Abstract
Background:
We assessed the occurrence of dengue fever in association with travel in a non-endemic hilly region. The clinical presentation and laboratory parameters of febrile patients with a travel history to an endemic region were studied, and the role of the laboratory in the diagnosis was affirmed.
Materials and Methods:
Febrile patients presenting with clinical features defining dengue with a history of travel to an endemic area constituted the study group. Serum samples were tested for dengue-specific NS1 antigen and IgM, IgG antibodies. The demographic data were retrieved from the hospital information system. A hematological and biochemical workup was done and the results analyzed using percentage, proportion, mean, and median.
Results:
Out of 189 febrile patients, 58 were reactive to serological tests for dengue, with 47 (81%) males. The presenting features were chills and rigors, myalgia, cough, sweating, and vomiting. Thrombocytopenia (74.35%), lymphopenia (52.94%), and leucopenia (47.05%) were present in early disease, with AST >34 IU/L in 58.97% of the patients. The NS1 antigen was detectable between three and seven days of fever and the IgM antibodies after five days. The positivities to only NS1, both NS1 and IgM, and IgM alone were 60.34, 27.58, and 10.34%, respectively, and the median duration of fever was five, seven, and ten days, respectively. One case of dengue hemorrhagic fever and one of probable secondary dengue infection with detectable IgG were encountered.
Conclusion:
Dengue fever remains unsuspected in febrile cases in non-endemic regions. History of travel is an essential criterion to suspect dengue. A non-specific clinical presentation eludes diagnosis. Serological tests for antigen and antibodies, and hematological and biochemical markers are vital for distinguishing the diagnosis.
Keywords: Dengue, dengue fever, IgM, IgM/IgG antibody, lymphopenia, neutrophilia, NS1 antigen, thrombocytopenia
INTRODUCTION
Dengue fever (DF) is an acute febrile, arboviral disease caused by a Flavivirus. It is transmitted by female Aedesaegypti mosquitoes. The disease is endemic in tropical and subtropical countries and accounts for 100 million cases annually.[1] An estimated 40% of the world population is at risk and dengue hemorrhagic fever (DHF) accounts for 5% of the mortality in most countries.[1,2]
A high fever (>100°F) of sudden onset, severe headache, myalgia, retro-orbital pain, lymphadenopathies, maculopapular rash, with or without respiratory involvement, nausea, vomiting and diarrhea are the usual clinical features. The most characteristic is muscle and joint pain, which earns it the name of break-bone fever or bone-crusher disease. Children and young adults may develop abdominal pain, hemorrhagic tendencies, with petechiae, bruises, hematuria, hematemesis, epistaxis, and melena. Thrombocytopenia and relative leucopenia with hemoconcentration define a probable case of dengue hemorrhagic fever.[3]
There are four serologically distinct types of dengue fever, with only partial protection against heterologous serotype infection.[4] Dengue hemorrhagic fever is usually associated with secondary infection with different serotypes, but can occur in primary dengue fever, especially in infants and children.[5,6] Early detection decreases chances of progression to dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), preventing the subsequent associated morbidity and mortality.[4,7]
The changing climatic conditions have made it conducive for the vector to survive in newer geographical regions and increased travel has significantly changed the geographical distribution of the cases detected. Unsuspected cases imported from endemic areas are reported from non-endemic areas. Considering such an emergent situation, we have evaluated the clinical features, and hematological and biochemical parameters of confirmed clinical suspects of DF attending various departments of our tertiary care institute, in a laboratory.
