Abstract
Background:
Sperm DNA in human beings and most vertebrates is packed by protamines into highly compact form of chromatin. There are many staining methods to assess sperm chromatin. Three different methods of staining were used simultaneously in this study and the goal was to determine which of these sperm tests has a relation with fertilization rate in intracytoplasmic sperm injection (ICSI).
Materials and Methods:
Thirty couples who referred to Yamagata University Hospital (Yamagata, Japan) for ICSI were included in this study. The greater part of semen was prepared for ICSI. The remaining part was used for staining with aniline blue, acridine orange, and chromomycin A3 (CMA3). For evaluation of abnormal morphology and abnormality of head, Papanicolaou-stained smears were used. The analysis of data was done using Spearman coefficient of correlation and logistic regression model. Receiver operator characteristic (ROC) curve was used for discrimination of CMA3 staining power to identify ICSI rates.
Results:
Percentage of CMA3 positivity, unlike those of aniline blue and acridine orange, showed significant negative correlation with fertilization rate. Moreover, the percentage of CMA3 positivity showed a positive correlation with the percentage of abnormal morphology and abnormality of head. By dividing patients into CMA3 <48% and CMA3> 48% groups, the area under the curve was 0.646.
Conclusions:
CMA3 staining (protamine deficiency) could be considered as a useful tool for evaluation of male fertility prior to infertility treatment.
Keywords: Acridine orange, aniline blue, chromomycin A3, intracytoplasmic sperm injection, sperm
INTRODUCTION
Semen quality is usually judged by the volume, motility, and morphology of spermatozoa in an ejaculate.[1] It is generally accepted that there is a relation between sperm parameters and fertilizing ability.[2] However, conventional sperm parameters cannot always predict the results of fertilization and this indicates that hidden anomalies lie at the sperm membrane or chromatin level.[3,4]
Since its first clinical success, intracytoplasmic sperm injection (ICSI) has been the most accepted assisted reproduction technology.[5] ICSI is the best technique for treatment of male factor infertile couples. When using ICSI, the sperm-oocyte membrane interaction is not important and further emphasis is on the quality of the sperm chromatin and the ability of the oocyte to initiate decondensation and pronuclear formation.[6]
A number of studies have shown that spermatozoa with abnormal nuclear chromatin organization are more frequent in infertile men.[7,8] In this regard, many functional tests have been developed to assess the relation between sperm chromatin status and the fertilizing ability of a semen sample.[7,8,9] Aniline blue discriminates between lysine-rich histones on the one hand and arginine- and cysteine-rich protamines on the other. The histone-rich nuclei of immature spermatozoa contain abundant lysine and react positively by taking up the aniline blue stain, whereas the protamine-rich nuclei of mature spermatozoa with abundant arginine and cysteine residues react negatively and remain unstained.[7] Also, acridine orange (AO) is a fluorochrome that fluoresces green when bound to native DNA (double stranded and normal) and red when bound to denatured DNA (single stranded).[8] Furthermore, chromomycin A3 (CMA3) is a fluorochrome that has been shown to compete with the protamines for binding to the minor groove of DNA and is a useful tool for assessing packaging quality of the chromatin in sperm and its protamine deficiency.[9]
As mentioned before, nowadays ICSI is a good technique for treatment of severe cases of infertility. Although the tests given above are useful for studying some aspects of chromatin abnormality in human sperms, the relation of them with each other, with conventional sperm parameters, and with the results of ICSI has not been studied in detail. Also, it is important to determine which test gives the most information about the fertility of spermatozoa in ICSI patients. So, in this study, aniline blue, AO, and CMA3 staining methods were used simultaneously for the first time to evaluate the potential value of them for prediction of fertilization power of sperms in ICSI cases.
