Abstract
Disseminated intravascular coagulation (DIC) is a reflection of an underlying systemic disorder which affects the coagulation system, simultaneously resulting in pro-coagulant activation, fibrinolytic activation, and consumption coagulopathy and finally may result in organ dysfunction and death. Though septicaemia is the most common cause of DIC, several other conditions can also lead to it. A diagnosis of DIC should be made only in the presence of a causative factor supported by repeated laboratory tests for coagulation profile and clotting factors. An effective scoring system helps to detect an overt DIC and a high score closely correlates with mortality. Treatment of DIC is aimed at combating the underlying disorder followed by supportive management. Low molecular weight heparin is advocated in special situations whereas anti-thrombin III and activated protein C are of doubtful value. Early diagnosis and prompt treatment backed by laboratory support can reduce the morbidity and mortality associated with it. The methodology of search for this review article involved hand search from text books and internet search using Medline (via PubMed) using key words DIC, thrombosis, fibrin degradation products, anti-thrombin and tissue factor for the last 25 years and also recent evidence-based reviews.
Keywords: Activated protein C, anti-thrombin, coagulation factors, disseminated intravascular coagulation, fibrin degradation products, thrombosis, tissue factor
INTRODUCTION
The coagulation system in the body consists of clotting and fibrinolytic mechanisms. The function of the former is to prevent excessive blood loss, whereas the latter is to ensure circulation within the vasculature. Following an insult, the activated coagulation cascade adequately balances the naturally occurring anti-coagulant systems and the fibrinolytic system (which generates plasmin) to maintain a normal circulation. In disseminated intravascular coagulopathy (DIC) like syndrome, there is widespread activation of the blood coagulation system leading to excessive generation and disseminated deposition of fibrin clots in small and midsize vessels, which alters the microcirculation leading to ischaemic necrosis in various organs particularly in kidney and lung resulting in organ failure.[1] There can be concomitant consumption of platelets and coagulation factors resulting in serious haemorrhagic complications which sometimes may be the most striking clinical presentation. Hence, a patient with DIC can present as thrombotic and bleeding problem simultaneously [Figure 1].
Figure 1.
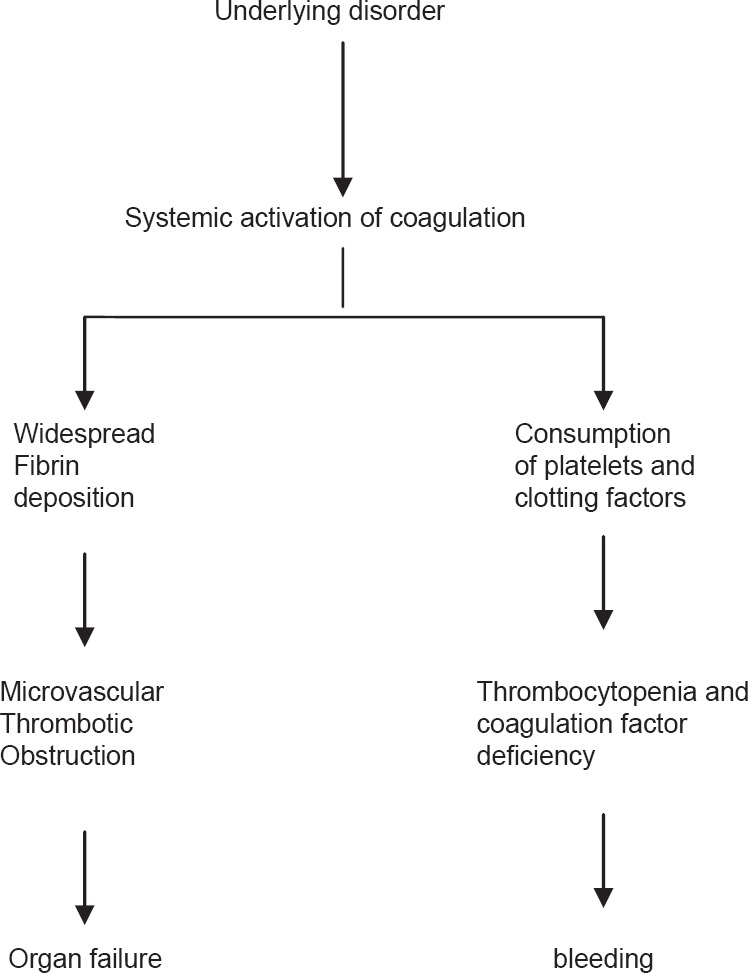
The clinical picture of disseminated intravascular coagulation
Disseminated intravascular coagulopathy should not be considered as a distinct disease entity but rather a sign of another disease. It has been associated with almost all life-threatening diseases. The clinical spectrum of DIC can range from a small decrease in platelet count and sub-clinical prolongation of prothrombin time (PT) and activated partial thromboplastin time (aPTT) to a fulminant DIC with widespread thrombosis and severe bleeding.[1] Any tissue insult sufficient enough to release tissue products or toxins into the circulation can result in DIC. This review will focus on definition, aetio-pathogenesis, diagnosis and management of DIC.