MATERIALS AND METHODS
A retrospective analysis was conducted of clinical suspects of dengue fever attending the tertiary care facility between September 2012 and April 2013. The study group included patients presenting with sudden onset of fever and history of travel to a dengue-endemic area. Any two or more of the following manifestations of headache, retro-orbital pain, myalgias, arthalgias, rash, and hemorrhagic tendencies defined a probable case of dengue fever. Dengue hemorrhagic fever was labeled in patients with recent fever, hemorrhagic tendencies, thrombocytopenia with platelet counts below 100,000/mm3, and hematocrit 20% above average for age, as per the World Health Organization (WHO) criteria.[2]
The serum samples submitted from the clinical suspects with dengue like illness between September 2010 and April 2013 were included in the retrospective study. The hematological and biochemical tests and demographic profile was retrieved from the medical charts and hospital information system. The qualitative determination of the antibodies and antigen in the serum samples was done using the Advantage Dengue NS1Ag and Ab Combi Card as per the manufacturer's instructions (J. Mitra and Co. Pvt. Ltd., New Delhi, India). It is a rapid, solid-phase, immunochromatographic test, intended for early diagnosis and presumptive differentiation of primary and secondary infections. The test kit has two devices for screening of NS1 antigen and IgM, IgG antibodies. The accuracy indices of the rapid device for detection, as claimed by the manufacturer are, sensitivity of 100 and 93% and specificity of 99.34 and 99.01% for antibody and antigen detection, respectively. The results were analyzed using percentage, mean, median, and proportion.
RESULTS
The serum samples were tested for presence of dengue virus — specific IgM, IgG antibodies and NS1 antigen between September 2010 and April 2013 [Table 1].
Table 1.
Month-wise distribution of dengue fever cases
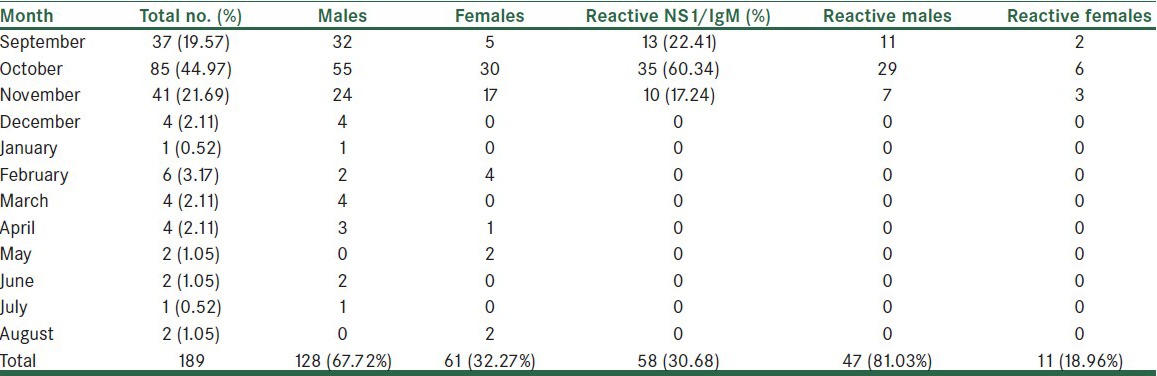
Out of the 189 clinical suspects, 128 were males and 61 females. Of these clinical suspects, 58 were reactive to either NS1 antigen or IgM/IgG antibody or both together, giving a laboratory incidence of 30.68%. There were 47 males and 11 females, and the male to female ratio of reactive cases was 4.27:1. Eighteen males and three females were hospitalized due to severity of symptoms. The hospital stay ranged from four to ten days, with a median stay of five days. No sample was received from children below the age of 18 years. Most of the patients were in the age range of 21 to 50 years and the mean age was 31.44 years.
