Abstract
Background:
Cardiovascular diseases (CVDs) are the leading cause of death in Iran. The present study evaluated the 7-year incidence of CVD risk factors among the participants of Isfahan cohort study (ICS).
Materials and Methods:
ICS was a longitudinal study on adults over 35 years of age from the urban and rural areas in three counties in central Iran. Data on clinical examination and blood measurements were collected in 2001. Subjects were followed and similar data were collected in 2007. Cumulative incidence was calculated through dividing new cases of each risk factor by the population free of that risk factor at baseline. Incidence proportion was determined for major CVD risk factors including hypertension (HTN), hypercholesterolemia (HC), hypertriglyceridemia (HTg), obesity, diabetes mellitus (DM), metabolic syndrome (MetS), and smoking.
Results:
A total number of 6323 adults free of CVDs were recruited. After 7 years of follow-up, 3283 individuals were re-evaluated in 2007. The participants’ age was 49.2 ± 10.3 years in 2001 (mean ± SD). The 7-year cumulative incidence of HTN, HC, HTg, overweight, obesity, DM, MetS, and smoking was 22.8%, 37.4%, 28.0%, 26.3%, 7.4%, 9.5%, 23.9%, and 5.9% in men and 22.2%, 55.4%, 33.5%, 35.0%, 18.8%, 11.3%, 36.1%, and 0.7% in women, respectively. Among those with overweight or obesity, 14.7% of men and 7.9% of women decreased their weight up to the normal level.
Conclusions:
The present study revealed a high incidence of CVD risk factors especially dyslipidemia, obesity, MetS and HTN. Therefore, the application of life-style modification interventions seems necessary.
Keywords: Cardiovascular disease, cumulative incidence, Iran, risk factor
INTRODUCTION
Non-communicable diseases (NCDs) have recently received significant attention in low-and middle-income countries. Over 50% of deaths in the mentioned countries are caused by NCDs, particularly cardiovascular diseases (CVD).[1] In fact, NCDs are predicted to be responsible for more than 3/4 of deaths in low- and middle-income countries by 2020.[2]
Hypertension (HTN), diabetes, dyslipidemia, obesity, and smoking are traditional risk factors for CVD. The World Health Organization (WHO) has identified obesity, HTN, hypercholesterolemia (HC), and smoking as the main risk factors for immature death, and disability.[3] The high prevalence of these factors in the Middle East has led to the higher incidence of CVDs in the region. On the other hand, the growing popularity of urbanization among the Middle Eastern youth would result in higher prevalence of the risk factors and thus, CVDs in the future.[4] NCDs, especially CVDs, are the main cause of death in Iran.[5,6]
Although numerous studies have evaluated the prevalence of CVD risk factors in Iran,[7,8,9,10,11] longitudinal studies that have assessed the incidence of CVD risk factors are scarce[12,13] which mostly assessed one risk factor. Isfahan cohort study (ICS) was a longitudinal study to evaluate the incidence of CVDs and their risk factors in central areas of Iran.[14] The present study reports the 7-year incidence of major CVD risk factors.
MATERIALS AND METHODS
The ICS is a population-based, ongoing longitudinal study of adults aged 35 years old or more, living in urban and rural areas of three counties in central Iran; who had participated in the baseline survey of a community trial for CVD prevention and control, entitled the Isfahan Healthy Heart Program.[15,16] The participants were recruited from January 2 to September 28, 2001.
The baseline survey was conducted in a representative population of adults who were living in urban and rural areas of Isfahan, Najafabad and Arak. Participants were selected by multistage random sampling and were recruited to reflect the age, sex, and urban/rural distribution of the community. Details of the sampling method were described in a former report.[14] Ethical approval was obtained from the Ethics Committee of Isfahan Cardiovascular Research Centre (a WHO collaborating center).
After obtaining informed written consent, medical interview, and physical examination were conducted. Measurements of blood pressure, anthropometric parameters as well as fasting blood tests were carried out following standard protocols and using calibrated instruments as has been described previously.[14] Diabetes mellitus (DM) was defined as fasting plasma glucose ≥126 mg/dL or 2-h postprandial plasma glucose ≥200 mg/dL or being on anti-diabetic agents. Total cholesterol ≥200 mg/dl and triglyceride ≥150 mg/dl or being on anti-lipidaemic agents was defined as HC and hypertriglyceridemia (HTg), respectively. Plasma low density lipoprotein cholesterol (LDL-C) ≥130 mg/dL or being on anti- lipidemic agents was considered as high LDL-C. High density lipoprotein cholesterol (HDL-C) <40 mg/dL in men and <50 mg/dL in women or being on anti-lipidemic agents was the definition for low HDL-C. Systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or being on antihypertensive drugs was defined as hypertensive (HTN). Body mass index (BMI) ≥25 kg/m2 and ≥30 kg/m2 were considered as overweight and obesity, respectively. The recommendation of International Diabetes Federation (IDF) for Middle Easterners to define central obesity as waist circumference ≥94 cm in men and ≥80 cm in women was used.[17] In addition, the local recommendation for Iranian population as the cutoff value of 95 cm for both men and women was employed[18] The updated Adult Treatment Panel III guideline of the National Cholesterol Education Program definition,[19] was used to define metabolic syndrome (MetS) as the presence of three or more of the following components: 1) serum triglycerides ≥150 mg/dL; 2) HDL-C <40 mg/dL for men and < 50 mg/dL for women; 3) plasma glucose ≥100 mg/dL fasting or on treatment; 4) blood pressure ≥130/85 mmHg or anti-hypertensive medication use, and 5) waist circumference ≥102 cm in men and ≥88 cm in women. Subjects who smoked daily were considered as current smoker.
