Abstract
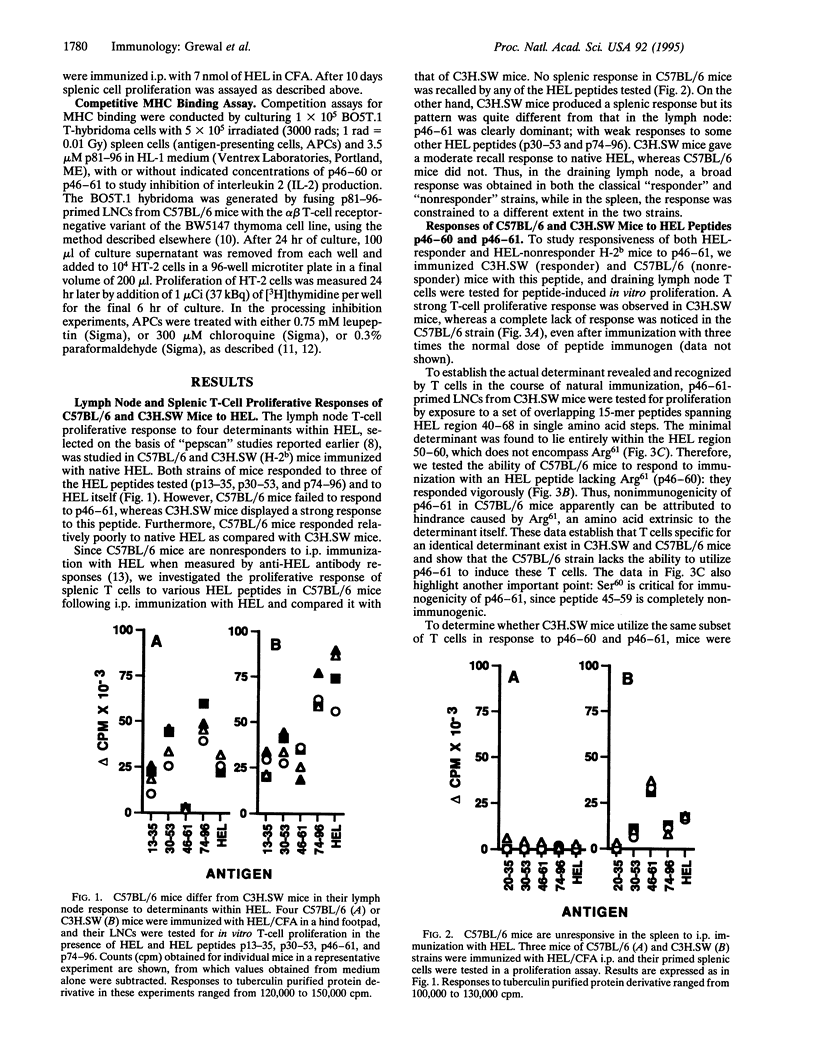
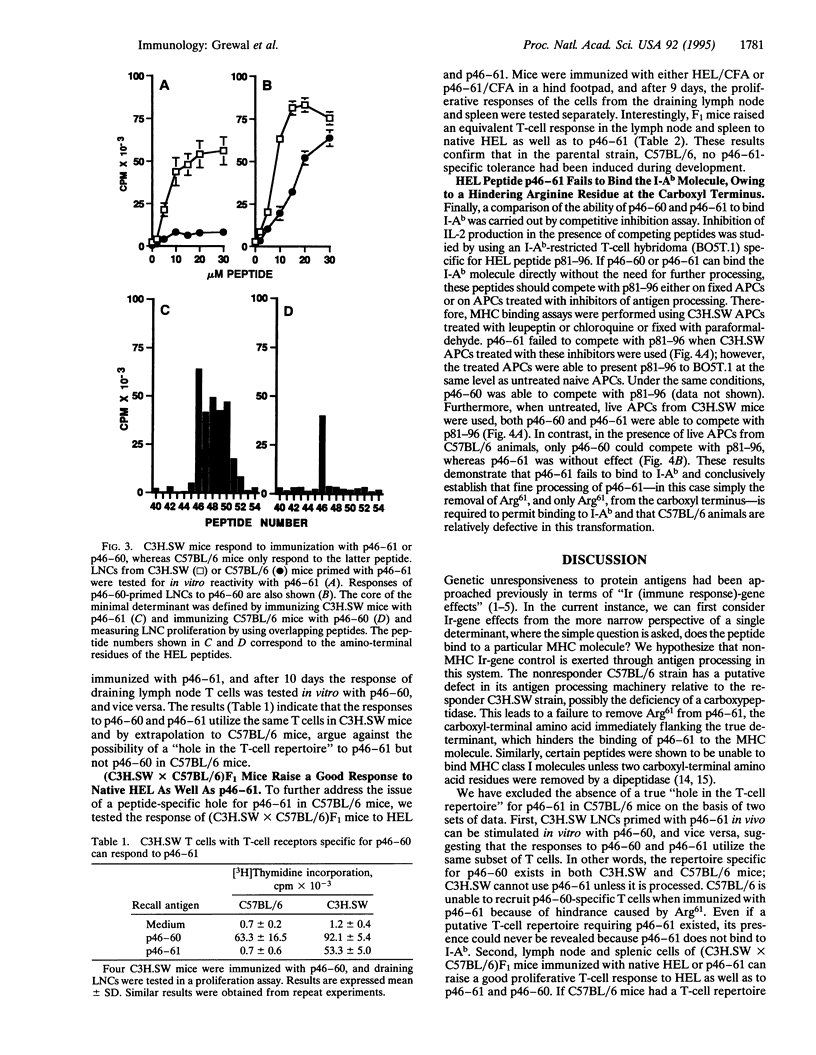
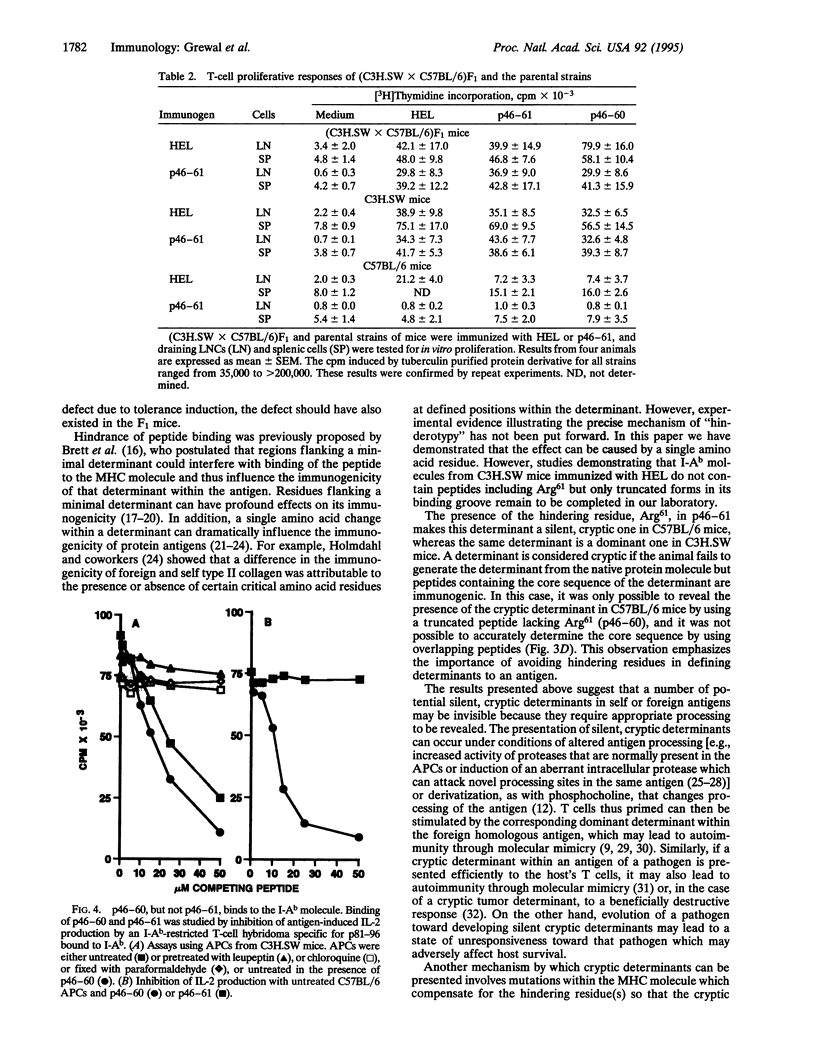
The immune system has evolved the potential to respond to a wide variety of antigens, yet unresponsiveness to many foreign determinants is encountered frequently. Here, we report a lack of response to a particular determinant, hen egg lysozyme (HEL)-(46-61)-peptide (p46-61), in C57BL/6 (H-2b) mice, whereas a strong T-cell response to this determinant is obtained in major histocompatibility complex (MHC)-identical C3H.SW mice. However, (C3H.SW x C57BL/6)F1 mice respond well to p46-61, suggesting the absence of a p46-61-specific "hole" in the T-cell repertoire in C57BL/6 mice. We further show that p46-61 cannot bind the I-Ab class II MHC molecule, whereas p46-60 lacking Arg61 exhibits good binding and is immunogenic in both strains. Thus, the presence of the hindering residue, Arg61, renders p46-61, a dominant determinant in C3H.SW, into a silent, cryptic determinant in C57BL/6 mice. Upon i.p. immunization with HEL, no T-cell responses to either HEL or p46-61 could be demonstrated in spleens of HEL-primed C57BL/6 mice, whereas a predominant response to p46-61 and HEL was demonstrated in C3H.SW mice. Evidently, C57BL/6 mice differ from C3H.SW in their ability to process p46-61 into an actual I-Ab binding determinant, indicating a putative enzymatic defect in the C57BL/6 strain. Furthermore, our results suggest that the inability of C57BL/6 mice to respond in the spleen to HEL is based upon its failure to generate a dominant immunogenic determinant from HEL, coupled with its pattern of susceptibility to regulatory effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneo B. A., Yowell R. L., Sercarz E. E. Recognition and display of the predominant idiotype among members of the regulatory circuitry controlling the anti-lysozyme immune response. J Immunol. 1985 Feb;134(2):1073–1079. [PubMed] [Google Scholar]
- Bhardwaj V., Kumar V., Geysen H. M., Sercarz E. E. Subjugation of dominant immunogenic determinants within a chimeric peptide. Eur J Immunol. 1992 Aug;22(8):2009–2016. doi: 10.1002/eji.1830220809. [DOI] [PubMed] [Google Scholar]