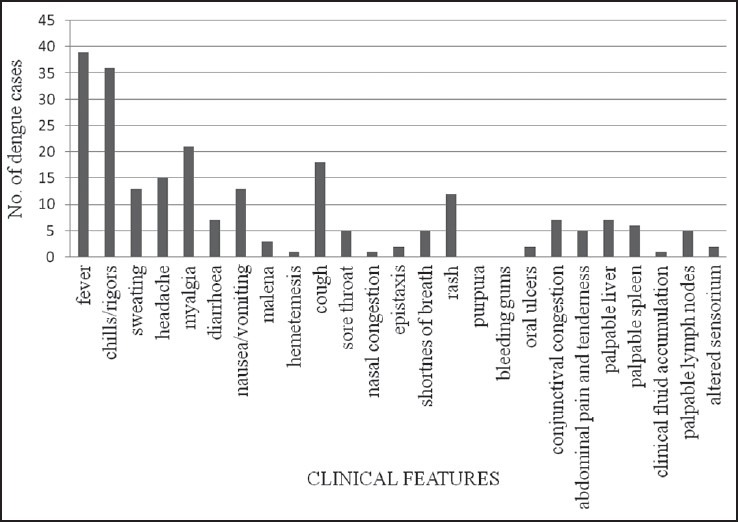
The clinical features seen in more than 50% of the patients were fever associated with chills and rigors, sweating, myalgia, and cough. Headache, nausea/vomiting and diarrhea were also significant presenting complaints. The frequency of clinical features in laboratory-proven dengue cases are represented in Figure 1.
Figure 1.
Reported clinical features and signs of dengue cases
In the present study, out of the 58 proven dengue cases, 35 (60.34%) patients were positive for NS1 antigen alone and median duration of their illness was four days (range three to five days). Antigen was detectable at the earliest on the third day after onset of fever and undetectable beyond seven days. NS1 antigen and IgM antibodies together were positive in 16 cases (27.58%). These patients had an onset of fever five to seven days earlier (median duration of seven days). IgM antibodies were detectable earliest on day five. The remaining seven patients presented with around 10 days of fever (7-10 days) and six (10.34%) were reactive only for IgM antibodies, whereas, one had IgM and IgG antibodies.
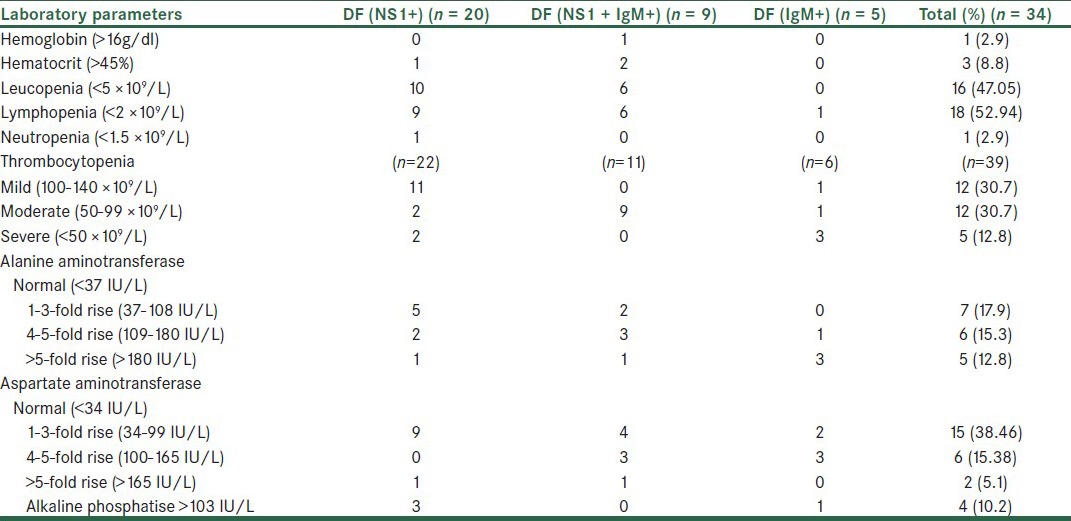
The platelet count was noted in 39 patients, out of which 43.58% had a platelet count <100 × 109/L. Patients with fever of five days, that is, acute phase, had normal counts of mild thrombocytopenia and those beyond seven days, that is, the convalescent phase, documented counts of <100 × 109/L. Leucopenia (<5 × 109 cells/L) was seen in 47.05% cases. Lymphopenia in the acute phase and increase in neutrophils in the late stage were observed in the present study. Elevated aspartate aminotransferase (AST >34 IU/L) was seen in 58.97% and alanine aminotransferase (ALT >37 IU/L) in 46.15%. The hematological and biochemical parameters in relation to the serological profile are represented in Table 2.
