Abstract
Background:
Keratoconus is an asymmetric, bilateral, progressive noninflammatory ectasia of the cornea that affects approximately 1 in 2000 of the general population. This may cause a significant negative impact on quality of life. Corneal collagen crosslinking (CXL) is one of the recently introduced methods that have been used to decrease the progression of keratoconus, in particular, as well as other corneal-thinning processes.
Materials and Methods:
A total of 44 keratoconic eyes of 22 patients were enrolled in this randomized prospective study, after obtaining informed consent. In the first group, the corneal epithelium were totally removed and in the second group, the central 3 mm of epithelium was kept intact and partial removal was performed. After collagen crosslinking in both groups, comprehensive ophthalmologic examination was performed on all patients before and 6 months after the surgery. This article is registered at www.clinicaltrial.gov with registration number NCT01809977.
Results:
The difference between the two groups was not statistically significant regarding postoperative corneal haziness, refraction, and visual acuity (P > 0.05). However, comparison of pre- and postoperative parameters within each group revealed that total removal of the cornea has resulted in significant improvement of K-max (P value: 0.01) and Q-value (P value: 0.009); while eyes in partial removal group had better improvement of corrected vision (P value: 0.006). Both methods had significant and similar increase in optical corneal density (P < 0.0001).
Conclusion:
In our study, keeping the central corneal epithelium intact was not beneficial for decreasing corneal haziness, however, this method caused better improvement in corrected vision. Total epithelium off technique resulted in better improvement of K-max and Q-value.
Keywords: Corneal haze, cross-linking, keratoconus, riboflavin, UVA
INTRODUCTION
Keratoconus is an asymmetric, bilateral, progressive noninflammatory ectasia of the cornea that affects approximately 1 in 2000 of the general population.[1,2,3] Although the exact cause of keratoconus is not clear,[4] it is believed that keratoconus is caused by gradual bio-mechanical weakening of the cornea due to changes in corneal collagen structure and organization, alterations of the extra-cellular matrix and keratocyte apoptosis.[5,6] Keratoconus may only lead to a slight irregular astigmatism or cause severe visual impairment.[4] It mostly affects patients during the second decade of life;[4,7] therefore, it may cause a significant negative impact on quality of life.[8] For this reason, several treatment options have been developed to stop the progression of keratoconus and to improve vision.
Corneal collagen crosslinking (CXL) is one of the recently introduced methods that has been used to decrease the progression of keratoconus, in particular, and in other corneal-thinning processes as well.[9] CXL predominantly affects the anterior 300 μm of the cornea.[10] In addition to stabilizing the corneal architecture, CXL can have beneficial effects on corneal optics and vision.[11,12,13,14] CXL results in improved corrected distance visual acuity (CDVA), uncorrected distance visual acuity (UDVA), maximum and average keratometry (K) values, several corneal topography indices, and corneal and optical higher-order aberrations (HOAs).[15]
However, similar to other surgical procedures, CXL may cause several short- and long-term complications including corneal haze, permanent scars, endothelial damage, treatment failure, sterile infiltrates, and herpes reactivation.[16]
Corneal haze is a relatively common complication that may occur in 10-90% of patients.[17] Post-CXL corneal haze extends into the anterior stroma to approximately 60% depth. The depth of crosslinking into the stroma, could be associated to the amount of keratocyte loss and methods used to remove corneal epithelium.[2,16,18] Corneal haze may affect corrected visual acuity, and therefore, cause deterioration of vision.[19] Given the proposed potential role for the method used to remove corneal epithelium in corneal haze, effects of corneal haze on visual outcomes and the vital role of central cornea in the quality of vision, this study was designed to quantitatively evaluate whether partial removal versus total removal of the corneal epithelium can lead to less corneal haze and better visual outcomes.
MATERIALS AND METHODS
After approval of the study by the ethic committee and obtaining informed consent, this clinical trial was performed on patients with the diagnosis of keratoconus.
