCR) was performed using the iCycler (Bio-Rad Laboratories Inc., Hercules, CA). Each reaction contained 200 nM of primer mix (1/1, v/v) and 2.5 µl of diluted (1/4, v/v) cDNA. Forward (5′>3′) and reverse (5′>3′) primer sequences were: 18S (gta-acc-cgt-tga-acc-cca-tt; cca-tcc-aat-cgg-tag-tag-cg), Reg3b (tac-tgc-ctt-aga-ccg-tgc-ttt-ctg; gac-ata-ggg-caa-ctt-cac-ctc-aca), and TNFα (cac-cac-cat-caa-gga-ctc-aa; agg-caa-cct-gac-cac-tct-cc), respectively. The PCR contained a denaturation step for 3 minutes at 95° and 45 cycles of 30 seconds at 95°, 45 seconds at gene-specific annealing temperature, and 30 seconds at 72° for primer extension. Comparative threshold cycle (Ct) method was used to calculate expressions relative to WT control groups. Briefly, Ct for the target amplicon and the internal control, 18S gene, were determined. Afterward all results were normalized to their own internal control 18S to remove the differences in the amounts of nucleic acid added due to any deficiency of the reverse transcriptase PCR step, the values were then normalized to the average of the actual reference control sample group. The final results were expressed as fold changes between the sample and the controls corrected with 18S (Lippai et al., 2013).
Micro-RNA Analysis
Tissue samples of the second cm of proximal small intestines were lysed in QIAzol Lysis reagent (Qiagen) and incubated on ice for 5 minutes followed by micro-RNA isolation using Direct-zol RNA MiniPrep kit with on column DNA digestion (Zymo Research Corp. Irvine, CA). Reverse transcription (30 minutes—16°C; 30 minutes—42°C; 5 minutes—85°C) was performed in Eppendorf Realplex Mastercycler (Eppendorf, New York, NY) using 10 ng RNA, TaqMan primers, and MicroRNA Reverse Transcription Kit followed by quantitative reverse transcription PCR (10 minutes—95°C; 40 cycles of 15 seconds—95°C; 1 minute—60°C) in iCycler (Bio-Rad Laboratories) using Taq-Man Universal PCR Master Mix and mouse primers for miR-155 and snoRNA202, as internal control. Relative expression was calculated by Ct method.
Electrophoretic Mobility Shift Assay
End labeling of double-stranded NF-κB oligonucleotide, 5′AGTTGAGGGGACTTTCGC3′ was accomplished by treatment with T4 polynucleotide kinase in the presence of γ32P-ATP (PerkinElmer, Waltham, MA), followed by purification on a polyacrylamide copolymer column (Bio-Rad Laboratories). Proximal intestinal whole cell lysates (5 µg) were incubated with 1 µl labeled oligonucleotide (50,000 cpm), 4 µl dI-dC (Affymetrix Inc., Santa Clara, CA), and 5× gel buffer (containing 20 mM HEPES pH 7.9 [Sigma, St. Louis, MO], 50 mM KCl [Sigma], 0.1 mM EDTA [Boston BioProducts Inc., Ashland, MA], 1 mM DTT [Sigma], 5% glycerol [Fisher Scientific, Fair Lawn, NJ], and 200 µg/ml bovine serum albumin in sterile water). A 20 µl final volume was reached by adding nuclease-free water. For cold competition reaction a 20-fold excess of specific unlabeled double-stranded probe was added to the reaction mixture 20 minutes prior to adding the labeled oligonucleotide. Samples were incubated at room temperature for 20 minutes. Reactions were run on a 4% polyacrylamide gel. Gels were then dried and exposed to an X-ray film at −80°C for 6 hours or overnight where appropriate. Kodak X-OMAT 2000A Processor (Kodak, Rochester, NY) was used for film development in the darkroom. The films were scanned and densitometry was performed on the images using Multi Gauge Ver.3.2 image software (Fujifilm Corp., Valhalla, NY) (Mandrekar et al., 2002).
Enzyme-Linked Immunosorbent Assay
Whole liver lysates were extracted from proximal intestine in RIPA buffer containing protease and phosphatase inhibitors (1 mM PMSF, 1 mM NaF, 2 mM Na3VO4, 20 mM Na4P2O7, and protease inhibitor tablet). First, the tissue was homogenized with stainless steel bead (Qiagen) in TissueLyser II (Qiagen) then centrifuged. The tissue lysate supernatant was stored at −80°. Protein level was measured by enzyme-linked immunosorbent assay (ELISA) reader using Bio-Rad protein assay dye reagent concentrate (Bio-Rad Laboratories). TNFα (BD Biosciences, San Diego, CA) was measured in whole tissue lysates, following the manufacturer’s instructions.
Endotoxin
Endotoxin levels were evaluated in serum with Limulus Amebocyte Lysate (LAL) assay (Lonza Group Ltd., Basel, Switzerland).