Table 2.
Laboratory results of confirmed dengue cases
DISCUSSION
Dengue fever is a viral disease ranging from asymptomatic, undifferentiated high-grade fever to severe, life-threatening, sequel-like DHF and DSS. The difficulties faced in diagnosing cases that mimic common febrile infections like leptospirosis, scrub typhus, and typhoid fever clinically call for essential laboratory identification.
Our study reported a laboratory incidence of 30.68% dengue fever in the clinically suspected cases. All reactive cases presented during September through October and early November highlighting a characteristic seasonal trend. Although the indigenous population of this hilly region are largely spared, those visiting these distant destinations for educational, vocational or business activities are at risk of acquiring the infection.
Travel to the adjoining endemic regions of Delhi, Chandigarh, and Ludhiana was the major antecedent event. Only two patients had infection after exposure in low-lying areas of Himachal Pradesh. Adult males were predominantly affected (81%) rather than women and children, as their nature of work required frequent travel.
The disease usually begins with a sudden, sharp rise in temperature, invariably high grade, and is associated with chills as reported in 80% by Abdul et al.[5] Fever was the presenting complaint in all our cases, with chills in 92.3% of the adults, usually associated with rigors. Other associated symptoms were headache, sweating, and myalgia, which corroborated with the previous studies.[5] Various studies have correlated gastrointestinal symptoms of diarrhea and vomiting with dengue. A high proportion of DHF patients with abdominal pain (18.7%), diarrhea (32%), and vomiting (68%) have been studied by other workers.[8] In our subjects, nausea, vomiting or diarrhea, and pain in the abdomen were significant presenting complaints.
Upper respiratory symptoms are a common accompaniment of DF. Cough should be considered an important predictive marker, as a high proportion (46%) of our patients presented with cough, whereas, soreness of throat and shortness of breath were seen in few. Similar findings have been recognized earlier where cough, sore throat, and nasal congestion constituted a notable clinical feature in 22.6, 51.6, and 20%, respectively.[9] DHF and DSS may be complicated with severe respiratory conditions like acute respiratory distress syndrome (ARDS), with pulmonary hemorrhage, with or without hemoptysis.[6] The occurrence of DHF was unusual in our subjects and life-threatening complications did not occur.
Hemorrhagic manifestations are considerably associated with platelet counts <50 × 109/L more so in patients of DHF. Different investigators report erythematous rash (14-25%), petechiae / ecchymosis (9-24.3%), epistaxis (9%), hematemesis/melena (6-8%), gum bleeding (3-9%), and vaginal bleeding (9.3%).[8] Cutaneous rashes are observed in more than 50% of the dengue fever cases during primary infection, including facial erythema, scarlatiniform or morbilliform exanthem and petechiae.[10] In our study, a transient, morbilliform exanthem was seen in 30.7% patients, typically on the trunk and joint flexors, and constituted a primary presenting complaint rather than fever. Melena, hematemesis, epistaxis, and oral ulcers rarely accompanied fever in our patients and did not attribute to disease suspicion. The three clinical characteristics of diarrhea, eye pain, and upper respiratory symptoms together, have been established as relevant in diagnosing dengue fever.[9] Eye pain and congestion was seldom reported in our cases, although diarrhea and cough were frequent features. Most subjects ascribed eye pain to fever and did not state the symptom, thus accounting for a low occurrence.
Dengue virus antigen has been found in Kupfer cells and sinusoidal lining cells in the liver, splenic cells, and lymph nodes.[6] Hepatomegaly has been reported in 1.8-45% of the adults hospitalized with dengue infection, that is, various workers from South East Asia.[8] Neurological manifestations constitute 20-25% in other studies.[5] Out of the hospitalized patients in this study, four had hepatomegaly, two each had splenomegaly and lymphadenopathy, whereas, one patient, who had dual infection of dengue and scrub typhus, had hepatosplenomegaly, lymphadenopathy (LAP), and altered sensorium with encephalopathy.
