Abstract
Background:
The Streptococci are the pioneer strains in plaque formation and Streptococcus mutans are the main etiological agent of dental plaque and caries. In general, biofilm formation is a step-wise process, which begins by adhesion of planktonic cells to the surfaces. Evidences show that expression of glucosyltransferase B and C (gtfB and gtfC) and fructosyltransferase (ftf) genes play critical role in initial adhesion of S. mutans to the tooth surface which results in formation of dental plaques and consequently caries and other periodontal disease.
Materials and Methods:
The aim of this study was to determine the effect of biosurfactants produced by a probiotic strain; Lactobacillus casei (ATCC39392) on gene expression profile of gftB/C and tft of S. mutans (ATCC35668) using quantitative real-time PCR.
Results:
The application of the prepared biosurfactant caused dramatic down regulation of all the three genes under study. The reduction in gene expression was statistically highly significant (for gtfB, P > 0.0002; for gtfC, P > 0.0063, and for ftf, P > 0.0057).
Conclusion:
Considerable downregulation of all three genes in the presence of the prepared biosurfactant comparing to untreated controls is indicative of successful inhibition of influential genes in bacterial adhesion phenomena. In view of the importance of glucosyltransferase gene products for S.mutans attachment to the tooth surface which is the initial important step in biofilm production and dental caries, further research in this field may lead to an applicable alternative for successful with least adverse side effects in dental caries prevention.
Keywords: Biosurfactants, Lactobacillus casei, oral streptococci, probiotics
INTRODUCTION
Dental plaque as a microbial biofilm is defined as the multi-species community of micro-organisms formed on the tooth surfaces, in a protective shelter of an extra cellular matrix of host and microbial polymers.
Dental caries are one of the most prevalent chronic human infectious diseases widely spread in all age groups.[1,2] Colonization of the teeth by S. mutans is a main etiology of dental caries in human beings.[3,4] These organisms acquired an evolutionary adaptability to enzymatic conversion of sucrose to extracellular glucans by a set of enzymes, glucosyltransferases (gtfs), produced by the bacterium.[5,6] The gtfs secreted by S. mutans (particularly GtfB and GtfC) may act in two ways; either bacterial colonization of the tooth surface or attachment of bacteria to each other, modulating the formation of tightly adherent biofilms, which is a main prerequisite of dental caries.[7,8,9,10,11,12] It is now a well-known fact that S. mutans adhesion to the tooth surface is vital for the initiation and progression of dental caries.[13,14] In vitro studies have indicated that gtfB and gtfC are essential for the sucrose-dependent attachment of S. mutans cells to hard surfaces,[15] but gtfD is dispensable.[16,17] Therefore, these enzymes being the main component in dental plaques and caries created much interest to be targeted in anti-caries therapeutic strategies.[18]
A variety of antiplaque agents are developed and tested for their ability to interfere with dental biofilm formation or metabolism. However, due to their inefficiency or for presence of some harmful side effects, efforts toward finding alternative efficient and safe antiplaque agents are in continued demands. Like all other areas of medical therapeutics that ultimately natural resources proved to be the best, it appears logical to look for a natural product to prevent bacterial biofilm production on teeth surfaces.[19,20]
Lactobacilli, as probiotic agents, are believed to interfere with pathogens through different mechanisms.[21] One of its proposed mechanisms is production of biosurfactants. These secondary products are structurally diverse group of surface-active molecules synthesized by some of the biosurfactant producing microorganisms, have received much attention in recent years because of their superiority over synthetic surfactants, such as low toxicity, biodegradability, and environmentally safe.[22] However, there are some extensive in vitro studies as well as clinical trials regarding the application of bacteriotherapy in caries prevention with several proposed mechanisms through which probiotics interfere with the cariogenic bacteria.[23] Since there are a number of inherent problems in application of direct live bacteria, it seems using probiotics byproducts would be much more desirable and safe.
Here, we investigated effects of biosurfactants produced by Lactobacillus casei (L.c.) on gtfB, gtfC, and ftf gen expression level in S. mutans (ATCC35668) using real-time RT-PCR.
MATERIALS AND METHODS
Bacteria and culture conditions
S. mutans strain ATCC35668 was cultured on blood agar and Mitis salivarius agar medium and incubated in a CO2-enriched atmosphere at 37°C. Lactobacillus casei (ATCC39392) as a probiotic source was cultured in the Man, Rogosa and Sharpe (MRS) broth or agar.