Lipopolysaccharide-Binding Protein Measurement
Serum-lipopolysaccharide-binding protein (serum-LBP) levels were detected using mouse LBP ELISA kit (Cellsciences.com, Canton, MA).
Western Blot
Tissue lysates were run on 15% polyacrylamide gel. Proteins were transferred to nitrocellulose membrane overnight then blocked for 2 hours in blocking buffer containing Tris-buffered saline, 0.1% TWEEN-20 and 5% bovine serum albumin. Primary antibodies against mouse Reg3b (R&D Systems Inc., Minneapolis, MN), Src homology 2-containing inositol phosphatase-1 (SHIP1) (Cell Signaling, Danvers, MA), cytochrome p450 E1 (Cyp2E1) EMD Millipore Corp., Billerica, MA), nd β-actin (Abcam, Cambridge, MA) were used overnight at 4° at different dilution rates varying from 1:5,000 to 1:30,000 in blocking buffer, followed by 3 washing steps. For detection, appropriate donkey anti-sheep or anti-mouse secondary HRP-linked antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA) were used for 1 hour at a dilution rate of 1:5,000 in blocking buffer. The immunoreactive bands were detected by chemiluminescence using Pierce ECL Western blotting substrate (Pierce Biotechnology, Rockford, IL) and LAS-4000IR Ver.2.02 (Fujifilm Corp.). The results were quantified by densitometric analysis using Multi Gauge Ver.3.2 image software (Fujifilm Corp.) (Lippai et al., 2013).
Statistical Analysis
Because the data were not normally distributed, statistical analysis was performed using Kruskal–Wallis nonparametric test. Data are shown as average ± standard error of the mean (SEM) and differences were considered statistically significant at p-value ≤0.05. The experiments were performed a minimum of 2 times.
RESULTS
Acute Alcohol Binge and Chronic Alcohol Feeding Differently Regulates Antibacterial Protein Expression in the SB
Endotoxin is increased in the sera of alcoholic patients (Fujimoto et al., 2000). Here, we found that both acute binge and chronic alcohol feeding increased serum endotoxin levels (Fig. 1A). However, the increase in serum-endotoxin level was transient after acute alcohol binge and returned to normal by 12 hours after alcohol intake. LBP, an acute phase protein produced by the liver, is induced by and binds to endotoxin (Summa et al., 2013). Serum-LBP was induced 12 hours after acute alcohol gavage and was also increased after 5 weeks of chronic alcohol feeding compared to nonalcohol controls (Fig. S1A).
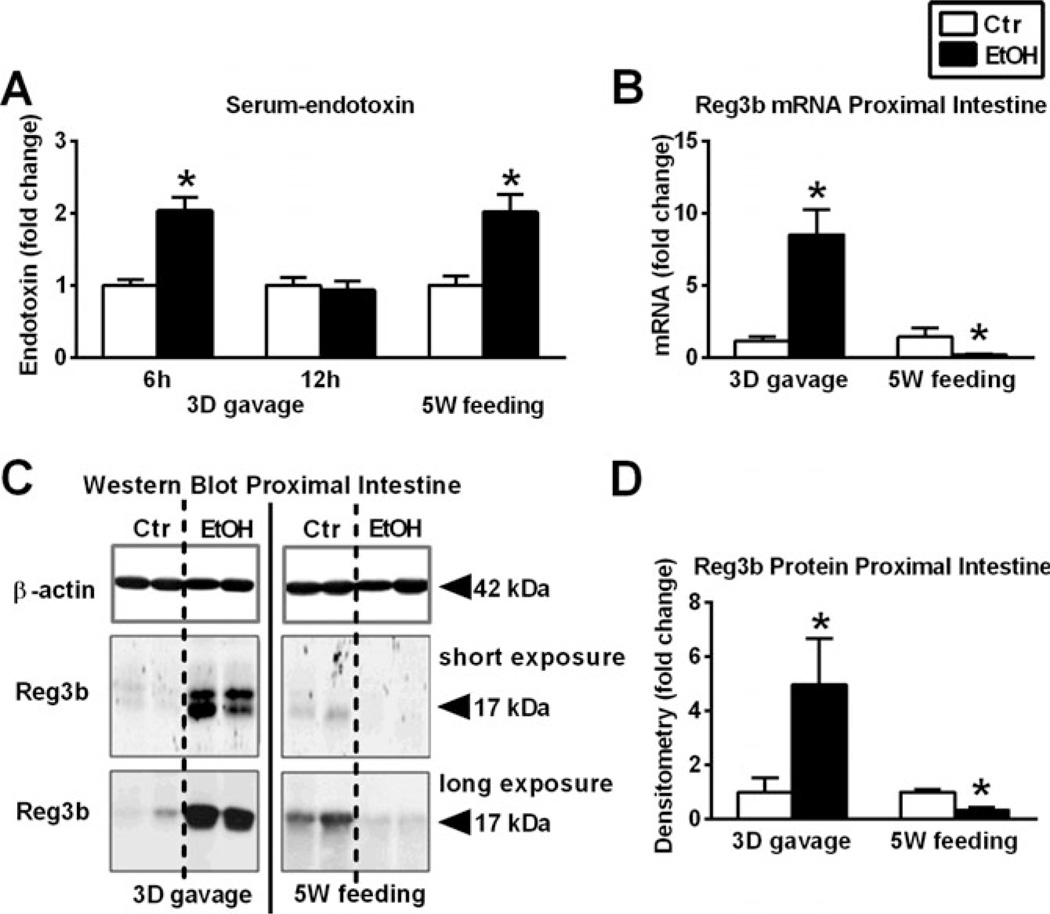
Fig. 1.