DEFINITION
A widely accepted definition put forward by the subcommittee on DIC of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis (ISTH) is as follows: DIC is an acquired syndrome characterised by the intravascular activation of coagulation with loss of localisation arising from different causes. It can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction.[2]
AETIO-PATHOGENESIS OF DISSEMINATED INTRAVASCULAR COAGULOPATHY
DIC occurs secondary to a clinical disorder which provides a key for appropriate investigation and management. The clinical spectrum includes sepsis, trauma, malignancy, liver disease, obstetric disorders, envenomation, vascular anomalies and major transfusion reactions [Table 1]. The pathogenesis may follow either or both of the following pathways:
Table 1.
Conditions associated with DIC

As a part of systemic inflammatory response, there is activation of cytokine network and thereby coagulation system as in sepsis or polytrauma and/or
Release of pro-coagulant material to the blood stream as in malignancies or obstetrical cases.
Bacterial septicaemia accounts for the major cause of DIC which may be due to either cell membrane components of the microorganism or bacterial exotoxins.[3] In severe trauma, release of phospholipids and fat (major fractures) into the circulation can cause haemolysis, endothelial damage and activation of the coagulation cascade.[4] Solid tumours and haematological malignancies particularly acute pro-myelocytic leukaemia and some forms of prostatic cancer are associated with DIC.[5] Tumour cells can produce various pro-coagulant molecules including tissue factor and a cancer pro-coagulant, which is a cysteine protease with factor X activating properties.[6] Compared to sepsis and trauma, DIC in cancer is found to have a less fulminant presentation. A more chronic and gradual systemic activation of coagulation leads to sub-clinical progression and finally bleeding at the site of the tumour. Acute obstetric complications such as abruptio placentae, amniotic fluid embolism, rarely pre-eclampsia and intrauterine death of the foetus can also lead to DIC. Release of thromboplastin-like material in abruptio placentae is the causative factor which correlates with the degree of separation. Aortic aneurysms and cavernous haemangiomas may promote DIC by producing vascular stasis or local activation of coagulation system; whereas snake bites cause exogenous toxins induced DIC. Though microangiopathic haemolytic anaemia can mimic DIC clinically, it can be differentiated from it by normal PT and aPTT which are usually prolonged in DIC.
Several mechanisms occurring simultaneously play an effective role in the development of DIC. Excess thrombin generation, as a result of bacteraemia or endotoxaemia, is insufficiently balanced by impaired anti-coagulant systems such as anti-thrombin and protein C. This results in excess fibrin generation and deposition in the vascular system. In the early phase of DIC, plasmin (naturally occurring fibrinolytic agent) produced by the activation of plasminogen activators from the endothelial cells causes fibrinolysis to maintain circulation. But, its effect is immediately offset by high circulating levels of plasminogen activator inhibitor-1, a fibrinolytic inhibitor. Bleeding manifestation, seen in some forms of DIC, is due to the excess fibrinolytic activity.