Clinical features are non-specific and variable, thus serology plays a vital role in establishing diagnosis. NS1 antigen is a non-structural glycoprotein, highly conserved and essential for viability and replication of the dengue virus. Detectable as early as one day post onset of fever up to 18 days, it expedites the time of diagnosis by 48-72 hours before appearance of antibodies with higher specificity.[1,11] This buys time for the treating physician to decide the further line of management. It was observed that the detection rate of the NS1 antigen decreased from 100% in early disease (≤5 days) to 61.53% in those presenting with seven or more days of fever, and thus, emphasized the role of antigen detection in early disease.
In primary dengue fever, the IgM antibodies appear after a latent period and in a low quantity. These are identifiable on day five of illness in 80% of all infected people and in 93-99% by days six to ten of the illness, and persist for 30-60 days.[11,12] The IgM antibody is evident in 14.28% of the cases with disease of duration up to five days and it increases to 84.61% in patients with one or more weeks of fever. This reiterates the usefulness of IgM detection in a later phase of the disease. In the late stage of the disease, the sensitivity of detecting NS1 is reduced, possibly due to immune complex formation, which decreases the antigen to below a quantifiable level.
IgG antibodies are slow to rise in primary infections reaching low detectable titers by the end of seven days. Rapidly rising high titers occur in secondary infection in the acute phase.[11] IgG antibodies were measurable in one subject, who had seven days of fever. This supported our assumption that except this case, probably of secondary dengue, the other 57 were primary dengue infections. We thus advocate incorporation of both antigen and antibody detection in all clinical suspects of dengue. Identification of both primary and secondary infection is of epidemiological significance and relevant to patient management.
Thrombocytopenia is the leading predictor of dengue fever, as documented in literature earlier.[8] In our experience 74.35% adults had low platelet counts. The levels correlated with the duration of disease and a consistent fall was observed with disease progression. Leucopenia may be another significant correlate of early dengue as seen in 47.05% of our cases. These findings are in agreement with the documentation by previous workers. Leucopenia with neutrophilia is commonly seen in the initial phase of dengue.[5] A fall in lymphocytes, primarily in patients presenting within one week of onset of disease, was present in 52.94% of the patients. In support are observations of Smith et al, and others.[5,13] Neutrophil counts were in normal range in a majority of the cases. Two patients developed neutrophilia, but they had dual infection, one with scrub typhus and the other concomitant falciparum malaria, thus it was difficult to comment on neutrophilia.
Thrombocytopenia, leucopenia, and elevated AST beyond 34 IU/L, together, have been elected to identify dengue infection with a sensitivity of 75% by earlier investigators.[13] Liver functions were deranged in 58.97% of the cases in the present study. Of these, 50% had at least one week duration of disease. Acute hepatitis showed a rise in aminotransferase levels, with peak levels on the ninth day and return to normal within three weeks. Damaged monocytes also released AST, accounting for higher levels of AST than ALT.[7] In our study, one case of primary dengue fever had DHF with a platelet count of 60,000/mm3, WBC of 4970/cmm, hemoglobin 17g/dl, hematocrit 52.2%, and AST and ALT 288 IU/L and 168 IU/L respectively. DHF occurred mostly in secondary dengue, but it was rarely documented in primary infections as seen in our study.[5]
Our experience reiterates the absence of dengue virus transmission at high altitudes, but low-lying areas are not free of disease transmission. Importation of cases from neighboring endemic areas is a consideration due to increased travel. We conclude that febrile patients presenting during fall and early winter, with less than one week history of chills, sweating, myalgia, vomiting, diarrhea, cough, and rash, with mild thrombocytopenia, fall in WBC counts, and lymphopenia should be suspected for dengue. Fever of a longer duration associated with hemorrhagic manifestations, hepatomegaly with impaired liver function, and a moderate-to-severe thrombocytopenia, with or without impairment of other hematological parameters may define a possible case.