Differences between acute alcohol binge and chronic alcohol feeding on serum endotoxin and gut antimicrobial protein expression. Wild-type mice were gavaged daily 50% 5 g/kg ethanol (EtOH; n = 6) or equal amount of saline (Ctr; n = 6) for 3 consecutive days (3D) or were fed with 5% EtOH containing (EtOH; n = 8) or isocaloric (Ctr; n = 7) diet for 5 weeks (5W). Serum-endotoxin measurement was executed using LAL-assay (A). Regenerating islet-derived 3-beta (Reg3b) mRNA was assessed by quantitative reverse transcription polymerase chain reaction in proximal small bowel (SB) using 18S internal control (B). Reg3b protein in whole SB lysates was assessed by Western blot using β-actin loading control (C), and further quantified by densitometry (D). Bars represent mean ± SEM (*p-value <0.05 relative to appropriate controls by Kruskal–Wallis nonparametric test).
Decreased expression of antibacterial protein was reported in chronic alcoholic patients in the SB (Yan et al., 2011). We found that acute alcohol binge increased the expression of the antibacterial protein, Reg3b, both at mRNA (Fig. 1B) as well as protein level (Fig. 1C,D) in the SB of mice. In contrast, chronic alcohol feeding decreased both the mRNA as well as the protein level of Reg3b (Fig. 1C,D) (Average; SEM of density values of saline vs. alcohol gavage 248; 98 vs. 1,226; 426 and PF vs. alcohol-feeding [longer exposure] 265; 20 vs. 107; 31, respectively).
Inflammatory Markers, NF-κB Activation and TNFα, Are Differently Regulated by Acute Alcohol Binge and Chronic Alcohol Feeding
Pro-inflammatory cytokine, TNFα, secreting cells are increased in the inflamed mucosa in inflammatory bowel disease (Breese et al., 1994). Up-regulation of TNFα involves activation of transcription factors, such as NF-κB (Liu et al., 2000) or AP-1 (Redhu et al., 2011). We found that while TNFα was increased at the mRNA level in the SB after both acute binge and chronic alcohol feeding (Fig. 2A), TNFα protein levels were increased only after chronic alcohol feeding compared to the corresponding pair-fed controls (Fig. 2B). We found that both acute binge and chronic alcohol feeding induced NF-κB activation and DNA binding in SB, however, the rate of increase was significantly greater in mice with chronic compared to acute binge alcohol administration (Fig. 2C,D).
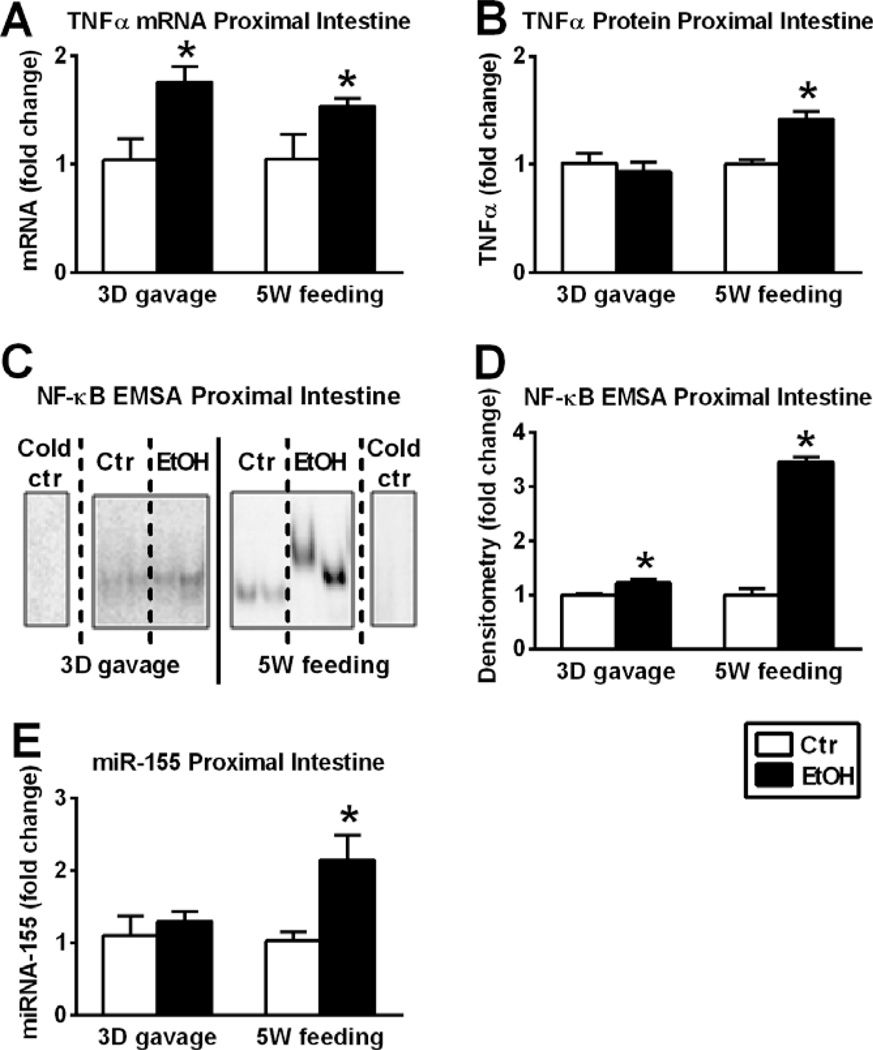
Fig. 2.