In order to determine CVD events, follow-up surveys were carried out almost biannually asking participants for any hospital admission or neurological symptoms. Detailed explanation for follow-up process is available elsewhere.[14] In 2007 (the 7th year of follow-up), participants were invited for repeated laboratory measurements, physical examination and interview. The subjects who attended measurements in 2007 were labeled as available subjects against those who were lost-to-follow-up or those who did not attend for repeated measurements. Laboratory measurement methods were similar in 2001[14] and 2007, but the used autoanalyzer was different (Eppendorf, Hamburg, Germany in 2001 vs. Hitachi 902, Japan in 2007). Both instruments were tightly qualified and were validated with the external standard laboratory center. The same definitions as what had been used at baseline were adopted for the new findings in 2007. Those with a diagnosis of HTN or diabetes were assumed to have the disease even if they did not fulfill the diagnostic criteria in 2007. However, dyslipidemia was defined only if subjects’ data met the definitions.
Statistical analysis
The cumulative incidence (incidence proportion) for CVD risk factors with 95% confidence interval was calculated as the number of new cases during the study period divided by the number of subjects at risk (free of risk factor of interest) at baseline. Subjects who had reported a positive history of CVD at baseline were excluded from the calculations. Average annual incidence was calculated as cumulative incidence divided by 6.8 years of follow-up, which was the follow-up duration of the study population. Numerical values of risk factors were compared between 2001 and 2007 using the paired t-test. Data entry was carried out using EPI info™. Data were analyzed using the SPSS for Windows (version 15; SPSS Inc., Chicago, IL., USA). For all analyses, statistical significance was assessed at a level of 0.05 (2-tailed). The population attributable risk was calculated from unadjusted incidence rate ratio for CVD events during 7 years of follow-up using Stata (version 11.2, StataCorp LP, TX).
Due to considerable rate of non-response in the second measurement, Heckman two-step method[20] was used to assess the selection bias owing to the possible effect of non-random selection. Two separate models for prediction of incident HTN and DM, as most significant risk factors, were employed using Heckprob command in Stata software (Stata/IC 11.0, StataCorp LP, TX, USA). The selection equation (first step, model of factors associated with non-response) was developed using a set of possible factors (age, sex, urban/rural, education, employment, smoking, BMI categories, blood pressure categories, and pulse rate) as well as prevalent DM for incident HTN and also prevalent HTN for incident DM. The same set of variables except employment was used to predict incident HTN and DM (second step, outcome models using probit regression) taking into account the error terms derived from the first step. Likelihood ratio test of independent equations with the hypothesis that rho statistics equals 0 was considered to show the selection bias. Accordingly, the statistically significant result of the test (reject rho = 0) indicates substantial bias.
RESULTS
At baseline evaluation (2001), 100 (3.2%) men and 81 (2.4%) women reported a history of CVD and were excluded from all further analyses. Among 6323 participants free of CVD, who aged 50.7 ± 11.6 years (mean ± SD) and 3255 (51.5%) were women, laboratory measurements and physical examinations were performed for 3283 participants in 2007 [Figure 1]. The average age of subjects with complete measurements in both assessments was increased from 49.2 ± 10.3 years in 2001 to 55.9 ± 10.4 in 2007, and 1679 (51.2%) of participants were women.
Figure 1.
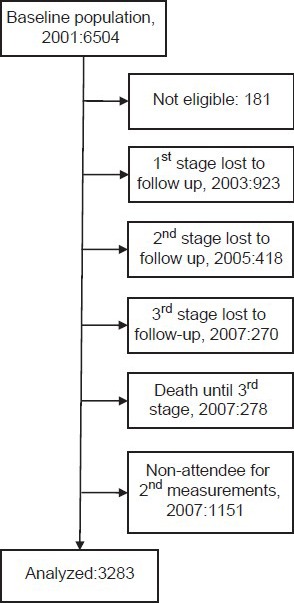
Study algorithm
Considering three categories of participants comprising of available subjects, those who were lost-to-follow-up and those who did not attend for repeated measurements after 7 years, HTN at baseline was significantly higher in the last category (25.4% vs. 25.6% vs. 32.6%, respectively; P < 0.001). Diabetes showed the same pattern but lower differences (9.2% vs. 8.4% vs. 11.3%, respectively; P = 0.034). Inversely, the available subjects who were included in the analysis were slightly more centrally obese (71.0% vs. 68.0% vs. 68.3%, respectively; P = 0.048) and overweight was more in this group (42.4% vs. 37.7% vs. 38.4%, respectively; P = 0.002). Age of the aforementioned groups was 49.2 ± 10.3, 50.4 ± 12.2 and 52.5 ± 12.7, respectively (P < 0.001). There were no further statistically significant differences in terms of sex, urban/rural, smoking, obesity, HC, HTg, low HDL-C, and MetS. Heckman two-step method did not show severe selection bias in the two different models for HTN (P = 0.115) and DM (P = 0.872).