- Bhayani H., Carbone F. R., Paterson Y. The activation of pigeon cytochrome c-specific T cell hybridomas by antigenic peptides is influenced by non-native sequences at the amino terminus of the determinant. J Immunol. 1988 Jul 15;141(2):377–382. [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Berzofsky J. A. Influences of antigen processing on the expression of the T cell repertoire. Evidence for MHC-specific hindering structures on the products of processing. J Exp Med. 1988 Jul 1;168(1):357–373. doi: 10.1084/jem.168.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Dos Reis G. A., Shevach E. M. Antigen-presenting cells from nonresponder strain 2 guinea pigs are fully competent to present bovine insulin B chain to responder strain 13 T cells. Evidence against a determinant selection model and in favor of a clonal deletion model of immune response gene function. J Exp Med. 1983 Apr 1;157(4):1287–1299. doi: 10.1084/jem.157.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan A., Regan J., Seamon K. B., Lindholm C. Identification of single amino acid substitutions in the staphylococcal nuclease protein that enhance and diminish T cell clone recognition of naturally processed peptides. Int Immunol. 1992 Dec;4(12):1399–1406. doi: 10.1093/intimm/4.12.1399. [DOI] [PubMed] [Google Scholar]
- Fox B. S., Chen C., Fraga E., French C. A., Singh B., Schwartz R. H. Functionally distinct agretopic and epitopic sites. Analysis of the dominant T cell determinant of moth and pigeon cytochromes c with the use of synthetic peptide antigens. J Immunol. 1987 Sep 1;139(5):1578–1588. [PubMed] [Google Scholar]
- Gammon G., Geysen H. M., Apple R. J., Pickett E., Palmer M., Ametani A., Sercarz E. E. T cell determinant structure: cores and determinant envelopes in three mouse major histocompatibility complex haplotypes. J Exp Med. 1991 Mar 1;173(3):609–617. doi: 10.1084/jem.173.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon G., Shastri N., Cogswell J., Wilbur S., Sadegh-Nasseri S., Krzych U., Miller A., Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev. 1987 Aug;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Kapp J. A., Pierce C. W. The role of suppressor T cells in the expression of immune response gene function. J Mol Cell Immunol. 