Differences between acute alcohol binge and chronic alcohol feeding on nuclear factor-κB (NF-κB) activation, tumor necrosis factor-α (TNFα) and micro-RNA-155 (miR-155) expression. Wild-type mice were gavaged daily 50% 5 g/kg ethanol (EtOH; n = 6) or equal amount of saline (Ctr; n = 6) for 3 consecutive days (3D) or were fed with 5% EtOH containing (EtOH; n = 8) or isocaloric (Ctr; n = 7) diet for 5 weeks (5W). TNFα mRNA was assessed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) in proximal small bowel (SB) using 18S internal control (A). TNFα protein in whole SB lysates was assessed by specific enzyme-linked immunosorbent assay (B). NF-κB activation of whole SB lysates was assessed by electrophoretic mobility shift assay (C), loading equal amounts of protein, and further quantified by densitometry (D). MiR-155 was assessed by qRT-PCR in SB using snoRNA202 internal control (E). Bars represent mean ± SEM (*p value <0.05 relative to appropriate controls by Kruskal–Wallis nonparametric test).
MiR-155 Was Increased After Chronic Alcohol Feeding in Murine Proximal Small Intestine
MiR-155 is a disease modifying micro-RNA and can increase the half-life of TNFα mRNA (Bala et al., 2011). Here, we found that acute binge had no effect on SB miR-155 levels, however, a significant increase in miR-155 was observed in chronic alcohol-fed mice compared to controls (Fig. 2E). To further investigate the mechanistic role of miR-155 in SB, we tested miR-155-KO mice.
MiR-155 Deficiency Protected Mice from Alcohol-Induced Alterations in Serum Endotoxin and SB Antibacterial Protein Expression
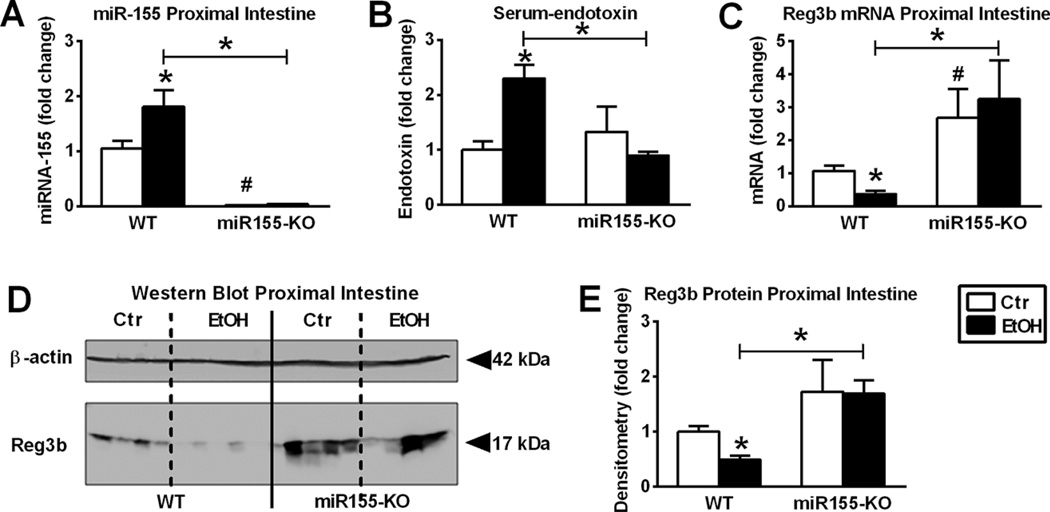
Consistent with the miR-155-deficient phenotype, there was no detectable miR-155 before or after alcohol feeding in miR-155-KO mice (Fig. 3A). We found that miR-155-KO mice were protected from chronic alcohol-induced increase in serum endotoxin compared to alcohol-fed WT mice (Fig. 3B). Whereas serum-LBP was induced by alcohol in both genotypes (Fig. S1B). This observation raised the question whether miR-155 deficiency could preserve the gut barrier. We found that unlike WT mice, chronic alcohol-fed miR-155-KO mice had no decrease in Reg3b mRNA (Fig. 3C) or protein (Fig. 3D,E) levels in the SB compared to pair-fed controls.