Biosurfactant production
L. casei cultured overnight (15 ml) was inoculated into 600 ml of MRS broth and incubated for 24 hours. The cells were harvested using centrifugation at 10,000 × g for 5 minutes at 10°C, washed twice with demineralized water, and resuspended in 100 ml of PBS. The lactobacilli were incubated at room temperature for 2 hours with gentle stirring for biosurfactant production. Subsequently, the bacterial cells were pelleted using centrifugation, and the supernatant was filtered through a 0.22-mm filter (Millipore). Then dialyzed against distilled water at 4°C in a Spectrapor membrane tube (molecular weight cutoff 6000 to 8000 Da; Spectrum Medical Industries, Inc.) and freeze-dried as described previously.[24]
Drop collapse method
To test whether the biosurfactant produced could decrease the surface tension between water and hydrophobic surfaces, its ability to collapse a droplet of water was tested. Briefly, extracted biosurfactant (25 μl) was pipetted as a droplet onto a piece of parafilm, and the flattening and spreading of the droplet on the parafilm surface was followed over seconds or minutes. Subsequently, methylene blue was added to the water and supernatants for photographic purposes. The droplet was allowed to dry, and the diameter of the dried droplet was recorded.[25,26]
Biofilm formation assay by microtiter plate method
To generate biofilms on microtiter plates, 20 μl of an overnight culture of S. mutans were inoculated in each well of a 24-well polystyrene plate and cultivated in 2 ml BHI broth supplemented with 1% sucrose. The plates were incubated at 37°C in an atmosphere enriched with 5% CO2. After incubation for 18 hours, the spent medium was aspirated, and the wells were washed with PBS to remove unattached cells. The biofilm was incubated again in fresh BHI with 1% sucrose; after 18-hour incubation, the spent medium was aspirated again. The cells were washed, and the biofilm was incubated again in fresh BHI broth with 1% sucrose supplemented with or without 2.5 mg per ml of freeze-dried biosurfactant. After incubation for 4 hours, the cells of the biofilms were dislodged and transferred to tubes containing 2 ml of PBS solution and vortexed.[14]
Extraction of total RNA
The prepared biofilm cells on microtiter plates (S. mutans ATCC 35668 with and without biosurfactant in three replicates) were used for RNA extraction. Cells were disrupted using a ribolyser instrument (Hybaid, UK) and the supplied kit according to the manufacturer's instructions. Briefly, RNA containing supernatant from the ribolyser tube was transferred to a new RNase-free microtube, centrifuged, treated with 300 μl of chloroform-isoamyl alcohol, vortexed, and centrifuged again. Then, total RNA was recovered by precipitation with isopropanol and dried under appropriate sterile conditions. Quantitative and qualitative evaluations of extracted RNA were performed using a spectrophotometer (Biophotometer, Eppendorf, Rs 232-C, Germany) and agarose gel electrophoresis.
Reverse transcription
Each reverse transcription reaction mixture (20 μl) containing 50 ng of random hexamers, 2 μg of total RNA sample, and up to 12 μl of RNase-free water was incubated at 70° C for 5 minutes to remove any secondary structure and placed on ice. Then, 5X RT buffer (4 μl), 2 U of ribonuclease inhibitor, and 10 mM dNTP mix were added to each reaction mixture. After incubation for 5 minutes at 37°C, 1 μl of reverse transcriptase was added. Then, the mixture was incubated at 42°C for 60 minutes. The reaction was terminated by heating the mixture at 70°C for 10 minutes, and the cDNA samples were stored at -70°C for further manipulation.