While systolic blood pressure slightly increased by the aging of subjects, diastolic blood pressure decreased to some extent [Table 1]. FPG showed a remarkable increase even in non-diabetics. LDL-C considerably increased by aging, but HDL-C and triglyceride significantly decreased. Despite small but significant reduction in waist circumference, BMI showed a slight increase over 7 years. Table 2 shows the population attributable risk of different CVD risk factors for CVD events.
Table 1.
Changes of blood pressure, lipid profile, fasting plasma glucose, and obesity indices from 2001 to 2007
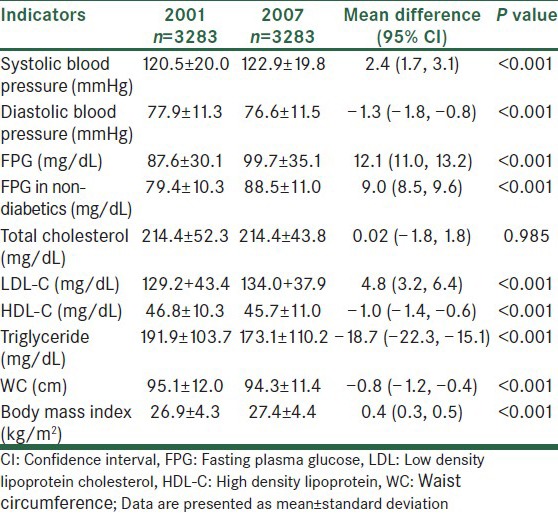
Table 2.
Population attributable risk
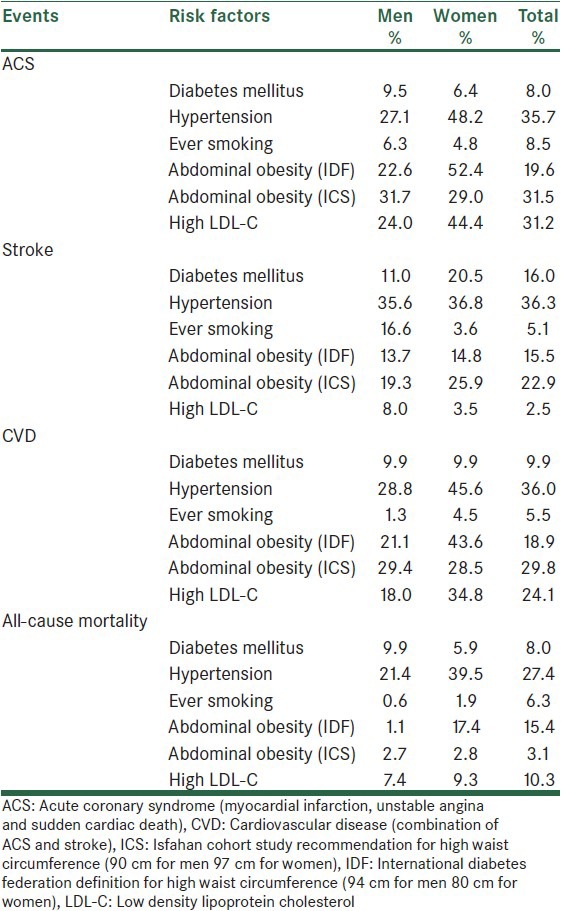
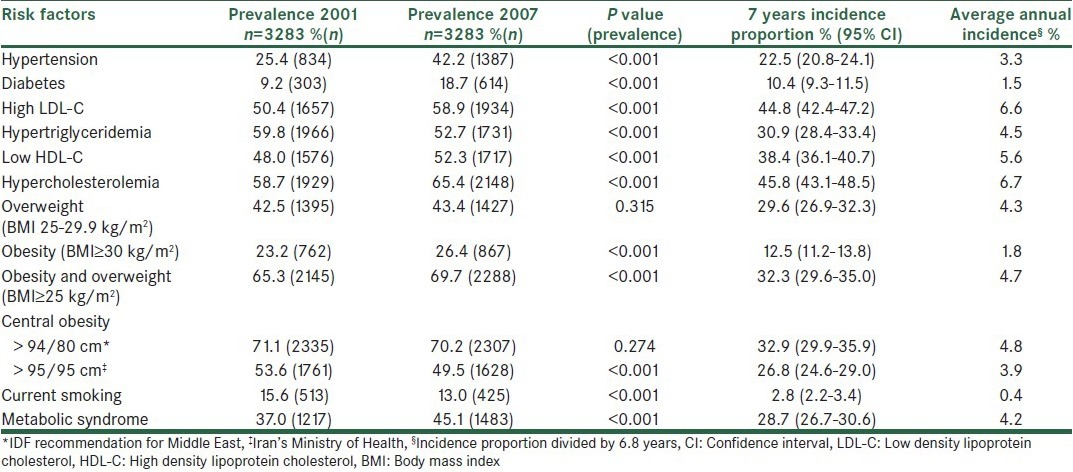
Table 3 shows that the highest incidence proportion in men was seen in high LDL-C followed by HC and low HDL-C. The prevalence of most risk factors significantly increased; however, the most dramatic increase was seen in the prevalence of diabetes followed by HTN. In women, the highest incidence was seen in central obesity (based on IDF definition), HC and high LDL-C and the same as men, prevalence of diabetes and HTN showed a remarkable increase [Table 4]. Despite the considerable incidence proportion, the prevalence of central obesity based on IDF definition, overweight and low HDL-C did not significantly changed in women. Table 5 shows the corresponding incidence and prevalence rates in the whole study population.