MATERIALS AND METHODS
Subjects and treatment
Thirty couples referring to Yamagata University Hospital (Yamagata, Japan) for ICSI were included in this study. Follicular growth was stimulated in the female partner using gonadotropin-releasing hormone (GnRH) agonist in combination with human menopausal gonadotropin (hMG). The response to treatment was monitored daily from day 7 of the administration of hMG by ultrasonography. Ovulation was triggered by administration of 10,000 IU human chorionic gonadotropin (hCG). Oocytes were collected 34-36 h post hCG using a simple lumen aspiration needle (Laborafoire C.C.D., Paris, France).
Semen samples were collected by masturbation after 3-4 days of abstinence on the day of oocyte recovery. Two milliliter of semen usually was prepared for ICSI using Percoll gradient (80%) techniques. The remaining part of semen was washed twice in Dulbecco's Ca–Mg free phosphate-buffered saline (PBS) and then was used for staining with aniline blue, AO, and CMA3. Several sperm smears were prepared for aniline blue and AO staining. One milliliter of washed spermatozoa was used for CMA3 staining.
Sperm routine tests assessment
Semen analysis was carried out according to the World Health Organization guidelines.[1] Sperm count was performed in a Makler counting chamber (count was expressed as million/ml). Motility was evaluated by direct microscopic examination. Percentages of abnormal morphology and abnormality of head were assessed by using Papanicolaou-stained smears according to strict criteria.[10]
Aniline blue staining
Immature sperm nuclear protein was stained with acidic aniline blue (Sigma, St Loius, MO, USA), as described by Terquem and Dadoune.[11] Sperm smears were prepared from washed spermatozoa and were fixed in 3% glutaraldehyde in PBS for 30 min. Then the slides were stained with 5% aqueous aniline blue in 4% acetic acid (pH = 3.5). The proportion of sperm stained with aniline blue was determined by counting 200 spermatozoa under oil immersion. The histone-rich nuclei of immature spermatozoa react positively by taking up the aniline blue stain and get blue, whereas the protamine-rich nuclei of mature spermatozoa react negatively and remain unstained.
AO staining
The normality of DNA was examined by AO fluorescence method described by Tejada et al.[8] Sperm smear was prepared from washed spermatozoa and fixed in Carnoy's solution (3 parts methanol and 1 part acetic acid) for 2 h.
The Carnoy's solution was prepared daily. Then the slides were washed in distilled water and allowed to air-dry for a few minutes before staining. The AO (Sigma) staining solution was prepared as follows. Ten milliliters of 1% AO solution in distilled water was added to a mixture of 40 ml of 0.1 M citric acid and 2.5 ml of 0.3 M Na2HPO4, 7H2O, pH 2.5. The 1% AO stock solution was stored in dark at 4°C for 4 weeks. Sperm smear was stained for 5 min. Then the slide was gently rinsed and mounted with distilled water. Slides were read on the same day with a fluorescent microscope (Olympus, Japan) using a 490 nm excitation filter and a 530 nm barrier filter. In each slide, 200 sperms were counted and the percentage of sperm with abnormal DNA was calculated. Sperms with normal (double-stranded) DNA fluoresce green and those with denatured or single-stranded DNA fluoresce red or yellow.
CMA3 staining
Washed spermatozoa were fixed in Carnoy's solution at 4°C for 5 min and then spread on slides. For CMA3 (Sigma) staining,[12] each slide was treated for 20 min with 100 μl of CMA3 solution (0.25 mg/ml in McIlvaine buffer (pH 7.0, containing 10 mM MgCl2). The slides were then rinsed in the buffer glycerol. At least 200 spermatozoa were randomly evaluated on each slide. Microscopic analysis of slides was performed under an objective lens of 100 × magnification in an Olympus (Japan) microscope using appropriate filters (460-470 nm). CMA3 staining evaluation was done by distinguishing between bright yellow stained spermatozoa (CMA3 positive) and dull yellow stained spermatozoa (CMA3 negative).