Inclusion criteria were age between 16 and 40 years, axial topography consistent with keratoconus, minimum corneal thickness more than 400 μm and a progression of keratoconus in past 12 months (an increase in maximum keratometry (K) of 1.00 diopter (D), an increase of refractive astigmatism of 1 D of or increase of refractive error of 0.5 D).[20]
In addition to patients with a history of ocular herpes or nonhealing corneal ulcers, subjects with current ocular infection, severe preoperative corneal haze or scar, severe ocular surface disease, autoimmune diseases, and pregnant women were excluded.
Finally, a convenient sample of 22 patients (44 eyes) who met the study criteria were enrolled for this study. Using computer generated random numbers, patients were randomly distributed into two treatment groups, and both eyes were treated with the same method [Figure 1].
Figure 1.
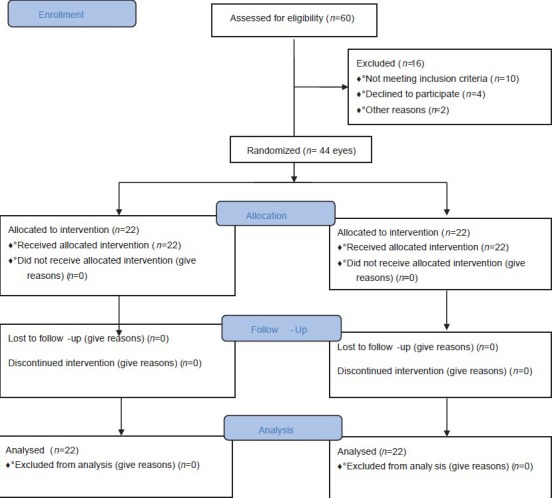
Study CONSORT flowchart
In order to record preoperative characteristics, a comprehensive ophthalmologic examination was performed on all patients before the surgery. The examination included best corrected visual acuity, Pentacam Scheimpflugimagery (OCULUS Optikgerate GmbH, Wetzlar, Germany) and refraction.
Pentacam was used to examine K-max (D), Q-value, Thinnest point, IVA (Index of vertical asymmetry), IHD (Index of height decentration), ISV (Index of surface variance), KI (Keratoconus index), CKI (Central keratoconus index), IHA (Index of height asymmetry), Rmin (Minimum radius of curvature).
Tonometry measurements were performed by Goldman Tonometry (Haag-Streit AG) using a sodium fluorescein solution.
Then, according to the treatment group, patients underwent CXL with either total or partial corneal epithelium removal. All operations were performed by the same surgeon.
The CXL procedure was performed under sterile condition. In order to reduce the risk of retinal and lens damage, all patients received Pilocarpine (eye drop 2%) preoperatively. Then, topical anesthesia was administered, and the corneal epithelium was removed by mechanical debridement. In the first group, total removal was performed by mechanical debridement of the corneal epithelium over the central 9 mm. In the second group, partial removal was performed by removing a 3 mm width ring and leaving the central 3 mm of the cornea intact [Figure 2]. Then, riboflavin (0.1% in 20% dextran solution) was administered topically every minute for 30 minutes. After that, a slit-lamp examination was performed to confirm riboflavin absorption throughout the corneal stroma and anterior chamber. Thereafter, 3 mW/cm2 ultraviolet-A (370 nm wavelength UVA) corresponding to a surface dose of 5.4 J/cm2) irradiation was applied to the cornea for 30 minutes at a working distance of 5 cm. AIROC UV-X™ 1000 device (IROC Innocross AG, Switzerland) was used as the UVA radiation source. During the procedure, riboflavin solution and topical anesthetic agent were administered every 2-3 minutes to moisten the cornea and to saturate it with riboflavin. Subsequently, a therapeutic contact lens was placed after the operation and maintained for 7 days.
Figure 2.
