Abstract
International Society of Blood Transfusion has recently recognized 33 blood group systems. Apart from ABO and Rhesus system, many other types of antigens have been noticed on the red cell membranes. Blood grouping and cross-matching is one of the few important tests that the anaesthesiologist orders during perioperative period. Hence, a proper understanding of the blood group system, their clinical significance, typing and cross-matching tests, and current perspective are of paramount importance to prevent transfusion-related complications. Nonetheless, the knowledge on blood group system is necessary to approach blood group-linked diseases which are still at the stage of research. This review addresses all these aspects of the blood groups system.
Keywords: ABO blood groups, antibody typing, blood group system, rhesus blood group, screening
INTRODUCTION
The term “blood group” refers to the entire blood group system comprising red blood cell (RBC) antigens whose specificity is controlled by a series of genes which can be allelic or linked very closely on the same chromosome. “Blood type” refers to a specific pattern of reaction to testing antisera within a given system. Over a period of time, our understanding on blood groups has evolved to encompass not only transfusion-related problems but also specific disease association with RBC surface antigens. Karl Landsteiner has been credited for the discovery of ABO blood group system in 1900.[1] His extensive research on serology based on simple but strong scientific reasoning led to identification of major blood groups such as O, A, and B types, compatibility testing, and subsequent transfusion practices. He was awarded Noble Prize in 1930 for this discovery. His obituary lists an immense contribution of more than 346 publications. Later, Jan Jansky described classification of human blood groups of four types.
BLOOD GROUPS
At present, 33 blood group systems representing over 300 antigens are listed by the International Society of Blood Transfusion.[2,3] Most of them have been cloned and sequenced. The genes of these blood group systems are autosomal, except XG and XK which are X-borne, and MIC2 which is present on both X and Y chromosomes. The antigens can be integral proteins where polymorphisms lie in the variation of amino acid sequence (e.g., rhesus [Rh], Kell), glycoproteins or glycolipids (e.g., ABO). Some of the important groups are mentioned here [Table 1].
Table 1.
Blood group systems
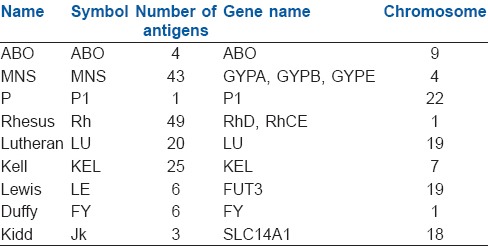
ABO system
Among the 33 systems, ABO remains the most important in transfusion and transplantation since any person above the age of 6 months possess clinically significant anti-A and/or anti-B antibodies in their serum. Blood group A contains antibody against blood group B in serum and vice-versa, while blood group O contains no A/B antigen but both their antibodies in serum.
H-antigen
H-antigen is the precursor to the ABO blood group antigens. It is present in all RBCs irrespective of the ABO system. Persons with the rare Bombay phenotype are homozygous for the H gene (HH), do not express H-antigen on their RBCs. As H-antigen acts as precursor, its absence means the absence of antigen A and B. However, the individuals produce isoantibodies to H-antigen as well as to antigens A and B.
Rhesus system
Rhesus-system is the second most important blood group system after ABO.[4] Currently, the Rh-system consists of 50 defined blood group antigens out of which only five are important. RBC surface of an individual may or may not have a Rh factor or immunogenic D-antigen. Accordingly, the status is indicated as either Rh-positive (D-antigen present) or Rh-negative (D-antigen absent). In contrast to the ABO system, anti-Rh antibodies are, normally, not present in the blood of individuals with D-negative RBCs, unless the circulatory system of these individuals has been exposed to D-positive RBCs. These immune antibodies are immunoglobulin G (IgG) in nature and hence, can cross the placenta. Prophylaxis is given against Rh immunization using anti-D Ig for pregnant Rh-negative mothers who have given birth to Rh-positive child.