Intracytoplasmic sperm injection
After oocytes’ collection, they were incubated in 80 IU hyaluronidase in human tubal fluid medium for about 1 min.[13] Then the oocytes were washed in fresh human tubal fluid medium and transferred to Hepes-buffered medium under oil in Petri dish. Prepared sperms were introduced into droplet of polyvinylpyrrolidone (Sigma). The sperm with the best morphology was selected. Each selected sperm was immobilized and sucked into an injection pipette. The sperm was injected into the oocytes.
Statistical analysis
All statistical calculations including Spearman coefficient of correlation and receiver operator characteristic (ROC) analysis were carried out using Statistical Package for Social Studies (SPSS Inc., Chicago, IL, USA) software.
RESULTS
A substantial variation in the percentage of CMA3 staining, aniline blue staining, AO staining, abnormal morphology, and abnormality of head was observed in the ejaculated spermatozoa in the range of 7.4-66.5, 5.9-82, 4.3-92.1, 35.1-95, and 25.5-91.7 respectively.
CMA3 positivity in relation to sperm concentration, motility, and morphology
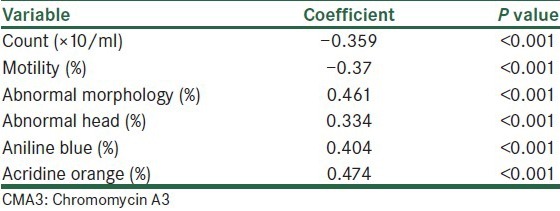
A substantial variation in the percentage of CMA3-stained cells was observed in the ejaculated human spermatozoa, varying between 7.4% and 66.5%. Value of these percentages to individual sperm parameters showed positive correlation between the percentage of CMA3-positive spermatozoa with percentage of abnormal morphology and abnormality of head. Also, there was a negative correlation with count and percentage of motility [Table 1].
Table 1.
Results of Spearman coefficient of correlation between CMA3 positivity and other sperm parameters and staining
CMA3 positivity in relation to aniline blue and AO staining
As seen in Table 1, the percentage of CMA3-positive spermatozoa had a positive significant correlation with the percentage of spermatozoa stained blue in aniline blue staining and with the percentage of spermatozoa stained yellow to red in AO staining [Table 1].
As shown in Table 1, the percentage of CMA3-positive spermatozoa had a positive significant correlation with the other sperm parameters (P < 0.001).
CMA3 positivity and other parameters in relation to fertilization rate in ICSI patients
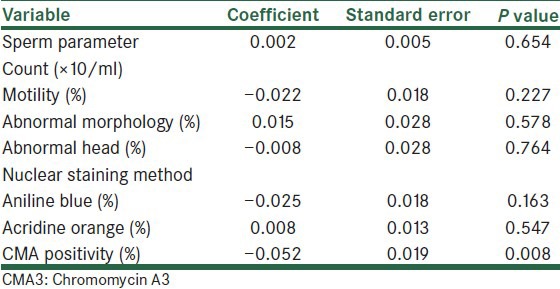
To determine which characteristics of sperm were related to fertilization rates, logistic regression analysis was used [Table 2]. The results showed that only the percentage of CMA3-postive spermatozoa in insemination medium had a negative significant relationship with fertilization rate.
Table 2.
Estimated coefficients of sperm parameters and several sperm nucleus staining methods in a multiple logistic regression model for the fertilization rate
As CMA3 positivity can affect the fertilization rate independently, the thresholds that have been suggested by Esterhuizen et al.[14] were chosen. Patients were grouped into CMA3 levels of < 48% and > 48%. The ROC analysis showed that the area under the curve was 0.646, which indicates that CMA3 is a moderate diagnostic factor [Figure 1].
Figure 1.