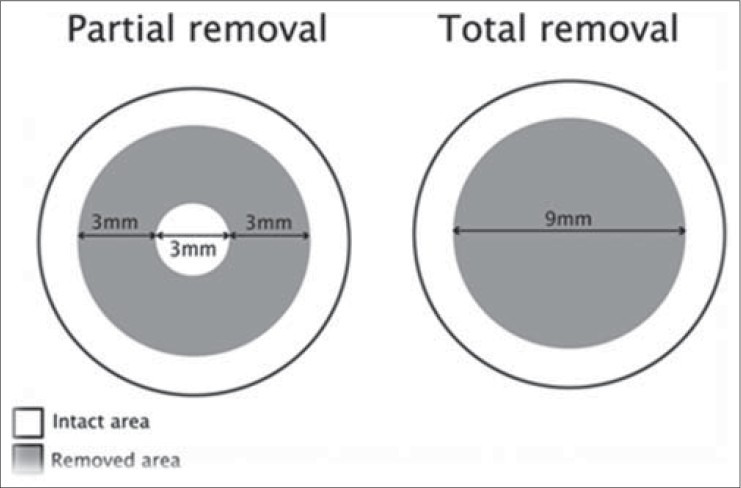
Schematic comparison of 2 surgical methods
After the surgery, all patients were applied with Betamethasone eye drop (1 drop every 3 hours) and Ciprofloxacin eye drop (1 drop every 6 hours) for 4 and 1 weeks, respectively.
Six months after the procedure, the aforementioned ophthalmologic examinations were repeated. Scheimpflug images of all eyes were taken with a Pentacam Scheimpflug camera (Oculus, Inc.). Grading of corneal haze at the slit lamp, however, is related to observer interpretation and is hard to quantify objectively. Therefore, to better the quantification of CXL-associated corneal haze, we used Scheimpflug densitometry module in this study to measure of CXL-associated corneal haze objectively. Corneal densitometry was measured over the central 3.0 mm along 1 meridian. The meridian of the image used was determined as the axis nearest to the maximum K value, and the Scheimpflug image at this axis was used for analysis.
Data were analyzed by the Statistical Package for the Social Sciences (SPSS) 20.0 (SPSS Inc., Chicago, IL, USA). Independent t-test, paired-t test, and Chi-square were used when appropriate. P < 0.05 were considered statistically significant.
RESULTS
The present study investigated 44 eyes of 22 patients (12 (27%) eyes of men and 32 (73%) eyes of women) with a mean age of 29.80 ± 9.93 years (range: 20-38 years). There was no significant difference between the two groups regarding demographic characteristics [Table 1].
Table 1.
Baseline characteristics
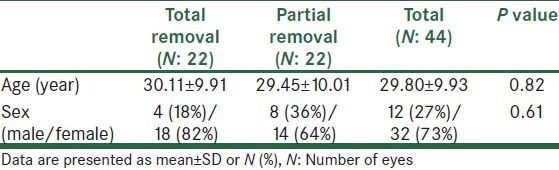
In addition, no significant difference was found between the two groups in preoperative best corrected visual acuity (BCVA), topography indices, and corneal density [Table 2].
Table 2.