MNS antigen system
MNS antigen system, first described by Landsteiner and Levine in 1927 is based on two genes: Glycophorin A and Glycophorin B. The blood group is under control of an autosomal locus on chromosome 4 and also under control of a pair of co-dominant alleles LM and LN. Anti-M and anti-N antibodies are usually IgM types and rarely, associated with transfusion reactions.
Lutheran system
Lutheran system comprised of four pairs of allelic antigens representing single amino acid substitution in the Lutheran glycoprotein at chromosome 19. Antibodies against this blood group are rare and generally not considered clinically significant.
Kell system
These erythrocyte antigens are the third most potent immunogenic antigen after ABO and Rh system, and are defined by an immune antibody, anti-K. It was first noticed in the serum of Mrs. Kellacher. She reacted to the erythrocytes of her newborn infant resulting in hemolytic reactions. Since then 25 Kell antigens have been discovered. Anti-K antibody causes severe hemolytic disease of the fetus and newborn (HDFN) and haemolytic transfusion reactions (HTR).
Duffy system
Duffy-antigen was first isolated in a patient called Duffy who had haemophilia. It is also known as Fy glycoprotein and is present in the surface of RBCs. It is a nonspecific receptor for several chemokines and acts as a receptor for human malarial parasite, Plamodium vivax. Antigens Fya and Fyb on the Duffy glycoprotein can result in four possible phenotypes, namely Fy(a+b−), Fy(a+b+), Fy(a−b+), and Fy(a−b−). The antibodies are IgG subtypes and can cause HTR.
Kidd system
Kidd antigen (known as Jk antigen) is a glycoprotein, present on the membrane of RBCs and acts as a urea transporter in RBCs and renal endothelial cells. Kidd antibodies are rare but can cause severe transfusion reactions. These antigens are defined by reactions to an antibody designated as anti-Jka, discovered in the serum of Mrs. Kidd who delivered a baby with HDFN. Jka was the first antigen to be discovered by Kidd blood group system, subsequently, two other antigens Jkb and Jk3 were found.
Agarwal et al.[5] carried out a study on automated analysis of blood groups in north Indian donor population and observed that the common blood groups in order of frequency were B, O, A, and AB; 94.4% being Rh-positive. In minor blood groups, the most commonly appearing phenotypes were Le (a−b−) for Lewis, Fy(a+b+) for Duffy, Jk(a+b+) for Kidd, and M+N+ for MNS system.
IMPORTANCE OF BLOOD GROUPS
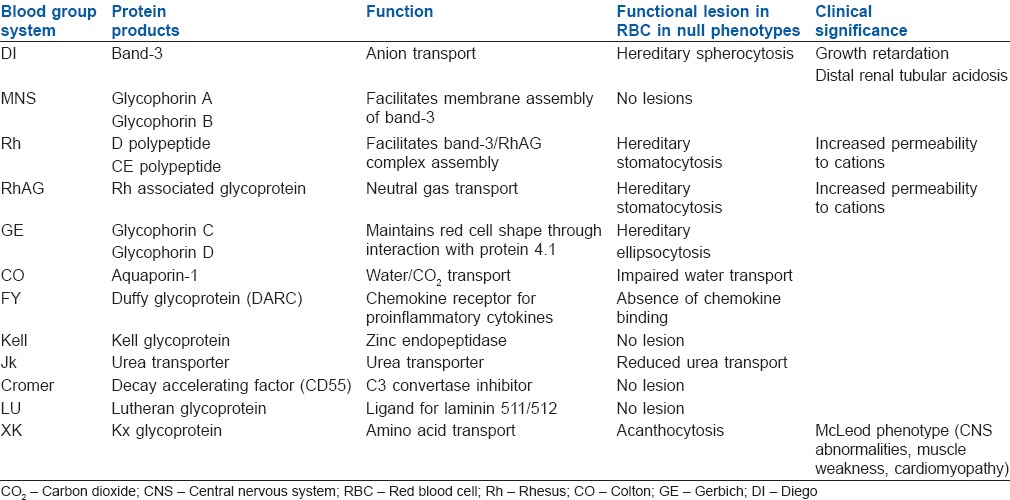
Structural lesions of red blood cell
Of the 33 blood group system antigens, five are defined by their carbohydrate structures (ABO, H, P1Pk, I, GLOB); two are obtained from the plasma (LE, CH⁄RG). The remaining 23 are characterized by the protein sequence of the RBC membrane protein,[6,7] five major proteins (DI, Rh, RhAG, MNS, GE, and CO) among them are expressed at higher levels and function as membrane transporters, whereas the functional importance of rest of 17 antigens is unknown. The proposed function of other antigens are mostly receptor/ligand signaling, enzymatic activity, and glycocalyx formation.[8] The null phenotype of the system, however, shows no immune system abnormalities when compared with mice except for a blunted neutrophil response on exposure to bacterial lipopolysaccharide.[9] Similarly, Knops blood group antigen has been associated with complement receptor 1[10] and Cromer system with decay acceleration factor.[11] Nevertheless, the clinical function of the null phenotypes of these blood groups still remains to be elucidated [Table 2].