Real-time quantitative RT-PCR
The amplification of the synthesized cDNAs was first optimized using conventional PCR. Real-time quantitative RT-PCR was performed using the ABI-step I plus (Applied Biosystems, USA) instrument and SYBR Green PCR Master Mix (Qiagen, Germany). The relative quantification of gtfB, gtfC, and ftf genes was performed using 16S rRNA as a reference gene. Three gene-specific primers for gtfB gene (F- AGCAATGCAGCCAATCTACAAAT and R-ACGAACTTTGCCGTTATTGTCA), gtfC (F-CTCAACCAACCGCCACTGTT, R-GGTTTAACGTCAAAATTAGCTGTATTAG), ftf (f-AAATATGAAGGCGGCTACAACG, R-CTTCACCAGTCTTAGCATCCTGAA), and 16SrRNA (F- CCTACGGGAGGCAGCAGTAG, R- CAACAGAGCTTTACGATCCGAAA) as an internal control were used in quantitation of genes under study.[14] The reaction mixture (20 μl) contained 1X SYBR Green PCR Master Mix (Qiagen), the appropriate forward and reverse PCR primers and 1 μl of the cDNA sample. The PCR program consisted of an initial denaturation at 95°C for 5 minutes, 40 cycles of denaturation at 95°C for 15 seconds, and annealing and extension at 60°C for 1 minute. Appropriate negative and positive controls were included. Using the two-step protocol described above, all primer pairs were evaluated for primer dimer formation without the addition of a template. As an additional control for each primer pair and each RNA sample, the cDNA synthesis reaction was performed without reverse transcriptase, to determine whether the RNA samples were contaminated with residual genomic DNA.
The critical threshold cycle (Ct) was defined as the cycle at which fluorescence became detectable above the background fluorescence and was inversely proportional to the logarithm of the initial number of template molecules.
RESULTS
Drop collapse assay
In the drop collapse assay, the biosurfactant droplets resulted in a collapsed droplet [Figure 1], indicating the effect of the biosurfactant on the reduction of surface tension. No activity was detected for distilled water.
Figure 1.
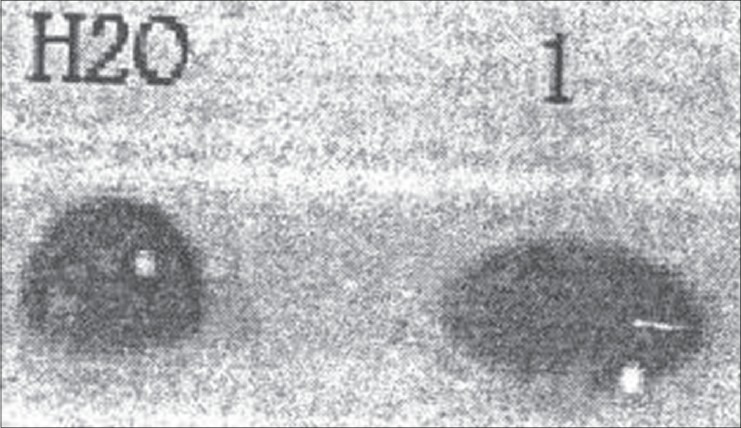
Drop collapse assay. Collapsed droplet (1) is the L.c.- biosurfactant and distilled water
Effects of the biosurfactant on gene expression profiles
For evaluation of the effect of L. casei biosurfactant on gtfB, gtfC, and ftf gene expression in S. mutans ATCC35668, Real-time RT-PCR was used.
The 16S rRNA gene was used as an endogenous reference [Figure 2]. The biosurfactant significantly reduced expression of all three genes under study in comparison to the reference gene. The P values obtained for each of the three genes (for gtfB, P > o.ooo2; for gtfC, P > 0.0063; and for ftf, P > 0.0057), is indicative of the high efficiency of the produced biosurfactant in downregulation of these adhesive promoting genes of S. mutans.
Figure 2.
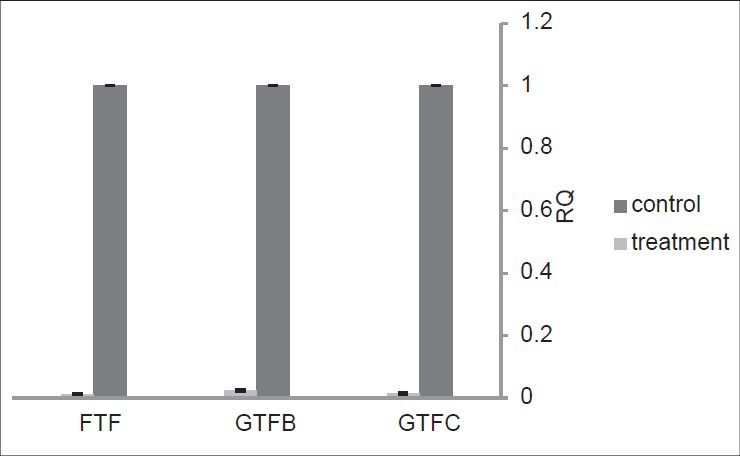
The effect of L.c.-derived biosurfactant on gtfB/C and ftf gene expression level in S. mutans ATCC35668
DISCUSSION
Dental caries are the worldwide serious problems involving all age groups in developing countries as well as developed ones. Since after incidence of dental caries treatments are involving high cost and successful eradication is almost impossible, prevention is most desirable option and recommended strategy. Frequent reports on development of resistance to various synthetic antimicrobials, prompted searching for suitable natural products,[27] such as herbicidal extractions or probiotic bacteria and their products. Lactic acid bacteria produce biosurfactant and one of the major roles known for surfactants is their inhibitory effect on other microbial species.[24,28,29,30] Here, we investigated the effects of biosurfactant extracted from L. casei on gene expression profile of standard biofilm producing strain of S. mutans ATCC35668. The selected genes studied here are all essential for initiation of bacterial adhesion and plaque formation.