1987;3(5):267–275. [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Glynne R., Radley E., Beck S., Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991 Oct 17;353(6345):667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- Kim B. S., Jang Y. S. Constraints in antigen processing result in unresponsiveness to a T cell epitope of hen egg lysozyme in C57BL/6 mice. Eur J Immunol. 1992 Mar;22(3):775–782. doi: 10.1002/eji.1830220322. [DOI] [PubMed] [Google Scholar]
- Kozlowski S., Corr M., Takeshita T., Boyd L. F., Pendleton C. D., Germain R. N., Berzofsky J. A., Margulies D. H. Serum angiotensin-1 converting enzyme activity processes a human immunodeficiency virus 1 gp160 peptide for presentation by major histocompatibility complex class I molecules. J Exp Med. 1992 Jun 1;175(6):1417–1422. doi: 10.1084/jem.175.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- McElligott D. L., Sorger S. B., Matis L. A., Hedrick S. M. Two distinct mechanisms account for the immune response (Ir) gene control of the T cell response to pigeon cytochrome c. J Immunol. 1988 Jun 15;140(12):4123–4131. [PubMed] [Google Scholar]
- Michaëlsson E., Andersson M., Engström A., Holmdahl R. Identification of an immunodominant type-II collagen peptide recognized by T cells in H-2q mice: self tolerance at the level of determinant selection. Eur J Immunol. 1992 Jul;22(7):1819–1825. doi: 10.1002/eji.1830220722. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Moudgil K. D., Ametani A., Grewal I. S., Kumar V., Sercarz E. E. Processing of self-proteins and its impact on shaping the T cell repertoire, autoimmunity and immune regulation. Int Rev Immunol. 1993;10(4):365–377. doi: 10.3109/08830189309061711. [DOI] [PubMed] [Google Scholar]
- Moudgil K. D., Sercarz E. E. Can antitumor immune responses discriminate between self and nonself? Immunol Today. 1994 Aug;15(8):353–355. doi: 10.1016/0167-5699(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Moudgil K. D., Sercarz E. E. Dominant determinants in hen eggwhite lysozyme correspond to the cryptic determinants within its self-homologue, mouse lysozyme: implications in shaping of the T cell repertoire and autoimmunity. J Exp Med. 1993 Dec 1;178(6):2131–2138. doi: 10.1084/jem.178.