Table 3.
Incidence of cardiovascular disease risk factors in men
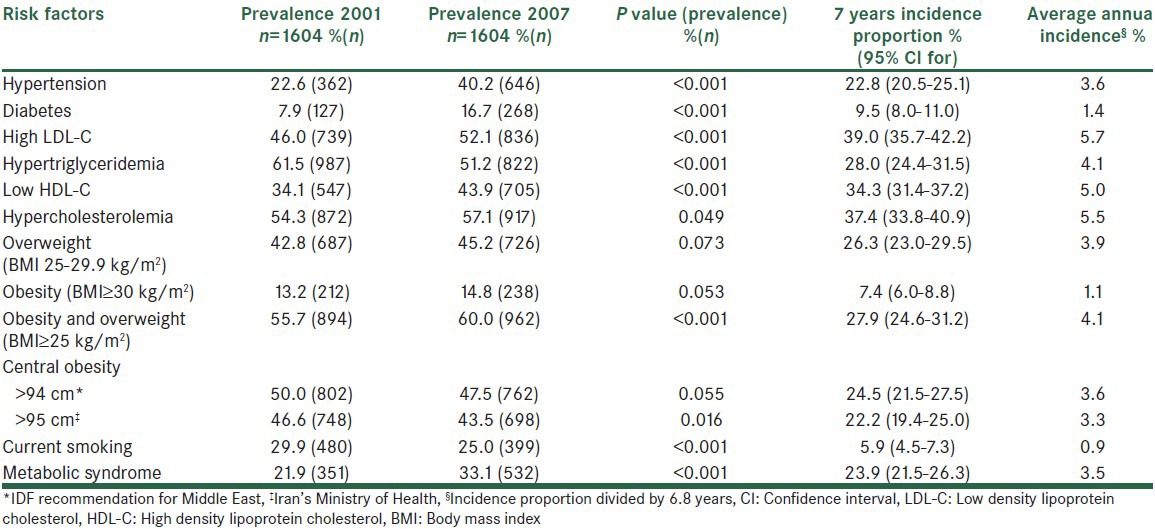
Table 4.
Incidence of cardiovascular disease risk factors in women
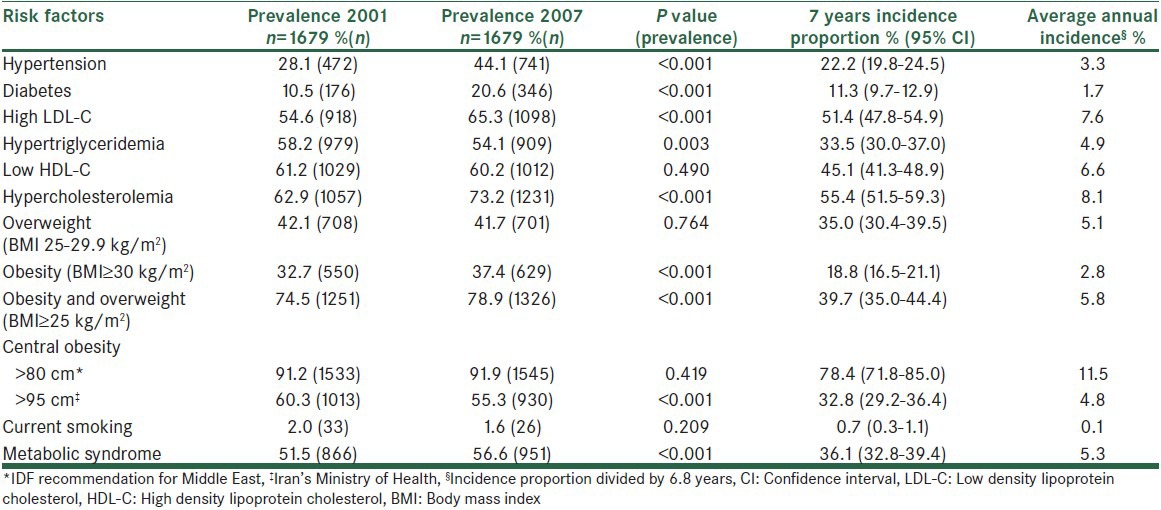
Table 5.
Incidence of cardiovascular disease risk factors in total population
DISCUSSION
Considerable incidence proportion was found for most major CVD risk factors in this study. To the best of our knowledge, the present study is among the few studies to assess the incidence of CVD risk factors in Iran and probably in the Middle East region. Most studies in the field have only evaluated the point prevalence of risk factors. On the other hand, none of the scarce published papers from Iran have considered the incidence of all CVD risk factors concurrently, i.e., they focused mainly on one risk factor.