The expression of glucosylteransferases like gtf B and C by biofilm producing bacteria and the production of insoluble extracellular glucans mediate the attachment of S. mutans not only to surfaces but also to other active types of bacteria and microorganisms which are in favor of persistent colonization of tooth surfaces by organisms.[31] Additionally, gtf genes are known virulence factors associated with the pathogenesis of dental caries and a high content of insoluble glucans in dental plaque, which is related to an elevated risk of biofilm cariogenicity in humans.[7] Several environmental factors can influence the expression and activity of the gtf enzymes. The existence of at least three enzymes acting on sucrose for production of glucans in addition to factors that involve post-translational modifications of the GTF enzymes, have traditionally complicated the understanding of regulatory studies.[32,33,34]
It is assumed that either the L. casei biosurfactant itself or a putative signaling molecule in the applied surfactant act upon gene expression level in the treated bacteria and hence reducing the process of S. mutans attachment and biofilm formation. Another alternative hypothesis could also put forward stating that biosurfactant may interfere with gtf enzyme activity from insoluble glucan production to water-soluble glucans, hence accounting for reduced S. mutans biofilm adherence. This notion needs confirmation through further studies in the future.
Most studies have focused on the production and gene regulation of virulence factors, such as gtfs, which play an important role in biofilm formation by S. mutans, for controlling dental caries.[35,36] The ability of S. mutans to produce extracellular polysaccharides from dietary sucrose has been demonstrated to significantly enhance its cariogenicity. Thus, the less extracellular polysaccharide produced, the lower the cariogenicity of S. mutans. It is demonstrated that chemical surfactants exerted different effects on the synthesis of glucosyltransferases in S. mutans; Tween 80 significantly increased the level of gtfs, while Triton X-100 decreased gtf levels.[37] In general, the data suggest that biosurfactant treatment can provide an option for controlling biofilm development and also influence the adhesive ability of bacterial pathogens.[38]
Apart from biosurfactants, much efforts were employed to evaluate effects of different natural or synthetic products like different polyphenols,[39] cocoa polyphenols,[40,41] plant extracts,[42] Lectins,[43] ursolic acid (UA)-containing composites,[44] Cranberry A-type proanthocyanidins,[45] mushroom, and chicory extracts,[46] chitosan containing composite resin.[47] However, compared to our study which is providing an insight on mode of inhibitory effect of the applied substances through gene expression profile study, the aforementioned works although proved the inhibitory effects on S. mutans adhesion or biofilm production but providing no information on detail about the underlying molecular mechanism through which this inhibitory effect is exerted.