There are three major natural anti-coagulants in the vascular system. They are anti-thrombin III, protein C and the tissue factor pathway inhibitor (TFPI). Studies have shown that thrombin generation in DIC is tissue factor driven (tissue factor/factor VIIa) with intrinsic pathway playing a minor role.[7,8] Anti-thrombin III is the most important inhibitor of thrombin in the coagulation cascade. Markedly reduced concentration of this factor in sepsis has been implicated in the pathogenesis of DIC and organ dysfunction.[9] Impairment of protein C pathway is mainly caused by down-regulation of thrombomodulin expression on endothelial cells by pro-inflammatory cytokines like tumour necrosis factor-alpha and interleukin-I beta.[10,11] This has been confirmed in meningococcal sepsis also.[12] Pro-inflammatory cytokines along with low levels of zymogen protein C leads to reduced protein C activation resulting in a pro-coagulant state. TFPI is the third significant inhibitor of coagulation and studies have shown that pharmacological doses of TFPI can reduce the mortality associated with systemic infection and inflammation.[13]
DIAGNOSIS OF DISSEMINATED INTRAVASCULAR COAGULOPATHY
A diagnosis of DIC should be made only in the presence of a clinical condition (causative factor) supported by relevant laboratory results. A combination of tests when repeated in a patient with a clinical condition known to be associated with DIC can be used for the diagnosis with a reasonable certainty.[14,15] Degree of coagulation factor consumption and activation can be screened by tests such as PT and aPTT or platelet count. A measurement of D-dimer levels in blood can be assayed which provides an indirect measure of fibrin formation. The laboratory abnormalities reported in DIC listed in the decreasing order of frequency are thrombocytopenia, elevated fibrin degradation products (FDPs), prolonged PT, aPTT and a low fibrinogen.[16] In early DIC, the platelet count and fibrinogen levels may remain within the normal range, albeit reduced from baseline levels.
THROMBOCYTOPENIA
Low platelets or a rapidly progressing thrombocytopenia is a key finding in DIC. Moderate (<1 lakh/mm3) to severe thrombocytopenia (<50,000/mm3) is seen in majority of patients and those with <50,000 have a 4-5 fold increased haemorrhagic complications compared to those with normal count. It is a sensitive but not a specific sign of DIC. Thrombocytopenia is seen in about 98% of cases, with <50,000/mm3 in about half the cases. It correlates well with thrombin generation as thrombin-induced platelet aggregation is mainly responsible for its consumption.[17] A declining trend rather than a single value is most indicative of DIC. At the same time, it should be remembered that a declining trend is not very specific for DIC because conditions associated with DIC such as acute leukaemia and sepsis can also have thrombocytopenia in the absence of DIC. At the same time, a stable platelet count is an indirect measure to suggest that thrombin formation has stopped.[17]
FIBRIN DEGRADATION PRODUCTS AND D-DIMERS
Fibrin degradation product is a measure of increased fibrinolytic activity which is also increased in DIC. New assays have developed which specifically detects the neo-antigens on degraded cross-linked fibrin called the D-dimer. Its level is also found to the elevated in conditions like trauma, recent surgery or venous thromboembolism and hence not a specific test for DIC. It can also be raised in liver and renal impairment as a result of its impaired metabolism and excretion.[18] Hence, it is of value when associated with a declining platelet count and prolonged PT and aPTT. Though much debate is underway regarding the cut-off level of D-dimer, clinician's experience, available circumstance and other supportive laboratory values are crucial to the diagnosis of DIC. Soluble fibrin monomer offers a theoretical advantage over FDP in the diagnosis of DIC as it is produced only intravascularly and not found rose in local inflammation and trauma. Though it has a high sensitivity (90-100%), the specificity is very low. However, its incorporation into the ISTH scoring system instead of D-dimer, can improve the specificity of diagnosing DIC.
PROTHROMBIN TIME AND ACTIVATED PARTIAL THROMBOPLASTIN TIME
Both PT and aPTT seem prolonged in about 50% of DIC cases which is attributed to the consumption of coagulation factors but can also be prolonged in impaired synthesis of coagulation factors and in massive bleeding.[19] At the same time, at least in half the patients with DIC, PT and aPTT are found normal or shortened due to the presence of circulating activated clotting factors like thrombin or Xa. Thus, a normal PT or aPTT do not exclude DIC and repeated monitoring is required.
FIBRINOGEN
Fibrinogen is an acute phase reactant and its plasma level can remain elevated for prolonged periods despite ongoing consumption in DIC. Hence, hypofibrinogenaemia for diagnosis of DIC carries very low sensitivity and was associated only with severe forms of DIC.[14] Fibrinogen level can be normal in nearly half the patients, and hence, serial measurements are indicated.