HTN has been previously reported by the ICS as the most important risk factor for CVD events and death due to CVDs.[14] According to our findings, the 7-year incidence proportion of HTN was 22.5%. It is predicted that in 2025, four out of three hypertensive patients would be living in developing countries.[21] The Framingham study showed the 10-year incidence of HTN in 55-65 year-old men and women as 56% and 52%, respectively.[22] The incidence rate (1000 person/years) in Tehran, the Iran's capital was reported to be 29 for women and 30 for men in a population with a mean age of 42 years,[12] which was lower than findings of the current study. The incidence proportions among individuals over 35 years of age in India and rural areas of China were considerably higher than the present study.[23,24] A Croatian study found that the 5-year incidence among the population over 18 years old to be as high as 34.1%.[25] A Canadian and Korean studies on people older than 20 years of age reported similar rates to ours.[26,27,28] Given the higher ages in our study (over 35 years), this similarity suggests lower incidence in ICS population. However, HTN had one of the highest population attributable risks in our study. It shows that almost one third of CVD events and about 27% of mortality could be eliminated if we were able to prevent HTN.
Obesity is considered as an epidemic now.[29] The overall 7-year incidence of overweight and obesity was 32.3% in our population with the rate being 12% higher in women. Such gender-related difference was also reported by studies in Croatia (with the 5-year incidence of 8.7% and 20.4% in men and women, respectively)[30] and Southern Europe (with the incidence being 2 times more in women).[31] In contrast, the ATTICA study in Greece found the incidence of obesity to be higher in adults men over 18 years old compared to women. It also showed the overall 5-year incidence of obesity and overweight as 8% and 45.4%, respectively.[32] In ICS, with the older population and longer follow-up duration, the incidence of obesity was expectedly higher, but in terms of overweight, it was considerably lower in our population. In an Australian study; however, the 5-year incidence of obesity and overweight was reported as low as 18.1%, which was almost half of its incidence in ICS.[33] To the best of our knowledge, no similar reports about the incidence of obesity are available from other parts of Iran. In addition to being an independent risk factor, obesity contributes to the most other CVD risk factors[34] and further increase CVD risk through them (as mediators).[35]
Central obesity is known to be a better risk factor compared to overall obesity, however, its classification was recommended to be defined locally.[36] As the proposed cutoff point of waist circumference for women in Iran[18] considerably differs from IDF recommendation for the region, our findings were substantially different based on the employed cutoff point. Nevertheless, abdominal girth reduction to the normal level based on IDF, led to a constant prevalence of central obesity during 2001-7. Accordingly, an important priority in the field of central obesity is to reach an agreement in terms of its definition in the region. The incidence of MetS was almost higher than most previous studies for women but not for men.[36] Furthermore, our findings were in line with studies that showed a higher incidence in women, neither those showing similarity in gender nor those showing superiority in men.[36]
Among the various figures that have been reported for the incidence of diabetes in different countries and regions, one of the highest belongs to Asia.[37] Several studies have demonstrated the increasing trend of diabetes incidence during the past decades.[38,39] While the incidence of diabetes in Aboriginal Australians older than 35 years of age was estimated as a little more than 2%,[40] another Australian study reported the rate as 0.8%.[38] A rate of 1% was calculated in the Spanish people aging over 30 years old.[41] European studies have indicated the annual incidence rate of diabetes as 7.6-10.8/1000.[41,42,43,44] A study in Tehran, Iran, revealed the 6-year incidence of diabetes among individuals over 20 years old to be 6.4%.[13] The lower incidence proportion in Tehran can be justified by the younger age. The higher incidence obtained in the present study might be due to demographic differences. Nonetheless, the calculated incidence was as high as the developed countries, which Western life-style is known as the cause. Owing to the importance of diabetes for CVD risk and other health burden, its pre-disposing factors such as obesity should be controlled.
Among the evaluated risk factors, dyslipidemia had the highest incidence proportion. In the present study, the 7-year incidence of HC was much higher in women compared to men (55.4% vs. 37.4%). We could not find any previous Iranian report on the incidence of HC or other kinds of dyslipidemia. Our findings were significantly higher than the 5-year incidence in the ATTICA study in Greece (17.7% in women and 23.7% in men); where in contrast to our study, the incidence was higher in men and the increased incidence after the age of 55 was found.[45] A Portuguese study also reported a similar phenomenon.[46] It suggests age and menopause as possible effective factors on the incidence of HC.
Despite the high incidence of HTg in this study, a significant reduction was observed in its prevalence after 7 years among both genders and more in men. It indicates the dynamic nature of HTg; while a considerable number of new cases emerge by aging, more known patients control it without medication, perhaps by life-style modifications. The prevalence of HTg has been reported as 37.2% in Korea,[47] 30.5% in Turkey,[48] and 24.9% in China.[49] Therefore, the prevalence of this complication among our participants, even after the 7-year reduction, was higher than other parts of the world. On the other hand, an American study suggested prevalence of 53.1% among individuals over 20 years old.[50] Overall, the high prevalence and incidence of HTg and factors affecting its dynamic status need further investigation.
We detected a 4% decrease in the prevalence of smoking among our male participants during the 7-year course of study. Compared to other risk factors, the incidence of smoking was inconsiderable in women and had the lowest value in men. Some other studies have also reported a decreased prevalence of smoking during the same period of time. They suggested anti-smoking regulations to be responsible for such reduction.[51,52,53] A Croatian cohort study found the 5-year incidence of smoking in men and women as 4.9% and 1.1%, respectively.[54] The incidence rate among the 30-39 year-old population was 2.3-7.9% in women and 3.7-8% in men.[55] Smoking thus does not seem to have been more prevalent among our participants compared to other populations. Previous studies have identified the highest incidence of smoking between the ages of 20 years and 29 years.[55] Consequently, low incidence could be because that our participants were not at the age of start smoking; however, long-term adverse effects of smoking on health necessitates the efforts to control smoking be continued.