In view of the results obtained in the present study, it is proposed that L. casei biosurfactant is highly effective in downregulating three proadhesive genes in S. mutans. Therefore, it would be an efficient alternative to the present therapeutics options generally considered for dental caries treatment. However, more elaborated experiments would be necessary to not only elucidate the molecular basis of biosurfactant mechanism in downregulation of the genes under study but also to make a suitable ground for moving toward clinical application of this strategy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Petersen PE, Bourgeois D, Ogawa H, Estupinan Day S. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Dye BA, Nowjack-Raymer R, Barker LK, Nunn JH, Steele JG, Tan S, et al. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003-04. J Public Health Dent. 2008;68:218–26. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 3.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol. 2005;33:248–55. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–7. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozen R, Bachrach G, Bronshteyn M. The role of fructans on dental biofilm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomyces viscosus. FEMS Microbiol Lett. 2001;195:205–10. doi: 10.1111/j.1574-6968.2001.tb10522.x. [DOI] [PubMed] [Google Scholar]
- 7.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. The role of sucrose in cariogenic dental biofilm formation-new insight. J Dent Res. 2006;85:878–87. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vacca-Smith AM, ScottAnne K, Whelehan MT, Berkowitz RJ, Feng C, Bowen WH. Salivary glucosyltransferase B as a possible marker for caries activity. Caries Res. 2007;41:445–50. doi: 10.1159/000107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jae-Gyu J, Klein MI, Xiao J, Gregoire S, Rosalen PL, Koo H. Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiol. 2009;9:228–38. doi: 10.1186/1471-2180-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192:3024–32. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata RM, Branco-de-Almeida EM, Franco R, Yatsuda MH, dos Santos SM, de-Alencarc H, et al. Inhibition of Streptococcus mutans biofilm accumulation and development of dental caries in vivo by 7-epiclusianone and fluoride. Biofouling. 2010;26:865–72. doi: 10.1080/08927014.2010.527435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao J, Koo H. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J Appl Microbiol Rev. 2010;108:2103–13. doi: 10.1111/j.1365-2672.2009.04616.x. [DOI] [PubMed] [Google Scholar]
- 13.Caglar E, Kargul B, Tanbogaet I. Bacteriotherapy and probiotics role on oral health. Oral Dis. 2005;11:1–7. doi: 10.1111/j.1601-0825.2005.01109.x. [DOI] [PubMed] [Google Scholar]
- 14.Tam A, Shemesh M, Wormser U, Sintov A, Steinberg D. Effect of different iodine formulations on the expression and activity of Streptococcus mutans glucosyltransferase and fructosyltransferase in biofilm and planktonic environments. J Antimicrob Chemother. 2006;57:865–71. doi: 10.1093/jac/dkl085. [DOI] [PubMed] [Google Scholar]
- 15.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu HK. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–94. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida A, Kuramitsu HK. Multiple Streptococcus mutans genes are involued in biofilm formation. Appl Environ Microbiol. 2002;68:6283–91. doi: 10.1128/AEM.68.12.6283-6291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida A, Ansai T, Takehara T, Kuramitsu HK. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol. 2005;71:2372–80. doi: 10.1128/AEM.71.5.2372-2380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chia JS, Hsu TY, Teng LJ, Chen JY, Hahn LJ, Yang CS. Glucosyl transferase gene polymorphism among Streptococcus mutans strains. Infect Immun. 1991;59:1656–60. doi: 10.1128/iai.59.5.1656-1660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briand JF. Marine antifouling laboratory bioassays: An overview of their diversity. Biofouling. 2009;25:297–311. doi: 10.1080/08927010902745316. [DOI] [PubMed] [Google Scholar]
- 20.Sendamangalama V, Choi OK, Kim D, Seo Y. The anti-biofouling effect of polyphenols against Streptococcus mutans. Biofouling. 2011;27:13–9. doi: 10.1080/08927014.2010.535897. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues L, Banat IM, Teixeira J, Oliveria R. Biosurfactants: Potential application in medicine. J Antimicrob Chemother. 2006;57:609–18. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 22.Vater J, Kablitz B, Wilde C, Franke P, Mehta N, Cameotra SS. Matrix-assisted laser desorption ionization -time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol. 2002;68:6210–9. doi: 10.1128/AEM.68.12.6210-6219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Twetman S. Are we ready for caries prevention through bacteriotherapy? Braz Oral Res. 2012;26(Suppl 1):64–70. doi: 10.1590/s1806-83242012000700010. [DOI] [PubMed] [Google Scholar]
- 24.Velraeds MC, Mei HC, Reid G, Busscher HJ. Inhibition of Initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol. 1996;62:1958–63. doi: 10.1128/aem.62.6.1958-1963.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuiper I, Lagendijk EL, Pickford R, Derrick JP, Lamers GE, Thomas-Oates JE, et al. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol Microbiol. 2004;51:97–113. doi: 10.1046/j.1365-2958.2003.03751.x. [DOI] [PubMed] [Google Scholar]
- 26.Tugrul T, Cansunar E. Detecting surfactant -producing microorganisms by the drop collapse test. World J Microbiol Biotechnol. 2005;21:851–3. [Google Scholar]
- 27.Allakera RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues L, Teixeira JA, Oliveira R. Modeling of biosurfactant production by Lactobacillus strains [CDROM] Anais do XV Simposio Nacional de bioprocessos – SINAFERM, Recife. 2005. [Last cited on 2010 Mar 16]. Available from: http://hdl.handle.net/1822/3463 .