Comparison of pre- and postoperative parameters within and between 2 groups
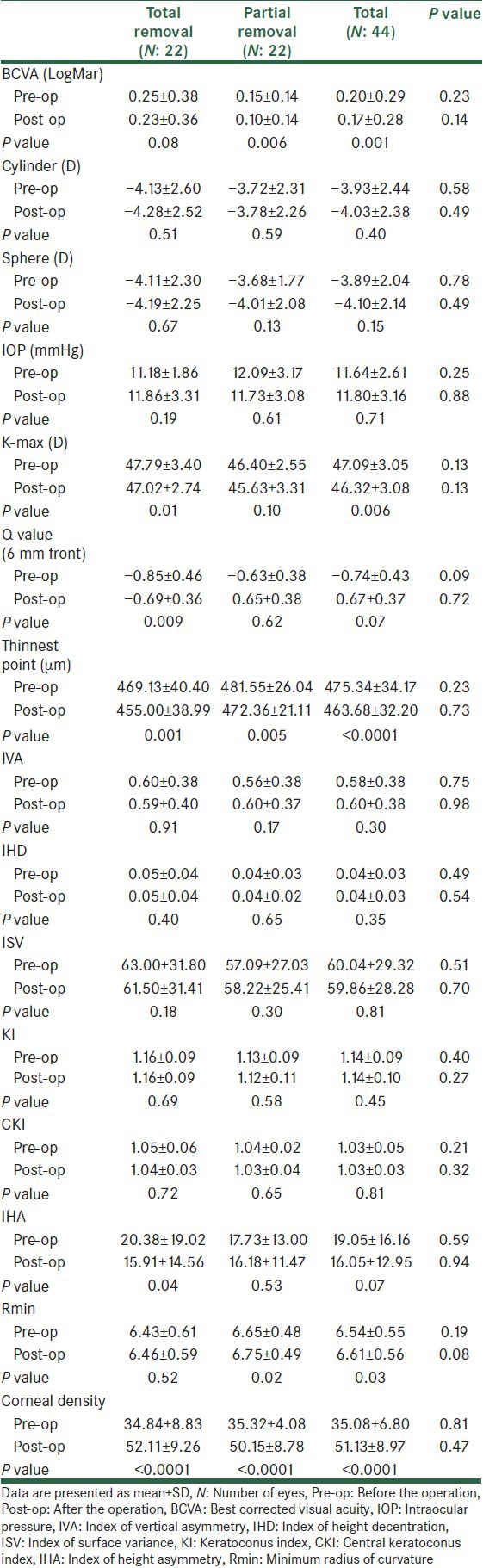
BCVA (LogMar) improved in both treatment groups after the operation. However, the improvement was statistically significant only in patients treated with partial removal method [Table 2].
Although mean of postoperative BCVA in the partial removal group was better than the total removal group, the difference was not statistically significant [Table 2].
Neither the total removal nor the partial removal group revealed significant improvement of mean of sphere, cylinder, and axis after the procedure. In addition, there was no statistical difference between groups in mean of postoperative sphere, cylinder, and axis [Table 2].
Mean of postoperative intraocular pressure (IOP) did not differ significantly between the two groups. Similarly, no statistical difference was found between pre- and postoperative IOP values within each group [Table 2].
There was no significant difference between groups regarding postoperative topography indices [Table 2]. However, comparison of pre- and postoperative values within each group showed significant decrease of K-max, Q-value, and index of height asymmetry (IHA) after total removal CXL, significant increase of R-min after partial removal CXL and significant decrease of the average of thinnest point after CXL with both methods [Table 2].
Corneal haziness was assessed by Scheimpflug densitometry. At the end of the 6 month follow-up, there was no significant difference between groups regarding the postoperative peak corneal density. However, postoperative corneal density was significantly higher than the preoperative value in both groups [Table 2].
None of the patients developed any serious complications.