The main limitation of the present study was the high number of individuals who did not accept to participate in the second measurements. Although, no significant differences were found between this group and others in most variables, the higher prevalence of overweight in the available population could have overestimated the incidence of other risk factors. The lower response rate in those with diabetes or HTN could be attributed to the fact that these subjects should inevitably have been under medical supervision and thus had less motivation to participate. Although, this could not have effect on incidence calculation for diabetes or HTN but these subjects may have constituted a high-risk population. However, MetS, as the most important clustering of risk factors, was not significantly different between the available population and others. It is thus, unlikely that lower prevalence of HTN and diabetes in available subjects compared to those who were lost-to-follow-up and non-attendees have severely biased the estimation of either these two or other risk factors. Another limitation was the two point measurements instead of repeated measurements. The latter would have provided more accurate estimate for the time that each risk factor was emerged and consequently, the calculated incidences were more precise. On the other hand, the way that average annual incidence was calculated, inevitably assumes constant rates over the follow-up period, which is wrong due to the population aging. However, considering the difficulties in the determination of the exact time that a risk factor emerges in a subject, average annual incidence is the best estimation for incidence rate (1000 person/year). The laboratory autoanalyzer was changed in 2007. Although both instruments were certified by external standard laboratory, it might be considered as a possible limitation.
In conclusion, the results of this study confirmed a generally high incidence of CVD risk factors, especially in dyslipidemia, obesity, MetS, and HTN. The incidence rate was generally higher in women than men except HTN. The present study revealed the alarming incidence of overweight and obesity especially, among women, which needs to be considered in health policy-making in Iran. On the other hand, sliming occurred considerably. Hence, although public health programs have been to some extent successful in controlling obesity, more endeavor is still required for wide adoption of a healthy life-style including diet modification and increased physical activity. In addition, the prevalence of many risk factors was shown to increase by increasing age. Life-style modification programs are thus required to be designed and promoted to control the incidence of these risk factors. Such interventions are mostly simple and inexpensive and can be expanded to cover the whole society. They would reduce the incidence of CVD risk factors and their associated diseases and thus save treatment costs. Further studies are indeed necessary to evaluate the factors affecting the incidence of CVD risk factors.
ACKNOWLEDGMENTS
This project would not have succeeded without the sincere efforts of the ICS staffs especially, Mansoureh Boshtam. We would like to express our thanks to our field managers Dr. Yahya Zhand (Arak), Hossein Balouchi (Isfahan) and Ahmadreza Ghasemi (Najafabad) who assisted us in administering the project in 2007. The baseline survey was supported by grant number 31309304. The Isfahan Cardiovascular Research Center, affiliated to Isfahan University of Medical Sciences, supported the biannual follow-ups.
Footnotes
Source of Support: Isfahan Cardiovascular Research Institute
Conflict of Interest: None declared.
REFERENCES
- 1.Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation. Geneva: World Health Organisation; 2011. NCD mortality and morbidit. [Google Scholar]
- 3.World Health Organisation. Geneva: World Health Organisation; 2012. Reducing Risks, Promoting Healthy Life. [Google Scholar]
- 4.Motlagh B, O’Donnell M, Yusuf S. Prevalence of cardiovascular risk factors in the Middle East: A systematic review. Eur J Cardiovasc Prev Rehabil. 2009;16:268–80. doi: 10.1097/HJR.0b013e328322ca1b. [DOI] [PubMed] [Google Scholar]
- 5.Talaei M, Sarrafzadegan N, Sadeghi M, Oveisgharan S, Marshall T, Thomas GN, et al. Incidence of cardiovascular diseases in an Iranian population: The Isfahan Cohort Study. Arch Iran Med. 2013;16:138–44. [PubMed] [Google Scholar]
- 6.Naghavi M, Abolhassani F, Moradi Lakeh M, Jafari N, Vaseghi S, Kazemeini H. Tehran: Ministry of Health; 2003. National burden of dieases, injuries, and risk factors and disability adjusted life expectancy in Islamic Republic of Iran in 2003. [Google Scholar]
- 7.Ayatollahi SM, Ghoreshizadeh Z. Prevalence of obesity and overweight among adults in Iran. Obes Rev. 2010;11:335–7. doi: 10.1111/j.1467-789X.2010.00725.x. [DOI] [PubMed] [Google Scholar]