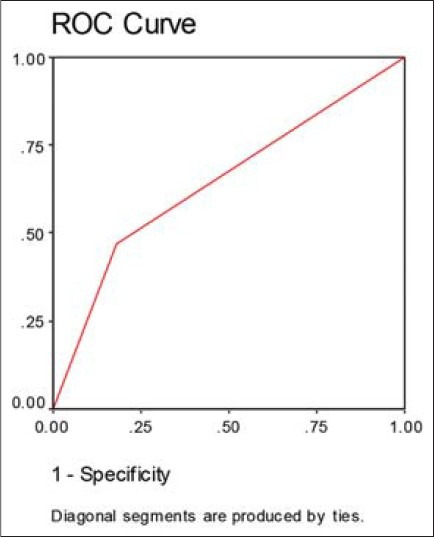
Receiver operator characteristic curve for fertilization rate based on CMA3 staining
DISCUSSION
Sperm nucleus and its chromatin have a special structure that is different from other cells of the human body. The substitution of the chromatin proteins (especially histones) by protamines is one of the central events of spermiogenesis. This event changes the structural organization of highly condensed sperm nucleus and also protects sperm DNA against the enzymatic attack of nucleases and polymerases.[15]
Sperm quality has been conventionally judged by parameters such as concentration, motility, and morphology. As the fertilization barrier is bypassed in ICSI, the sperm chromatin quality and the decondensation and pronuclear formation of oocytes are more important. Chromatin of mature spermatozoa has been shown to possess a varying binding capacity for several dyes and stains. The binding capacity is believed to reflect anomalies in packing quality due to the modifications of the nucleoprotein components occurring during spermiogenesis.[16] Some of these tests involve staining with aniline blue,[7] Methyl Green,[17] Giemsa,[18] and ethidium bromide.[19]
Among these tests, aniline blue discriminates between histone-rich (immature) and protamine-rich (mature) spermatozoa.[11] Also, there are some reports saying that assessment of sperm deoxyribonucleic acid (DNA) normality by staining with AO may help to predict the results of in vitro fertilization (IVF) and ICSI.[8,20] Due to tight packaging afforded by protamines, any modification or absence of these proteins could lead to an anomaly in the packaging process of sperm nucleus and influence the sperm quality (morphology) and fertilizing capacity.[9] In this regard, some researchers previously reported that there is a close relationship between CMA3 staining and fertilization ability of spermatozoa in IVF and ICSI cases.[9,21,22,23,24,25]
This is the first study in which three methods of sperm chromatin assessment were used at the same time in ICSI cases (these tests were directly related to sperm nucleus), while the others usually have one or two methods in their researches. These methods were used simultaneously to evaluate their superiority and ability to predict the results of fertilization rate post ICSI procedure. One of these fluorochromes, CMA3, has been found to be a useful tool for detection of protamine-deficient loosely packed chromatin.[9] Also, in ICSI, sperm is injected directly into the oocyte cytoplasm, and therefore, the interaction of membranes has limited importance. However, the results of this study suggest that CMA3 staining is the only method that can predict the results of ICSI. This result is similar to the results of Tavalaee et al. and Razavi et al.[21,23] Razavi et al. used CMA3, aniline blue, and Sodium dodecyl sulfate methods for prediction of fertilization rate post ICSI. Similarly, they also found that only CMA3 staining has a relation with percentage of fertilized oocytes post ICSI. They also did not see any relation between the percentage of sperms that stained with aniline blue and the fertilization rate in vitro post ICSI. Their results were similar to our findings. Also, Nasr-Esfahani et al. reported that the mean value of fertilization rate was significantly different in patients grouped for CMA3 positivity of 40%.[22] The results of Tavalaee et al. are similar to those of Razavi et al. which indicate the relation of CMA3 positivity with fertilization rate post ICSI. However, there was not any relation between DNA fragmentation and ICSI results.