Fig. 3.
Chronic alcohol increases micro-RNA-155 (miR-155) in small bowel (SB) and miR-155 deficient (miR-155-knockout [KO]) mice are protected from alcohol-induced serum endotoxin increase and suppression of antibacterial protein in the proximal small intestine. Wild-type (WT (n = 6 or 7) or miR-155-KO (n = 5 or 10) mice were fed with ethanol (EtOH) or isocaloric (Ctr) diet for 5 weeks, respectively. MiR-155 was assessed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) in proximal SB using snoRNA202 internal control (A). Serum-endotoxin measurement was executed using LAL-assay (B). Regenerating islet-derived 3-beta (Reg3b) mRNA was assessed by qRT-PCR in SB using 18S internal control (C). Reg3b protein in whole SB lysates was assessed by Western blot using β-actin loading control (D), and further quantified by densitometry (E). Bars represent mean ± SEM (*,#p value <0.05 relative to appropriate isocaloric or WT controls, respectively, by Kruskal–Wallis nonparametric test).
MiR-155 Deficiency Protected Mice from Alcohol-Induced TNFα Increase and NF-κB Activation
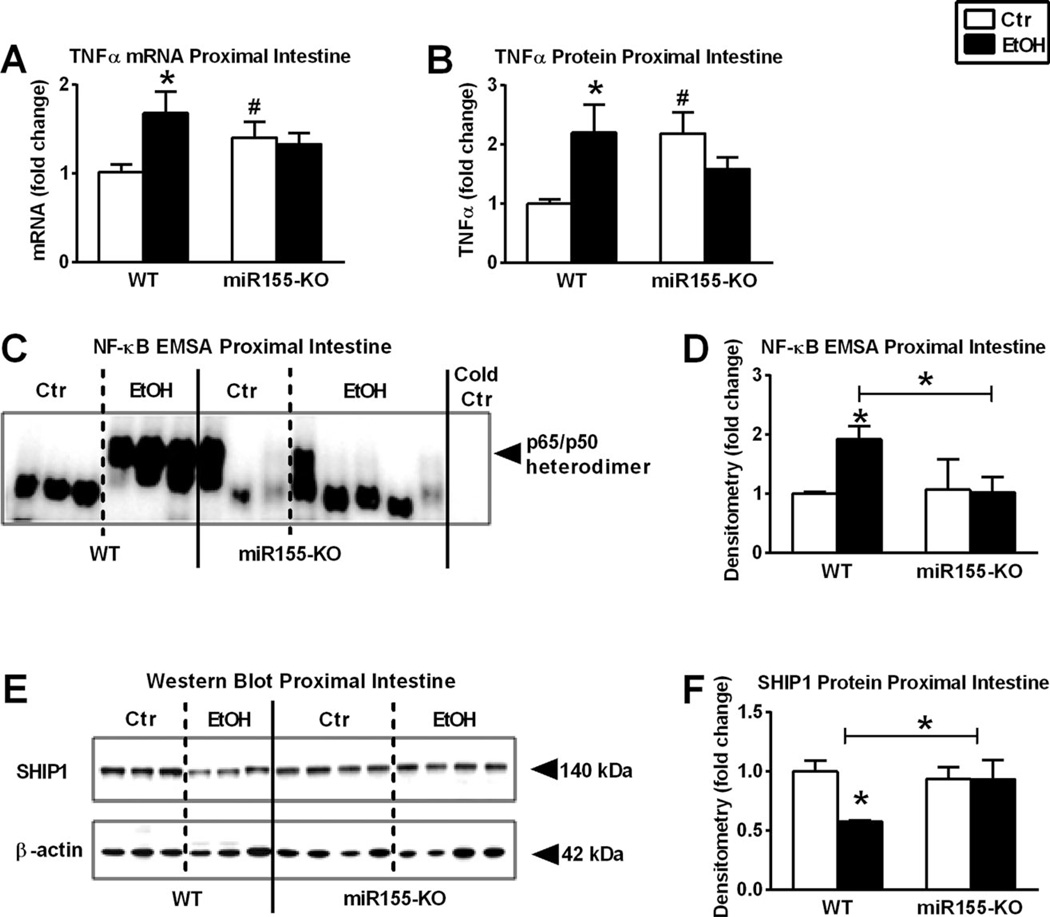
Deficiency in miR-155 also prevented NF-κB activation (Fig. 4C,D) and TNFα mRNA (Fig. 4A) or protein (Fig. 4B) increases in the SB after chronic alcohol feeding compared to alcohol-fed WT or pair-fed controls. MiR-155 negatively regulates and decreases the anti-inflammatory protein, SHIP1 (Kurowska-Stolarska et al., 2011). We found decreased SHIP1 protein levels in alcohol-fed WT but not in alcohol-fed miR-155-KO murine SB compared to pair-fed controls (Fig. 4E,F).