DISCUSSION
Keratoconus is a progressive corneal disorder characterized by bilateral conical protrusion and corneal thinning.[21] Although there are various conservative and surgical therapeutic options such as glasses, contact lenses, intracorneal rings, epi-keratoplasty, thermal keratoplasty, and lamellar or penetrating keratoplasty, these methods can only correct the refractive effect temporarily but do not stop the progression of keratoconus.[22]
CXL is the only available treatment directed at corneal stromal biomechanical and structural instability, which is the underlying pathology of keratoconus.[16] This technique was introduced by Wollensak et al. in 2003 for the first time, and is based on the combined use of the photo-sensitizer riboflavin and UVA light of 370 nm.[16]
Although CXLwas primarily developed to mitigate progression of ectatic corneal processes, it has also resulted in improvement of visual acuity and corneal topography characteristics in some patients.[11,12,13,14,22,23,20,24] For this reason, CXL has been considered as a new promising modality that can stabilize the cornea in keratoconus[2] and ectasia.[9,25] CXL increases the biomechanical stiffness of the cornea.[26,27] Therefore, it slows the progression of keratoconus, and may improve the visual and topographic outcomes in many patients.[11,12,13,14,22] However, like other corneal procedures, CXL may cause corneal haze. Corneal haze after CXL is different in clinical character from the haze after other procedures. The former is a dust-like change in the corneal stroma or a mid-stromal demarcation line, whereas the appearance of the latter one is a more reticulated sub-epithelial.[28,29,30,31]
Post-CXL corneal haze usually extends into the anterior stromato approximately 60% depth, which is on average equal to an absolute depth of 300 μm.[28,29,30,31] Corneal haze may significantly affect the quality of vision[19] specially if it involves the center of the cornea. Post-CXL haze could be associated with the depth of CXL into the corneal stroma as well as the amount of keratocyte loss. In addition, it was reported that the intact corneal epithelium may play an important role in prevention of corneal haze.[32] Based on the above evidence, it was proposed that partial removal of the cornea and leaving the central portion of the cornea intact could improve the outcome of CXL. Hence, we investigated this new method to see whether partial removal of the corneal epithelium could lead to less corneal haze and better outcome or not. To the best of our knowledge, this is the first study that has compared conventional CXL with a new method of partial removal of the cornea.
Present study demonstrated that comparing with the conventional CXL method in which the cornea is totally removed, partial removal of the corneal epithelium did not result in significantly different subjective and objective outcomes. However, comparison of pre- and postoperative parameters within each group revealed that total removal of the cornea has resulted in significant improvement of K-max, Q-value, and IHA; while partial removal has improved BCVA significantly. Both methods have significantly increased corneal density, and decreased the thinnest point 6 months postoperatively in a similar way.
As mentioned earlier, we found that the average of BCVA significantly improved after partial removal of the cornea. The significant BCVA improvement in the partial removal group may be due to leaving the central area of the cornea intact. However, postoperative BCVA was not significantly different from the preoperative values in patients treated with total removal method.
The improvement in vision after CXL could be caused by decrease in astigmatism and corneal curvature, topographical homogenization of the cornea following the increased rigidity in the cross-linked cornea and the improved fitting of contact lenses.[22] In contrast, post-CXL corneal haze may have a negative influence on vision.[19] We found no significant differences between the groups in either topographic indices or corneal optical density; therefore, it is not surprising not to observe a significant difference in postoperative BCVA. Although we have no definite answer to explain the significantly improved BCVA in the partial removal group, this finding could be due to leaving the central portion of the cornea intact and the significant flattening of the cornea after partial CXL that is measured by the minimum radius of curvature (R-min). The increase of R-min means further flattening of the cornea, and we observed a significant increase in the mean of the minimum radius of curvature (R-min) in the partial removal group. Greenstein et al. and Hersh et al. reported significant improvement of R-min following CXL.[33]
Raiskup-Wolf and co-workers conducted a study on 480 eyes of 272 patients, and investigated long-term results of total removal of CXL in patients with keratoconus. They followed participants for at least 6 months (26.7 ± 16.2 months), and reported significant BCVA improvement in the majority of patients during the follow-up period.[4] The difference between Raiskup-Wolf findings and what we have found could be due to the different follow-up durations. Since post-CXL corneal haze is usually a temporary complication,[16] the duration of follow-up can play an important role in the long-term outcome.
Despite no significant difference between groups regarding the postoperative K-max, a significant improvement of K-max was observed in patients treated with total removal CXL. The improvement of K-max in our study is consistent with the decreases in the K-max value after CXL in several previous studies.[12,13,14,22] Patients treated with the new method of partial removal of the corneal epithelium did not reveal a significant improvement of K-max.
The Q value reflects corneal asphericity, and is negative for most normal eyes.[34] The significant improvement of Q-value was observed in the total removal CXL group (P value 0.009).