In our study, AO staining which shows DNA denaturation in sperm chromatin was used. This staining method does not have any relation with ICSI results. Lin et al. also showed that AO staining and DNA fragmentation do not have any relation with ICSI results.[26] The results indicate that DNA condition could not be the problem. In this regard, several investigators have shown that there are significant differences between fertile and nonfertile men in CMA3 staining, aniline blue staining, and DNA damage,[27,28] and also, there is a higher percentage of CMA3- and aniline blue-stained sperms (but not AO-stained) in couples with unexplained recurrent abortion.[29]
However, protamination of sperm DNA is more important because CMA3 staining shows the number of sperms that have proper protamination.[30]
In our previous study, it was shown that both sperm morphology and sperm protamine deficiency affect the fertilization rate independently in IVF cases.[25] But the results of this work show that only protamine deficiency has an effect on fertilization in ICSI. It could be related to the selection of the best morphological sperms in ICSI.
It is observed from our results that CMA3 staining showed positive significant relation with the percentage of abnormality and also abnormality of sperm heads. This fact probably shows that protamine deficiency can cause abnormality of head and abnormality of other parts of sperm. Although there is a relationship between abnormality of sperm head and CMA3 staining, this relation does not have any effect on the fertilization rate in ICSI cases.
The discriminative power of nuclear maturity, as recorded by CMA3 staining to identify ICSI rates was investigated by dividing the patients into CMA3 < 48% and CMA3 > 48%.[14] The area under the ROC curve was 0.646. This value is not so strong, but shows that the patients with percentages under 48 may have better results in ICSI technique. In conclusion, protamine deficiency of sperm chromatin should be considered as an important defect of sperm; so, we can conclude that among the different routine and nuclear sperm assessment methods, CMA3 staining could be a precious method for predicting the results of ICSI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.3rd ed. Cambridge: Cambridge University Press; 1989. World Health Organization: Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucous Interaction. [Google Scholar]
- 2.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 3.Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: Biological and clinical aspects. Asian J Androl. 2006;8:11–29. doi: 10.1111/j.1745-7262.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 4.Leahy T, Gadella BM. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction. 2011;142:759–78. doi: 10.1530/REP-11-0310. [DOI] [PubMed] [Google Scholar]
- 5.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 6.Van Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenwillen C, Tournaye H, et al. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod. 1993;8:1055–60. doi: 10.1093/oxfordjournals.humrep.a138191. [DOI] [PubMed] [Google Scholar]
- 7.Foresta C, Zorzi M, Rossato M, Varotto A. Sperm nuclear instability and staining with aniline blue: Abnormal persistence of histones in spermatozoa in infertile men. Int J Androl. 1992;15:330–7. doi: 10.1111/j.1365-2605.1992.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 8.Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 1984;42:87–91. doi: 10.1016/s0015-0282(16)47963-x. [DOI] [PubMed] [Google Scholar]
- 9.Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi PG, Shoukir Y, et al. Sperm nuclear DNA damage and altered chromatin structure: Effect on fertilization and embryo development. Hum Reprod. 1998;13(Suppl 4):11–9. doi: 10.1093/humrep/13.suppl_4.11. [DOI] [PubMed] [Google Scholar]
- 10.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 11.Terquem A, Dadoune JP. Aniline blue staining of human spermatozoa chromatin: Evaluation of nuclear maturation. In: Andre J, editor. The Sperm Cell. London: Martinus Nijhoff Publishers; 1983. pp. 696–701. [Google Scholar]