- 29.Rodrigues L, Teixeira JA, Mei HC, Oliveria R. Isolation and partial characterization of a biosurfactant produced by Streptococcus thermophilus A. Colloids Surf B Biointerfaces. 2006;53:105–12. doi: 10.1016/j.colsurfb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int J Antimicrob Age. 2006;28(Suppl 1):30–4. doi: 10.1016/j.ijantimicag.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Schilling KM, Bowen WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992;60:284–95. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banas JA, Vickerman MM. Glucan binding proteins of the oral streptococci. Crit Rev Oral Biol Med. 2003;14:89–99. doi: 10.1177/154411130301400203. [DOI] [PubMed] [Google Scholar]
- 33.Wen ZT, Burne RA. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J Bacteriol. 2004;186:2682–91. doi: 10.1128/JB.186.9.2682-2691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen ZT, Yates D, Ahn SJ, Burne RA. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111–20. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamwsada M, Kawabata S. Synergistic effects of streptococcal glucosyltransferase on adhesive biofilm formation. J Dent Res. 2004;83:874–9. doi: 10.1177/154405910408301110. [DOI] [PubMed] [Google Scholar]
- 36.Huang M, Meng L, Fan M, Hu P, Bian Z. Effect of biofilm formation on virulence factor secretion via the general secretory pathway in Streptococcus mutans. Arch Oral Biol. 2008;53:1179–85. doi: 10.1016/j.archoralbio.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Tomita Y, Watanabe T, Takeuchi T, Nanbua A, Shinozaki N, Kemi T, et al. Effects of surfactants on glucosyltransferase production and in vitro sucrosedependent colonization by Streptococcus mutans. Arch Oral Biol. 1998;43:735–40. doi: 10.1016/s0003-9969(98)00065-x. [DOI] [PubMed] [Google Scholar]
- 38.Ofek I, Hasty DL, Sharon N. Anti-adhesion therapy of bacterial diseases: Prospects and problems. FEMS Immunol Med Microbiol. 2003;38:181–91. doi: 10.1016/S0928-8244(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 39.Sendamangalam V, Choi OK, Kim D, Seo Y. The anti-biofouling effect of polyphenols against Streptococcus mutans. Biofouling. 2011;27:13–9. doi: 10.1080/08927014.2010.535897. [DOI] [PubMed] [Google Scholar]
- 40.Percival RS, Devine DA, Duggal MS, Chartron S, Marsh PD. The effect of cocoa polyphenols on the growth, metabolism, and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur J Oral Sci. 2006;114:343–8. doi: 10.1111/j.1600-0722.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 41.Ferrazzano GF, Amato I, Ingenito A, De Natale A, Pollio A. Anti-cariogenic effects of polyphenols from plant stimulant beverages (cocoa, coffee, tea) Fitoterapia. 2009;80:255–62. doi: 10.1016/j.fitote.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342–9. doi: 10.1159/000104791. [DOI] [PubMed] [Google Scholar]
- 43.Klafke GB, Borsuk S, Gonçales RA, Arruda FV, Carneiro VA, Teixeira EH, et al. Inhibition of initial adhesion of oral bacteria through a lectin from Bauhinia variegata L. var. variegata expressed in Escherichia coli. J Appl Microbiol. 2013;115:1222–30. doi: 10.1111/jam.12318. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Song M, Roh BD, Park SH, Park JW. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor Dent Endod. 2013;38:65–72. doi: 10.5395/rde.2013.38.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng G, Klein MI, Gregoire S, Singh AP, Vorsa N, Koo H. The specific degree-of-polymerization of A-type proanthocyanidin oligomers impacts Streptococcus mutansglucan-mediated adhesion and transcriptome responses within biofilms. Biofouling. 2013;29:629–40. doi: 10.1080/08927014.2013.794456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Signoretto C, Marchi A, Bertoncelli A, Burlacchini G, Milli A, Tessarolo F, et al. Effects of mushroom and chicory extracts on the shape, physiology and proteome of the cariogenic bacterium Streptococcus mutans. BMC Complement Altern Med. 2013;13:117. doi: 10.1186/1472-6882-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JS, Shin DH. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor Dent Endod. 2013;38:36–42. doi: 10.5395/rde.2013.38.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]