Table 2.
Pathology associated with the null phenotype of the RBC antigen
Blood groups and disease association
The ABO blood groups have a profound influence on haemostasis.[12] They exert major quantitative effects on plasma levels of von Willebrand factor and factor VIII. Increased association of myocardial infarction, ischemic stroke, and venous thromboembolism is seen with blood groups A and AB[13] possibly through functional ABO glycol transferases modulation of thrombosis. A higher risk of cerebral venous thrombosis has been reported in non-O groups.[14] Significant association of ABO groups with the prevalence of preeclampsia has been reported, where AB group was found to be associated with an increased risk of 2.1-folds.[15] Preliminary studies suggested an association of ABO system with malignancies. A positive correlation has been shown between blood group A with chronic hepatitis-B infection and pancreatic cancer;[16] and blood group B with ovarian cancer.[17] Protection against falciparum malaria can be achieved with group O by reducing rosette formation.[18] Blood group O increases the severity of infection in Vibrio cholerae strains (O1 El Tor and O139).
BLOOD REQUISITION
After the decision to transfuse blood is taken the next step should be to order a requisition during which the following steps need to be remembered.
Blood grouping and cross-matching
The most fatal of all transfusion-related reaction is ABO incompatibility causing complement-mediated intravascular hemolysis. Hence, correct blood grouping and typing, and cross-checking with the blood requisition form is of utmost importance. ABO typing is carried out by testing RBCs for the A and B antigens and the serum for the A and B antibodies before transfusion. The next step involves Rh typing with only 15% of the population being Rh-negative.
Cross-matching
Cross-matching involves mixing of donor RBCs with the recipient serum to detect fatal reactions.[19] It has three phases in which the first phase (1-5 min) involves detection of ABO incompatibility and detection of antibody against MN, P, and Lewis systems. The second phase (30-45 min in albumin and 10-20 min in low ionic salt solution) involves incubation of first phase reactants at 37°C for detection of incomplete antibodies of Rh system. The third phase consists of the addition of antiglobulin sera to the incubated second phase reactants to detect incomplete antibodies of Rh, Kidd, Kell and Duffy. Among the three phases, the first two phases are more important as they detect those involved in fatal HTR. The total time taken for all the three phases is in between 45 and 60 min.
Antibody screening
Here, commercially prepared RBCs with all the antigens, which direct production of antibodies causing hemolytic reactions, are mixed with the recipient's serum to detect the presence of those very antibodies. It is also carried out with the donor's serum.