Fig. 4.
Micro-RNA-155 deficient (miR-155-knockout [KO]) mice are protected from alcohol-induced tumor necrosis factor-α (TNFα) increase and nuclear factor-κB (NF-κB) activation. Wild type (WT; n = 6 or 7) or miR-155-KO (n = 5 or 10) mice were fed with ethanol (EtOH) or isocaloric (Ctr) diet for 5 weeks, respectively. TNFα mRNA was assessed by quantitative reverse transcription polymerase chain reaction in proximal small bowel (SB) using 18S internal control (A). TNFα protein in whole SB lysates was assessed by specific enzyme-linked immunosorbent assay (B). NF-κB activation of whole SB lysates was assessed by electrophoretic mobility shift assay (C), loading equal amounts of protein, and further quantified by densitometry (D). Src homology 2-containing inositol phosphatase-1 (SHIP1) protein in whole SB lysates was assessed by Western blot using β-actin loading control (E), and further quantified by densitometry (F). Bars represent mean ± SEM (*,#p-value <0.05 relative to appropriate isocaloric or WT controls, respectively, by Kruskal–Wallis nonparametric test).
DISCUSSION
Alcohol use, both acute binge and chronic alcohol intake, has numerous negative health effects on different organs including the intestine (Bode and Bode, 2003; Lippai et al., 2013; Szabo et al., 2010). Our data confirmed previous reports of increased endotoxin levels in chronic alcohol feeding (Keshavarzian et al., 2001) and demonstrated that increase in serum endotoxin after acute alcohol binge was transient in mice. Serum-LBP, an acute phase protein, is produced by hepatocytes and has been shown to be induced in alcoholics (Summa et al., 2013). Here, we found that both acute and chronic alcohol intake increased endotoxin as well as LBP in the serum of mice. These observations implied that both acute binge and chronic alcohol intake affects the gut mucosal barrier function.
Several studies have shown that alcohol disrupts intestinal barrier integrity in the colon. Here, we focused on the proximal SB which has a different pattern of gene activation and is in the first line of contact with alcohol. Important elements of the mucosal innate immune system are antibacterial proteins that are reportedly decreased in chronic alcoholics (Yan et al., 2011). Similarly, chronic alcohol feeding decreased the antibacterial protein, Reg3b, levels in the SB. Interestingly, however, we found that acute alcohol binge increased Reg3b levels while serum endotoxin was increased. We speculate that the increased Reg3b after an acute alcohol binge may represent a compensatory mechanism to maintain intact gut barrier after an acute alcohol binge. After repeated and sustained alcohol exposure as it is in chronic alcohol feeding, this mechanism is likely to be exhausted and result in reduced Reg3b levels as we and others found after chronic alcohol feeding. We noticed that while loading the same amount of SB protein from acute gavage or chronic feeding to the gels, the control group of the chronic feeding had higher amount of Reg3b protein (longer exposure). The increased Reg3b production in the chronic feeding versus the acute gavage model might be attributable to the high fat content of the isocaloric diet, applied in chronic feeding, as in high-fat diet receiving mice the transcription of Reg3b of the neighbor mesenteric adipose tissue is induced (Hageman et al., 2010). It was also observed that Reg3b was highly induced at mRNA compared to protein levels. We cannot rule out the possibility of posttranscriptional or posttranslational modifications of Reg3b in our models.
Previous studies have shown that NF-κB activation and TNFα release play an important role in regulating intestinal epithelial function in inflammatory bowel disease (Atreya et al., 2008; Suenaert et al., 2002). Despite the fact that TNFα protein was increased only in chronic alcohol feeding in the SB, both acute and chronic alcohol consumption increased TNFα mRNA and yet acute binge only minimally increased NF-κB. As NF-κB is not the sole mediator of TNFα induction in our acute binge model there could be other transcription factors involved in the up-regulation of TNFα mRNA, such as AP-1 (Redhu et al., 2011).
Although acute alcohol increased TNFα mRNA, it did not affect TNFα protein while TNFα protein levels were increased after chronic alcohol feeding. This phenomenon could be in part due to miR-155 induction in chronic alcohol feeding in contrast to the lack of induction of miR-155 in the acute binge drinking model. It has been shown that miR-155 is a positive regulator of TNFα by increasing its mRNA stability in macrophages (Bala et al., 2011). Our data suggested that chronic alcohol leads to a robust inflammatory response in SB characterized by increased TNFα, NF-κB activation, and reduced expression of the antimicrobial protein, Reg3b. To the best of our knowledge, our results indicate for the first time that in contrast to chronic alcohol feeding, acute alcohol binge resulted in a transient increase in serum endotoxin and a further increase in Reg3b leading to the speculation that rapid restoration of the gut barrier may occur after barrier disruption caused by acute alcohol binge, whereas chronic alcohol-fed mice might not have this reserve capacity.