Keeping in view the disease prevalence and recent identification from non-endemic regions like Shimla, institution-based surveillance activities are recommended as per the Integrated Disease Surveillance and National Vector Borne Disease Control Programs of the Government of India. In our view, laboratory workup is of diagnostic significance in the scenario of clinical ambiguity as delay in diagnosis may overburden the healthcare system on the one hand and risk the outcome on the other.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shrivastava A, Dash PK, Tripathi NK, Sahni AK, Gopalan N, LakshmanaRao PV. Evaluation of a commercial Dengue NS1 enzyme-linked immunosorbent assay for early diagnosis of dengue infection. Indian J Med Microbiol. 2011;29:51–5. doi: 10.4103/0255-0857.76525. [DOI] [PubMed] [Google Scholar]
- 2.da Silva-Voorham JM, Tami A, Juliana AE, Rodenhuis-Zybert IA, Wilschut JC, Smit JM. Dengue: A growing risk to travellers to tropical and sub-tropical regions. Ned TijdschrGeneeskd. 2009;153:A778. [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Guidelines for Dengue Fever and Dengue Hemorrhagic Fever/Dengue Shock Syndrome for Health Care Providers. Caribbean Epidemiology Center (CAREC)/Pan American Health Organization/World Health Organization. DenguideJCP/VW/ gc. [Last accessed on 2000 Nov 21]; [Google Scholar]
- 4.Lanciotti RS, Tsai TF. Arboviruses. In: Murray PR, Baron EJ, Landry ML, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed. Chapter 97. Washington DC: ASM Press; 2007. pp. 1486–8. [Google Scholar]
- 5.Khan AH, Hayat AS, Masood N, Solangi NM, Shaikh TZ. Frequency and clinical presentation of dengue fever at tertiary care hospital of Hyderabad/Jamshoro. JLUMHS JLUMHS. 2010;9:88–94. [Google Scholar]
- 6.Gulati S, Maheshwari A. Atypical manifestations of dengue. Trop Med Int Health. 2007;12:1087–95. doi: 10.1111/j.1365-3156.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooks GF, Carroll KC, Butel JS, Morse SA, editors. Jawetz, Melnick, Adelberg's Medical Microbiology. 24th ed. Chapter 38. New York: McGraw-Hill; 2007. Arthropod -Borne and rodent -borne viral diseases; pp. 511–32. [Google Scholar]
- 8.Malavige GN, Velathanthiri VG, Wijewickrama ES, Fernando S, Jayaratne SD, Aaskov J, et al. Patterns of disease among adults hospitalized with dengue infections. QJM. 2006;99:299–305. doi: 10.1093/qjmed/hcl039. [DOI] [PubMed] [Google Scholar]
- 9.Ramos MM, Tomashek KM, Arguello DF, Luxemburger C, Quiñones L, Lang J, et al. Early clinical features of dengue infection in Puerto Rico. Trans R Soc Trop Med Hyg. 2009;103:878–84. doi: 10.1016/j.trstmh.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Stephan L, Eggert S. Human herpes viruses. In: Burgdorf WH, Plewig G, Wolff HH, Landthaler M, editors. Braun-Falco's Dermatology. 3rded. Chapter 9. New York: Springer MedizinVerlag Heidelberg; 2009. pp. 74–96. [Google Scholar]
- 11.CDC -Laboratory Guidance and Diagnostic Testing. Centres for Disease Control and Prevention cdc. [Last accessed on 2012 Sept 27]. info@ cdc.gov .
- 12.Sekaran DS, Lan EW, Subramaniam G. Comparison of five serological diagnostic assays for the detection of IgM and IgG antibodies to dengue virus. African J Microbiol Res. 2008;2:141–7. [Google Scholar]
- 13.Wilder-Smith W, Earnest A, Paton NI. Use of simple laboratory features to distinguish the early stage of severe acute respiratory syndrome from dengue fever. Clin Infect Dis. 2004;39:1818–23. doi: 10.1086/426029. [DOI] [PMC free article] [PubMed] [Google Scholar]