- 8.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third national surveillance of risk factors of non-communicable diseases (SuRFNCD-2007) in Iran: Methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janghorbani M, Amini M, Gouya MM, Delavari A, Alikhani S, Mahdavi A. Nationwide survey of prevalence and risk factors of prehypertension and hypertension in Iranian adults. J Hypertens. 2008;26:419–26. doi: 10.1097/HJH.0b013e3282f2d34d. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghi M, Roohafza HR, Kelishadi R. Blood pressure and associated cardiovascular risk factors in Iran: Isfahan healthy heart programme. Med J Malaysia. 2004;59:460–7. [PubMed] [Google Scholar]
- 11.Sadeghi M, Roohafza H, Shirani S, Poormoghadas M, Kelishadi R, Baghaii A, et al. Diabetes and associated cardiovascular risk factors in Iran: The Isfahan healthy heart programme. Ann Acad Med Singapore. 2007;36:175–80. [PubMed] [Google Scholar]
- 12.Bozorgmanesh M, Hadaegh F, Mehrabi Y, Azizi F. A point-score system superior to blood pressure measures alone for predicting incident hypertension: Tehran lipid and glucose study. J Hypertens. 2011;29:1486–93. doi: 10.1097/HJH.0b013e328348fdb2. [DOI] [PubMed] [Google Scholar]
- 13.Harati H, Hadaegh F, Saadat N, Azizi F. Population-based incidence of Type 2 diabetes and its associated risk factors: Results from a six-year cohort study in Iran. BMC Public Health. 2009;9:186. doi: 10.1186/1471-2458-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarrafzadegan N, Talaei M, Sadeghi M, Kelishadi R, Oveisgharan S, Mohammadifard N, et al. The Isfahan cohort study: Rationale, methods and main findings. J Hum Hypertens. 2011;25:545–53. doi: 10.1038/jhh.2010.99. [DOI] [PubMed] [Google Scholar]
- 15.Sarraf-Zadegan N, Sadri G, Malek Afzali H, Baghaei M, Mohammadi Fard N, Shahrokhi S, et al. Isfahan healthy heart programme: A comprehensive integrated community-based programme for cardiovascular disease prevention and control. Design, methods and initial experience. Acta Cardiol. 2003;58:309–20. doi: 10.2143/AC.58.4.2005288. [DOI] [PubMed] [Google Scholar]
- 16.Sarrafzadegan N, Baghaii A, Sadri G, Kelishadi R, Malekafzali H, Boshtam M, et al. Isfahan healthy heart program: Evaluation of comprehensive, community-based interventions for non-communicable disease prevention. Prev Control. 2006;2:73–84. [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 18.Azizi F, Khalili D, Aghajani H, Esteghamati A, Hosseinpanah F, Delavari A, et al. Appropriate waist circumference cut-off points among Iranian adults: The first report of the Iranian National Committee of Obesity. Arch Iran Med. 2010;13:243–4. [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American heart association/National heart, lung, and blood institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Grotzinger KM, Stuart BC, Ahern F. Assessment and control of nonresponse bias in a survey of medicine use by the elderly. Med Care. 1994;32:989–1003. doi: 10.1097/00005650-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 22.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–10. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 23.Sathish T, Kannan S, Sarma PS, Razum O, Thankappan KR. Incidence of hypertension and its risk factors in rural Kerala, India: A community-based cohort study. Public Health. 2012;126:25–32. doi: 10.1016/j.puhe.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Zheng L, Detrano R, Zhang X, Xu C, Li J, et al. Incidence and predictors of hypertension among rural Chinese adults: Results from Liaoning province. Ann Fam Med. 2010;8:19–24. doi: 10.1370/afm.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erceg M, Ivicević-Uhernik A, Kern J, Vuletić S. Five-year cumulative incidence of hypertension in adult Croatian population: The CroHort study. Coll Antropol. 2012;36(Suppl 1):83–7. doi: 10.5671/ca.2012361s.83. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Yang DH, Park HS, Cho Y, Jun JE, Park WH, et al. Incidence of hypertension in Korea: 5-year follow-up study. J Korean Med Sci. 2011;26:1286–92. doi: 10.3346/jkms.2011.26.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robitaille C, Dai S, Waters C, Loukine L, Bancej C, Quach S, et al. Diagnosed hypertension in Canada: Incidence, prevalence and associated mortality. CMAJ. 2012;184:E49–56. doi: 10.1503/cmaj.101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu K, Chen Z, Lipscombe LL. Canadian Hypertension Education Program Outcomes Research Taskforce. Prevalence and incidence of hypertension from 1995 to 2005: A population-based study. CMAJ. 2008;178:1429–35. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Geneva: World Health Organization; 2011. Obesity and overweight. Report No: 311. [Google Scholar]
- 30.Milanović SM, Uhernik AI, Fister K, Mihel S, Kovac A, Ivanković D. Five-year cumulative incidence of obesity in adults in Croatia: The CroHort study. Coll Antropol. 2012;36(Suppl 1):71–6. [PubMed] [Google Scholar]
- 31.Ortiz-Moncada R, García M, González-Zapata LI, Fernandez E, Alvarez-Dardet C. Incidence of overweight and obesity in a Mediterranean population-based cohort: The Cornellà health interview survey follow-up study (CHIS.FU) Prev Med. 2010;50:45–9. doi: 10.1016/j.ypmed.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Yannakoulia M, Panagiotakos D, Pitsavos C, Lentzas Y, Chrysohoou C, Skoumas I, et al. Five-year incidence of obesity and its determinants: The ATTICA study. Public Health Nutr. 2009;12:36–43. doi: 10.1017/S1368980008001900. [DOI] [PubMed] [Google Scholar]