Small noncoding RNAs, micro-RNAs, play an important role in fine-tuning of gene expression (Liu and Abraham, 2013). A previous study linked alcohol-induced disruption of gut epithelial tight junctions to miR-212-mediated decrease in zonula occludens-1 expression (Tang et al., 2008). MiR-155 is a regulator of Toll-like receptor-induced inflammation (Tili et al., 2009) and it increases TNFα by stabilizing its mRNA (Bala et al., 2011). Moreover, miR-155 is increased in inflammatory intestinal diseases including ulcerative colitis (Takagi et al., 2010), acute graft-versus-host disease (Ranganathan et al., 2012), and in the liver in alcoholic liver disease (Bala et al., 2011). Therefore we hypothesized that miR-155 contributes to alcohol-induced SB inflammation. Acute binge had no effect on SB miR-155 levels, however, chronic alcohol feeding significantly increased miR-155 levels in murine SB.
Furthermore, miR-155-deficient mice were protected from chronic alcohol-induced increase in serum endotoxin levels, which observation raised the question whether miR-155 deficiency could preserve the gut barrier. Despite no increase in serum-endotoxin, alcohol-fed miR-155-KO mice had increased level of serum-LBP. LBP is produced mainly by hepatocytes as an acute phase protein; however, other cell types including epithelial cells of the intestine are also involved in its production (Vreugdenhil et al., 1999). Transcription factors such as STAT3 or AP-1, which can be induced by alcohol in monocytes (Norkina et al., 2007), can induce LBP (Schumann et al., 1996). It is plausible that alcohol itself might contribute to LBP release via activation of transcription factors; this requires further investigation.
Blood alcohol content of mice from both genotypes were significantly increased upon chronic alcohol feeding; however, miR-155-KO mice had lower alcohol content than their WT controls (Fig. S2A). As alcohol consumption was the same among different genotypes, this decrease could be attributable to an increased metabolism. To rule out the possibility of increased metabolism in miR-155-KO mice, the rate of alcohol metabolism was determined after a single alcohol oral gavage (5 g/kg 50% ethanol). Our data suggest that alcohol metabolism (blood alcohol levels) was comparable between the genotypes (Fig. S2B). It is noteworthy that serum alcohol levels were higher in miR-155-KO mice than WT mice after a single alcohol oral gavage (Fig. S2B). The discrepancy in the blood alcohol levels after chronic alcohol feeding or a single alcohol oral gavage in miR-155-KO mice deserves further investigation. Hepatic Cyp2E1 enzyme contributes to the degradation of ethanol via oxidative mechanism. Hepatic Cyp2E1 levels were increased in both alcohol-fed WT and miR-155-KO mice; however, alcohol-fed miR-155-KO mice had lower expression of Cyp2E1 than their WT controls (Fig. S2C,D).
Deficiency in miR-155 prevented Reg3b decrease, NF-κB activation, and TNFα increase in SB after alcohol feeding. We observed a high background of TNFα protein in miR-155-deficient mice, which might be the result of either the absence of miR-155 or some other pathways being altered in miR-155-deficient mice. Furthermore, unlike in WT mice, the miR-155 target, SHIP1 did not decrease upon chronic alcohol feeding in miR-155-deficient mice, suggesting preservation of anti-inflammatory targets in alcohol-fed miR-155-KO mice. Together, these novel findings suggested that inflammatory changes in the gut could be partially attributed to miR-155 in chronic alcohol consumption.
Amplification of TNFα production by chronic alcohol in Kupffer cells involves alcohol-induced induction of NF-κB activation (Bala et al., 2011). Here, we found that the differences between the effects of acute binge and chronic alcohol intake might be in part related to miR-155 that was increased only after chronic alcohol feeding. Based on the regulatory effects of miR-155 on dendritic cell, B- and T-cell functions (Rodriguez et al., 2007) it is likely that some of these cell types contribute to the protection of barrier function in miR-155-KO mice. MiR-155-KO mice are prone to Salmonella typhimurium and Helicobacter pylori infection and are less likely to develop chronic gastritis or dextran sodium sulfate–induced colitis due to impaired B- and T-cell development (Bujanda, 2000; Oertli et al., 2011). The role of miR-155 in intestinal inflammation and colon tumors, which have been linked previously (Bakirtzi et al., 2011) and are increased in alcoholics (Bujanda, 2000), deserve further investigation.
In summary, our data suggest that both acute and chronic alcohol intake impair intestinal barrier function; the increased serum endotoxin after acute alcohol binge is transient and up-regulation of Reg3b is present in SB. In contrast, increased serum endotoxin after chronic alcohol administration is associated with reduced Reg3b and increased inflammatory cascade activation in SB that are dependent on miR-155 expression. The results suggest that miR-155 contributes to differences between acute binge and chronic alcohol feeding in the SB and may have an important role in regulation of gut barrier integrity.
ACKNOWLEDGMENTS
We thank Abhishek Satishchandran for his assistance with the oral alcohol gavage. This study was funded by NIH-NIAAA grant (AA020744).