CHANGING PRACTICES IN BLOOD GROUPING
There are controversies regarding the best method for procurement of blood during elective and emergency situations: (a) It can be done by routinely asking for grouping and cross-matching in elective surgical patients. Many scientific articles disputed the relevance of preoperative arrangement of blood in surgeries where blood loss is not anticipated to be significant.[20,21] (b) Blood may be ordered without full set of investigations.[19] ABO-Rh typing alone results in a 99.8% chance of a compatible transfusion. Antibody screening increases this safety margin up to 99.94%, and an additional cross-match further increases the compatibility to 99.95%. In absence of cross-matching, there is a possibility of missing the antigens on donor cells, but in clinical practice, they are of less importance. Hence, “screening and typing” alone should be carried out. Other methods include “type and partial cross-match,” which includes the immediate phase of cross-match; “type and uncross match,” for those recipients who have never been transfused before, the chance of detection of antibody with each cross-match is 1:1000; “type O Rh-negative uncross match,” it is performed in emergency situation when the time for these procedures is limited. In the latter condition, type O Rh-negative packed RBCs, that is, the universal donor can be used as they will have a negligible amount of hemolytic anti-A/anti-B antibodies against the recipient RBCs.
CURRENT TRENDS AND FUTURE AREAS OF RESEARCH
Three main antigen-modulation strategies have been proposed to prevent immune recognition of incompatible RBCs and to avoid haemolytic reactions due to alloimmunization. The first approach relies on enzymatic conversion of specific blood group antigens, that is, manipulation of the ABO system. Goldstein and Lenny achieved a remarkable milestone with the development of technology named “enzyme converted group O-RBC (ECO-RBC) concept” where the B antigen is replaced with O using galactosidase.[22] This treatment leaves fewer than 2000 antigenic sites per RBC without affecting membrane deformability, gas exchange, or expression of the RhD, C and E, MNS, Lewis, Kell, Lutheran, Duffy, and Kidd blood group systems as their antigenicity do not depend on the terminal galactose residues. In contrast with the B antigen, enzymatic conversion of A antigen was difficult due to existence of two Type-A blood group structures (A2 and A1).[23] Two new enzymes, N-acetylgalactosaminidase and a-galactosidase have been identified for removal of antigens A and B, respectively; and tested for their ability to generate ECO-RBCs from A1, A2, B, or AB donor units.[24] The enzyme conversion strategy has also been proposed to resolve ABO incompatibility issues in the field of organ transplantation.[25] The second approach is to mask antigens by treatment of RBCs with polyethylene glycol; also known as the stealth RBC concept. The third approach involves in vitro production of RBCs with a predefined antigenic profile from genetically manipulated stem cells.[26] Such cells could be used for the generation of “universal-donor” RBCs.
SUMMARY
Currently, our knowledge on blood groups goes beyond the usual tests of agglutination and transfusion to the better understanding of RBC antigens in light of their association with multiple diseases and the scope of use of this knowledge to modulate the disease processes. In this context, the role of adequate understanding of screening, typing, and cross-matching apart from awareness on evolving trends, for every clinician, may not be overemphasized.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Owen R. Karl Landsteiner and the first human marker locus. Genetics. 2000;155:995–8. doi: 10.1093/genetics/155.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lögdberg L, Reid ME, Lamont RE, Zelinski T. Human blood group genes 2004: Chromosomal locations and cloning strategies. Transfus Med Rev. 2005;19:45–57. doi: 10.1016/j.tmrv.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Lögdberg L, Reid ME, Zelinski T. Human blood group genes 2010: Chromosomal locations and cloning strategies revisited. Transfus Med Rev. 2011;25:36–46. doi: 10.1016/j.tmrv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Westhoff CM. The Rh blood group system in review: A new face for the next decade. Transfusion. 2004;44:1663–73. doi: 10.1111/j.0041-1132.2004.04237.x. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal N, Thapliyal RM, Chatterjee K. Blood group phenotype frequencies in blood donors from a tertiary care hospital in north India. Blood Res. 2013;48:51–4. doi: 10.5045/br.2013.48.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anstee DJ. The functional importance of blood group-active molecules in human red blood cells. Vox Sang. 2011;100:140–9. doi: 10.1111/j.1423-0410.2010.01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Daniels G, Reid ME. Blood groups: The past 50 years. Transfusion. 2010;50:281–9. doi: 10.1111/j.1537-2995.2009.02456.x. [DOI] [PubMed] [Google Scholar]