- 12.Bianchi PG, Manicardi GC, Urner F, Campana A, Sakkas D. Chromatin packaging and morphology in ejaculated human spermatozoa: Evidence of hidden anomalies in normal spermatozoa. Mol Hum Reprod. 1996;2:139–44. doi: 10.1093/molehr/2.3.139. [DOI] [PubMed] [Google Scholar]
- 13.Bos-Mikich A, Mattos AL, Ferrari AN. Early cleavage of human embryos: An effective method for predicting successful IVF/ICSI outcome. Hum Reprod. 2001;16:2658–61. doi: 10.1093/humrep/16.12.2658. [DOI] [PubMed] [Google Scholar]
- 14.Esterhuizen AD, Franken DR, Lourens JG, Van Zyl C, Müller II, Van Rooyen LH. Chromatin packaging as an indicator of human sperm dysfunction. J Assist Reprod Genet. 2000;17:508–14. doi: 10.1023/A:1009493824953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: Comparison with somatic cells. Biol Reprod. 1991;44:569–74. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- 16.Balhorn R. Mammalian protamine: Structures and molecular interactions. In: Adolph KW, editor. Molecular Biology of Chromosome Function. New York: Springer; 1989. pp. 366–95. [Google Scholar]
- 17.Godowicz B. Histochemical and autoradiographic studies of abnormal spermatozoa in mice of the inbred KE strain. Folia Histochem Cytochem (Krakow) 1977;15:211–5. [PubMed] [Google Scholar]
- 18.Windt ML, De Beer PM, Franken DR, Rhemrev J, Menkveld R, Lombard CJ, et al. Sperm decondensation and semen parameters: Utilization of a simple staining technique for the evaluation of human sperm decondensation. Andrologia. 1994;26:67–72. doi: 10.1111/j.1439-0272.1994.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 19.Filatov MV, Semenova EV, Vorob’eva OA, Leont’eva OA, Dorbchenko EA. Relationship between abnormal sperm chromatin packing and IVF results. Mol Hum Reprod. 1999;5:825–30. doi: 10.1093/molehr/5.9.825. [DOI] [PubMed] [Google Scholar]
- 20.Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A. The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril. 1996;66:634–9. doi: 10.1016/s0015-0282(16)58581-1. [DOI] [PubMed] [Google Scholar]
- 21.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–26. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 22.Nasr-Esfahani MH, Razavi S, Tavalaee M. Failed fertilization after ICSI and spermiogenic defects. Fertil Steril. 2008;89:892–8. doi: 10.1016/j.fertnstert.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Razavi S, Nasr-Esfahani MH, Mardani M, Mafi A, Moghdam A. Effect of human sperm chromatin anomalies on fertilization outcome post-ICSI. Andrologia. 2003;35:238–43. doi: 10.1046/j.1439-0272.2003.00566.x. [DOI] [PubMed] [Google Scholar]
- 24.Lolis D, Gergiou I, Syrrou M, Zikopoulos K, Konstantelli M, Messinis I. Chromomycin A3-staining as an indicator of protamine deficiency and fertilization. Int J Androl. 1996;19:23–7. doi: 10.1111/j.1365-2605.1996.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 25.Iranpour FG, Nasr-Esfahani MH, Valojerdi MR, al-Taraihi TM. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist Reprod Genet. 2000;17:60–6. doi: 10.1023/A:1009406231811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin MH, Kuo-Kuang Lee R, Li SH, Lu CH, Sun FJ, Hwu YM. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril. 2008;90:352–9. doi: 10.1016/j.fertnstert.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi MR, Lakpour N, Heidari-Vala H, Hodjat M, Amirjannati N, Hossaini Jadda H, et al. Relationship between sperm chromatin status and ICSI outcome in men with obstructive azoospermia and unexplained infertile normozoospermia. Rom J Morphol Embryol. 2011;52:645–51. [PubMed] [Google Scholar]
- 28.Nili HA, Mozdarani H, Aleyasin A. Correlation of sperm DNA damage with protamine deficiency in Iranian subfertile men. Reprod Biomed Online. 2009;18:479–85. doi: 10.1016/s1472-6483(10)60123-x. [DOI] [PubMed] [Google Scholar]
- 29.Kazerooni T, Asadi N, Jadid L, Kazerooni M, Ghanadi A, Ghaffarpasand F, et al. Evaluation of sperm's chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J Assist Reprod Genet. 2009;26:591–6. doi: 10.1007/s10815-009-9361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52:864–7. doi: 10.1095/biolreprod52.4.864. [DOI] [PubMed] [Google Scholar]