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Alcohol induces LBP in the serum of WT and miR-155-KO mice.
Fig. S2. Alcohol absorption and metabolism are increased in alcohol-fed mice.
REFERENCES
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- Bakirtzi K, Hatziapostolou M, Karagiannides I, Polytarchou C, Jaeger S, Iliopoulos D, Pothoulakis C. Neurotensin signaling activates microRNAs-21 and-155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141:1749.e1–1761.e1. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor alpha (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck IT, Dinda PK. Acute exposure of small intestine to ethanol: effects on morphology and function. Dig Dis Sci. 1981;26:817–838. doi: 10.1007/BF01309614. [DOI] [PubMed] [Google Scholar]
- Bhonchal S, Nain CK, Prasad KK, Nada R, Sharma AK, Sinha SK, Singh K. Functional and morphological alterations in small intestine mucosa of chronic alcoholics. J Gastroenterol Hepatol. 2008;23:e43–e48. doi: 10.1111/j.1440-1746.2007.05080.x. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95:3374–3382. doi: 10.1111/j.1572-0241.2000.03347.x. [DOI] [PubMed] [Google Scholar]
- Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135:73–88. doi: 10.1111/j.1365-2567.2011.03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, Kojima H, Sakurai S, Tanaka R, Namisaki T, Noguchi R, Higashino T, Kikuchi E, Nishimura K, Takaya A, Fukui H. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- Hageman RS, Wagener A, Hantschel C, Svenson KL, Churchill GA, Brockmann GA. High-fat diet leads to tissue-specific changes reflecting risk factors for diseases in DBA/2J mice. Physiol Genomics. 2010;42:55–66. doi: 10.1152/physiolgenomics.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge T, Persson J, Danielsson D. Mucosal bacterial growth in the upper gastrointestinal tract in alcoholics (heavy drinkers) Digestion. 1997;58:591–595. doi: 10.1159/000201507. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Beier JI, Arteel GE, Falkner KC, Barve SS, McClain CJ. Ethanol and dietary unsaturated fat (corn oil/linoleic acid enriched) cause intestinal inflammation and impaired intestinal barrier defense in mice chronically fed alcohol. Alcohol. 2013;47:257–264. doi: 10.1016/j.alcohol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:170–177. doi: 10.1161/ATVBAHA.112.300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bellerose G, Szabo G. Inhibition of NF-kappa B binding correlates with increased nuclear glucocorticoid receptor levels in acute alcohol-treated human monocytes. Alcohol Clin Exp Res. 2002;26:1872–1879. doi: 10.1097/01.ALC.0000042220.48841.9E. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011;166:1–15. doi: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkina O, Dolganiuc A, Shapiro T, Kodys K, Mandrekar P, Szabo G. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J Leukoc Biol. 2007;82:752–762. doi: 10.1189/jlb.0207099. [DOI] [PubMed] [Google Scholar]
- Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Muller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic gastritis and colitis. J Immunol. 2011;187:3578–3586. doi: 10.4049/jimmunol.1101772. [DOI] [PubMed] [Google Scholar]
- Ranganathan P, Heaphy CE, Costinean S, Stauffer N, Na C, Hamadani M, Santhanam R, Mao C, Taylor PA, Sandhu S, He G, Shana’ah A, Nuovo GJ, Lagana A, Cascione L, Obad S, Broom O, Kauppinen S, Byrd JC, Caligiuri M, Perrotti D, Hadley GA, Marcucci G, Devine SM, Blazar BR, Croce CM, Garzon R. Regulation of acute graft-versus-host disease by microRNA-155. Blood. 2012;119:4786–4797. doi: 10.1182/blood-2011-10-387522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhu NS, Saleh A, Halayko AJ, Ali AS, Gounni AS. Essential role of NF-kappaB and AP-1 transcription factors in TNF-alpha-induced TSLP expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L479–L485. doi: 10.1152/ajplung.00301.2009. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann RR, Kirschning CJ, Unbehaun A, Aberle HP, Knope HP, Lamping N, Ulevitch RJ, Herrmann F. The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell Biol. 1996;16:3490–3503. doi: 10.1128/mcb.16.7.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS One. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Sarnow P, Bala S. MicroRNA silencing and the development of novel therapies for liver disease. J Hepatol. 2012;57:462–466. doi: 10.1016/j.jhep.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, Ando T, Oyamada Y, Isozaki Y, Hongo H, Uchiyama K, Handa O, Kokura S, Ichikawa H, Yoshikawa T. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25(Suppl 1):S129–S133. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390–394. [PubMed] [Google Scholar]
- Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Tili E, Croce CM, Michaille JJ. miR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol. 2009;28:264–284. doi: 10.1080/08830180903093796. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil AC, Dentener MA, Snoek AM, Greve JW, Buurman WA. Lipopolysaccharide binding protein and serum amyloid A secretion by human intestinal epithelial cells during the acute phase response. J Immunol. 1999;163:2792–2798. [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]