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda N. K., Apple R., Sercarz E. Limitations in plasticity of the T-cell receptor repertoire. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9503–9507. doi: 10.1073/pnas.88.21.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry as a mechanism for the cause and a probe uncovering etiologic agent(s) of autoimmune disease. Curr Top Microbiol Immunol. 1989;145:127–135. doi: 10.1007/978-3-642-74594-2_11. [DOI] [PubMed] [Google Scholar]
- Ronchese F., Brown M. A., Germain R. N. Structure-function analysis of the Abm12 beta mutation using site-directed mutagenesis and DNA-mediated gene transfer. J Immunol. 1987 Jul 15;139(2):629–638. [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Shastri N., Miller A., Sercarz E. E. Amino acid residues distinct from the determinant region can profoundly affect activation of T cell clones by related antigens. J Immunol. 1986 Jan;136(2):371–376. [PubMed] [Google Scholar]
- Sherman L. A., Burke T. A., Biggs J. A. Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J Exp Med. 1992 May 1;175(5):1221–1226. doi: 10.1084/jem.175.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar S., Sercarz E. E., Krzych U. The molecular context of determinants within the priming antigen establishes a hierarchy of T cell induction: T cell specificities induced by peptides of beta-galactosidase vs. the whole antigen. Eur J Immunol. 1989 Apr;19(4):681–687. doi: 10.1002/eji.1830190417. [DOI] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio M. S., Berzofsky J. A., Krzych U., Smith J. A., Hodes R. J., Finnegan A. Sequences outside a minimal immunodominant site exert negative effects on recognition by staphylococcal nuclease-specific T cell clones. J Immunol. 1989 Nov 1;143(9):2814–2819. [PubMed] [Google Scholar]
- Wraith D. C., Smilek D. E., Mitchell D. J., Steinman L., McDevitt H. O. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989 Oct 20;59(2):247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Waters J. B., Früh K., Peterson P. A. Proteasomes are regulated by interferon gamma: implications for antigen processing. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4928–4932. doi: 10.1073/pnas.89.11.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]