A significant decrease in the corneal thickness was observed after CXL by both methods. This finding is consistent with the report of Brook and colleagues; however, they reported a partial recovery of the corneal thickness toward the baseline after 1 year of follow-up.[15] Also this finding could be due to decreased precision and errors of optical method in measuring corneal thickness when significant haze is present.[35]
Similarly, both methods have resulted in significantly increased corneal density. We used densitometry measurements obtained from Scheimpflug imagery as an objective assessment modality for corneal haze, and found no significant difference between the groups in postoperative corneal density. It has been reported that from 6 months to 1 year after CXL there would be a continuous decrease in corneal haze severity.[16,36] Therefore, longer follow-up may result in more accurate findings regarding the long-term impact of each method on corneal haze.
Both corneal thinning and stromal haze may be caused by the complex structural and physiological wound-healing changes in the cornea after CXL. Hence, thinning and haze could be two distinct clinical components of the basic CXL healing process.[37]
In this study, all index measurements were carried out using Scheimpflug imagery reconstructed by the Pentacam software. Although Pentacam topography measurements have been validated in several previous studies,[35,38,39] its ultimate topography analysis may be artifactually affected by corneal haze and/or demarcation line after CXL.[17,28,40]
The present study demonstrated that although each method improved some subjective or objective parameters, there was no significant difference between these methods to make one of them more desirable. This is an initial investigation, and has several limitations including the small population, short follow-up duration, lack of classification of patients based on the baseline severity of keratoconus and artifactual effects of intervention on Pentacam Scheimpflug topography. Therefore, further clinical studies, after modification of these limitations, can provide more precise comparison between these methods regarding their long-term outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Coskunseven E, Jankov MR, 2nd, Hafezi F, Atun S, Arslan E, Kymionis GD. Effect of treatment sequence in combined intrastromal corneal rings and corneal collagen crosslinking for keratoconus. J Cataract Refract Surg. 2009;35:2084–91. doi: 10.1016/j.jcrs.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 3.Renesto Ada C, Melo LA, Jr, Sartori Mde F, Campos M. Sequential topical riboflavin with or without ultraviolet a radiation with delayed intracorneal ring segment insertion for keratoconus. Am J Ophthalmol. 2012;153:982–93. doi: 10.1016/j.ajo.2011.10.014. e3. [DOI] [PubMed] [Google Scholar]
- 4.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Radner W, Zehetmayer M, Skorpik C, Mallinger R. Altered organization of collagen in the apex of keratoconus corneas. Ophthalmic Res. 1998;30:327–32. doi: 10.1159/000055492. [DOI] [PubMed] [Google Scholar]
- 6.Smolek MK, Beekhuis WH. Collagen fibril orientation in the human corneal stroma and its implications in keratoconus. Invest Ophthalmol Vis Sci. 1997;38:1289–90. [PubMed] [Google Scholar]
- 7.Olivares Jiménez JL, Guerrero Jurado JC, Bermudez Rodriguez FJ, Serrano Laborda D. Keratoconus: Age of onset and natural history. Optom Vis Sci. 1997;74:147–51. doi: 10.1097/00006324-199703000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Kymes SM, Walline JJ, Zadnik K, Gordon MO. Collaborative Longitudinal Evaluation of Keratoconus study group. Quality of life in keratoconus. J Ophthalmol. 2004;138:527–35. doi: 10.1016/j.ajo.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–40. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Iomdina E, Dittert DD, Herbst H. Wound healing in the rabbit cornea after corneal collagen cross-linking with riboflavin and UVA. Cornea. 2007;26:600–5. doi: 10.1097/ICO.0b013e318041f073. [DOI] [PubMed] [Google Scholar]