- 33.Barr E, Cameron A. Results for New South Wales. New South Wales, Australia: International Diabetes Institute; 2005. The Australian Diabetes Obesity and Lifestyle Study (AusDiab). Five Year Follow-up. [Google Scholar]
- 34.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:663–84. doi: 10.1016/j.ecl.2008.06.004. ix. [DOI] [PubMed] [Google Scholar]
- 35.Talaei M, Thomas GN, Marshall T, Sadeghi M, Iranipour R, Oveisgharan S, et al. Appropriate cut-off values of waist circumference to predict cardiovascular outcomes: 7-year follow-up in an Iranian population. Intern Med. 2012;51:139–46. doi: 10.2169/internalmedicine.51.6132. [DOI] [PubMed] [Google Scholar]
- 36.Santos AC, Severo M, Barros H. Incidence and risk factors for the metabolic syndrome in an urban South European population. Prev Med. 2010;50:99–105. doi: 10.1016/j.ypmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Ubink-Veltmaat LJ, Bilo HJ, Groenier KH, Houweling ST, Rischen RO, Meyboom-de Jong B. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited: A prospective population-based study in The Netherlands (ZODIAC-1) Eur J Epidemiol. 2003;18:793–800. doi: 10.1023/a:1025369623365. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Hoy WE, Si D. Incidence of type 2 diabetes in Aboriginal Australians: An 11-year prospective cohort study. BMC Public Health. 2010;10:487. doi: 10.1186/1471-2458-10-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB Sr. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: The Framingham Heart Study. Circulation. 2006;113:2914–8. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 41.Valdés S, Botas P, Delgado E, Alvarez F, Cadórniga FD. Population-based incidence of type 2 diabetes in northern Spain: The Asturias Study. Diabetes Care. 2007;30:2258–63. doi: 10.2337/dc06-2461. [DOI] [PubMed] [Google Scholar]
- 42.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: The Bruneck study. Diabetes. 2004;53:1782–9. doi: 10.2337/diabetes.53.7.1782. [DOI] [PubMed] [Google Scholar]
- 43.Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of Type 2 diabetes in England and its association with baseline impaired fasting glucose: The Ely study 1990-2000. Diabet Med. 2007;24:200–7. doi: 10.1111/j.1464-5491.2007.02068.x. [DOI] [PubMed] [Google Scholar]
- 44.Leibson CL, O’Brien PC, Atkinson E, Palumbo PJ, Melton LJ., 3rd Relative contributions of incidence and survival to increasing prevalence of adult-onset diabetes mellitus: A population-based study. Am J Epidemiol. 1997;146:12–22. doi: 10.1093/oxfordjournals.aje.a009187. [DOI] [PubMed] [Google Scholar]
- 45.Panagiotakos DB, Pitsavos C, Skoumas Y, Lentzas Y, Papadimitriou L, Chrysohoou C, et al. Abdominal obesity, blood glucose and apolipoprotein B levels are the best predictors of the incidence of hypercholesterolemia (2001-2006) among healthy adults: The ATTICA study. Lipids Health Dis. 2008;7:11. doi: 10.1186/1476-511X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa J, Borges M, Oliveira E, Gouveia M, Carneiro AV. Incidence and prevalence of hypercholesterolemia in Portugal: A systematic review. Rev Port Cardiol. 2003;22(Part I):569–77. [PubMed] [Google Scholar]
- 47.Kwon HS, Park YM, Lee HJ, Lee JH, Choi YH, Ko SH, et al. Prevalence and clinical characteristics of the metabolic syndrome in middle-aged Korean adults. Korean J Intern Med. 2005;20:310–6. doi: 10.3904/kjim.2005.20.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erem C, Hacihasanoglu A, Deger O, Kocak M, Topbas M. Prevalence of dyslipidemia and associated risk factors among Turkish adults: Trabzon lipid study. Endocrine. 2008;34:36–51. doi: 10.1007/s12020-008-9100-z. [DOI] [PubMed] [Google Scholar]
- 49.Li G, de Courten M, Jiao S, Wang Y. Prevalence and characteristics of the metabolic syndrome among adults in Beijing, China. Asia Pac J Clin Nutr. 2010;19:98–102. [PubMed] [Google Scholar]
- 50.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169:572–8. doi: 10.1001/archinternmed.2008.599. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention (CDC). Current cigarette smoking prevalence among working adults – United States, 2004-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1305–9. [PubMed] [Google Scholar]
- 52.Langley TE, Szatkowski LC, Wythe S, Lewis SA. Can primary care data be used to monitor regional smoking prevalence? An analysis of The Health Improvement Network primary care data. BMC Public Health. 2011;11:773. doi: 10.1186/1471-2458-11-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mackay DF, Haw S, Pell JP. Impact of Scottish smoke-free legislation on smoking quit attempts and prevalence. PLoS One. 2011;6:e26188. doi: 10.1371/journal.pone.0026188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samardzić S, Vuletić G, Tadijan D. Five-year cumulative incidence of smoking in adult Croatian population: The CroHort study. Coll Antropol. 2012;36:99–103. doi: 10.5671/ca.2012361s.99. [DOI] [PubMed] [Google Scholar]
- 55.Menezes AM, Lopez MV, Hallal PC, Muiño A, Perez-Padilla R, Jardim JR, et al. Prevalence of smoking and incidence of initiation in the Latin American adult population: The PLATINO study. BMC Public Health. 2009;9:151. doi: 10.1186/1471-2458-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]