- 8.Denomme GA. The structure and function of the molecules that carry human red blood cell and platelet antigens. Transfus Med Rev. 2004;18:203–31. doi: 10.1016/j.tmrv.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol Cell Biol. 2000;20:3097–101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao N, Ferguson DJ, Lee SF, Telen MJ. Identification of human erythrocyte blood group antigens on the C3b/C4b receptor. J Immunol. 1991;146:3502–7. [PubMed] [Google Scholar]
- 11.Telen MJ, Hall SE, Green AM, Moulds JJ, Rosse WF. Identification of human erythrocyte blood group antigens on decay-accelerating factor (DAF) and an erythrocyte phenotype negative for DAF. J Exp Med. 1988;167:1993–8. doi: 10.1084/jem.167.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Mooney CJ, Reilly MP. ABO Blood Groups and Cardiovascular Diseases. Int J Vasc Med 2012. 2012:641917. doi: 10.1155/2012/641917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiggins KL, Smith NL, Glazer NL, Rosendaal FR, Heckbert SR, Psaty BM, et al. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J Thromb Haemost. 2009;7:263–9. doi: 10.1111/j.1538-7836.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufano A, Coppola A, Nardo A, Bonfanti C, Crestani S, Cerbone AM, et al. Non-O blood group as a risk factor for cerebral vein thrombosis. Thromb Haemost. 2013;110:197–9. doi: 10.1160/TH13-02-0112. [DOI] [PubMed] [Google Scholar]
- 15.Hiltunen LM, Laivuori H, Rautanen A, Kaaja R, Kere J, Krusius T, et al. Blood group AB and factor V Leiden as risk factors for pre-eclampsia: A population-based nested case-control study. Thromb Res. 2009;124:167–73. doi: 10.1016/j.thromres.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012;131:461–8. doi: 10.1002/ijc.26376. [DOI] [PubMed] [Google Scholar]
- 17.Gates MA, Wolpin BM, Cramer DW, Hankinson SE, Tworoger SS. ABO blood group and incidence of epithelial ovarian cancer. Int J Cancer. 2011;128:482–6. doi: 10.1002/ijc.25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115:4635–43. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 19.Miller RD. Transfusion therapy. In: Miller RD, Ericksson LI, Fleischer LA, Weiner-Kronish JP, Young LA, editors. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone Elsevier; 2010. pp. 1739–66. [Google Scholar]
- 20.Ghirardo SF, Mohan I, Gomensoro A, Chorost MI. Routine preoperative typing and screening: A safeguard or a misuse of resources. JSLS. 2010;14:395–8. doi: 10.4293/108680810X12924466007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onotai L, Lilly-Tariah OD. Adenoid and tonsil surgeries in children: How relevant is pre-operative blood grouping and cross-matching? Afr J Paediatr Surg. 2013;10:231–4. doi: 10.4103/0189-6725.120887. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein J, Siviglia G, Hurst R, Lenny L, Reich L. Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals. Science. 1982;215:168–70. doi: 10.1126/science.6274021. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein J. Conversion of ABO blood groups. Transfus Med Rev. 1989;3:206–12. doi: 10.1016/s0887-7963(89)70080-8. [DOI] [PubMed] [Google Scholar]
- 24.Liu QP, Sulzenbacher G, Yuan H, Bennett EP, Pietz G, Saunders K, et al. Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol. 2007;25:454–64. doi: 10.1038/nbt1298. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Liu D, Ogawa H, Miwa Y, Nagasaka T, Maruyama S, et al. Alternative strategy for overcoming ABO incompatibility. Transplantation. 2007;83:1284–6. doi: 10.1097/01.tp.0000260634.85690.c4. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi-Najafabadi S, Vasheghani-Farahani E, Shojaosadati SA, Rasaee MJ, Armstrong JK, Moin M, et al. A method to optimize PEG-coating of red blood cells. Bioconjug Chem. 2006;17:1288–93. doi: 10.1021/bc060057w. [DOI] [PubMed] [Google Scholar]