- 11.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: Preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–45. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 12.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: The Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Grewal DS, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: One-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35:425–32. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 14.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–78. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Brooks NO, Greenstein S, Fry K, Hersh PS. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38:615–9. doi: 10.1016/j.jcrs.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Shikha D, Kavita R, Sundaram N. Complications of corneal collagen cross-linking. J Ophthalmol 2011. 2011:869015. doi: 10.1155/2011/869015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36:2105–14. doi: 10.1016/j.jcrs.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 18.Jankov Li MR, Jovanovic V, Nikolic L, Lake JC, Kymionis G, Coskunseven E. Corneal collagencross-linking. Middle East Afr J Ophthalmol. 2010;17:21–7. doi: 10.4103/0974-9233.61213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemi HT, Taheri MR, Fotouhi A, Kheiltash A. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy in high myopia: a prospective clinical study. BMC Ophthalmol. 2004;4:12. doi: 10.1186/1471-2415-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:1282–90. doi: 10.1016/j.jcrs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Pouliquen Y. Doyne lecture keratoconus. Eye (Lond) 1987;1:1–14. doi: 10.1038/eye.1987.2. [DOI] [PubMed] [Google Scholar]
- 22.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Greenstein SA, Fry KL, Hersh MJ, Hersh PS. Higher-order aberrations after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38:292–302. doi: 10.1016/j.jcrs.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Greenstein SA, Fry KL, Hersh PS. Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg. 2012;28:397–405. doi: 10.3928/1081597X-20120518-02. [DOI] [PubMed] [Google Scholar]
- 25.Vinciguerra P, Camesasca FI, Albè E, Trazza S. Corneal collagen cross-linking for ectasia after excimer laser refractive surgery: 1-year results. J Refract Surg. 2010;26:486–97. doi: 10.3928/1081597X-20090910-02. [DOI] [PubMed] [Google Scholar]
- 26.Ahearne M, Yang Y, Then KY, Liu KK. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br J Ophthalmol. 2008;92:268–71. doi: 10.1136/bjo.2007.130104. [DOI] [PubMed] [Google Scholar]
- 27.Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–90. doi: 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 28.Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: In vivo confocal microscopic evaluation. Clin Experiment Ophthalmol. 2007;35:580–2. doi: 10.1111/j.1442-9071.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 29.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–62. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Hanna KD, Pouliquen YM, Waring GO, 3rd, Savoldelli M, Fantes F, Thompson KP. Corneal wound healing in monkeys after repeated excimer laser photorefractive keratectomy. Arch Ophthalmol. 1992;110:1286–91. doi: 10.1001/archopht.1992.01080210104035. [DOI] [PubMed] [Google Scholar]
- 31.Carr JD, Patel R, Hersh PS. Management of late corneal haze following photorefractive keratectomy. J Refract Surg. 1995;11:S309–13. doi: 10.3928/1081-597X-19950502-25. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Kurosaka D, Bissen-Miyajima H, Tsubota K. Intact corneal epithelium is essential for the prevention of stromal haze after laser assisted in situ keratomileusis. Br J Ophthalmol. 2001;85:209–13. doi: 10.1136/bjo.85.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:149–60. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Fan ZJ, Xu SJ, Jia ZH, Liu BC. Clinical significance of corneal Q value in myopia patients. Int J Ophthalmol. 2006;6:642–3. [Google Scholar]
- 35.Koller T, Iseli HP, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea. 2009;28:510–5. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 36.Lim LS, Beuerman R, Lim L, Tan DT. Late-onset deep stromal scarring after riboflavin-UV-A corneal collagen cross-linking for mild keratoconus. Arch Ophthalmol. 2011;129:360–2. doi: 10.1001/archophthalmol.2011.23. [DOI] [PubMed] [Google Scholar]
- 37.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 38.Shaw AJ, Collins MJ, Davis BA, Carney LG. Corneal refractive changes due to short-term eyelid pressure in downward gaze. J Cataract Refract Surg. 2008;34:1546–53. doi: 10.1016/j.jcrs.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Swartz T, Marten L, Wang M. Measuring the cornea: The latest developments in corneal topography. Curr Opin Ophthalmol. 2007;18:325–33. doi: 10.1097/ICU.0b013e3281ca7121. [DOI] [PubMed] [Google Scholar]
- 40.Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009;25:S824–8. doi: 10.3928/1081597X-20090813-12. [DOI] [PubMed] [Google Scholar]