More recently, an atypical light transmittance profile on the aPTT has been found to be associated with DIC. This biphasic waveform abnormality occurs independently of prolonged clotting times and studies show it to be a simple, rapid and robust indicator of DIC.[20,21] It also carries a high positive predictive value for DIC with increasing waveform abnormality.[20]
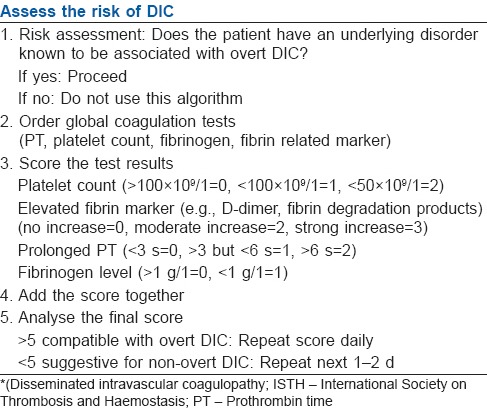
SCORING SYSTEM
The ISTH subcommittee has recommended a scoring system for overt DIC.[2] It is a five step diagnostic algorithm to calculate a DIC score based on simple laboratory results [Table 2]. For a diagnosis of overt DIC, a score of 5 or more from four parameters are required and was found to be sensitive to both infective and non-infective causes of DIC.[21] The score has a sensitivity of 91% and specificity of 97% and there exists a strong correlation between increasing DIC score and mortality. For each DIC point, increases in the odds of mortality of 1.25-1.29 have been demonstrated.[22] Several other studies have also confirmed the scoring algorithm as independent predictor of mortality.[23,24] They also showed that patients with sepsis and DIC have a higher mortality of 43% as compared with 27% in patients without DIC. Moreover, this scoring system has added prognostic value in better predicting mortality than the Acute Physiology and Chronic Health Evaluation (APACHE) II score as evidenced by an increase in odds ratio in the ISTH scoring.[25]
Table 2.
Diagnostic algorithm for DIC, proposed by ISTH
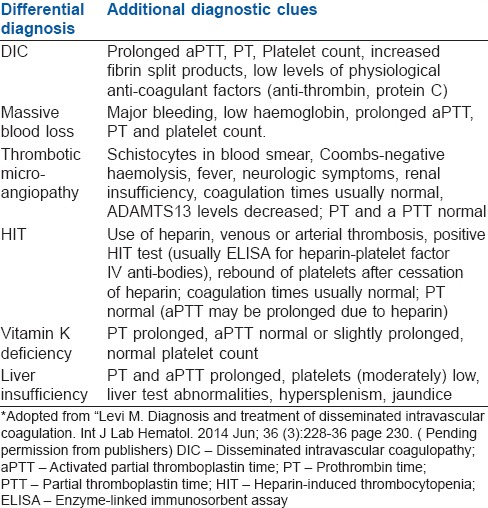
DIFFERENTIAL DIAGNOSIS OF DISSEMINATED INTRAVASCULAR COAGULOPATHY
Differential diagnosis of DIC includes:
Massive blood loss
Thrombotic microangiopathy
Heparin-induced thrombocytopenia
Vitamin K deficiency
Liver insufficiency.
Additional diagnostic clues are helpful in differentiating it from DIC [Table 3].
Table 3.
Differential diagnosis of DIC
TREATMENT
Key factor in DIC management is the proper treatment of the underlying cause and that may itself revert or prevent the development of DIC. But sometimes additional supportive treatment aimed at correction of coagulation abnormalities may be required. Blood component therapy should never be instituted on the basis of laboratory results alone, but reserved for patients with active bleeding or scheduled for surgery or an intervention associated with bleeding or those at risk of haemorrhagic events.[26] In DIC, platelet is given if there is bleeding with a count <50,000/cu.mm or in post-operative case, or requiring an invasive procedure or surgery.[27] In non-bleeding patients, transfusion may be started if count is <20,000/cu.mm. Fresh frozen plasma may be required to correct the coagulation defect. An initial dote of 15 ml/kg to start with and may require up to 30 ml/kg for a complete correction of coagulation factors.[26] Severe hypofibrinogenaemia (<1 g/l) that persists despite FFP replacement may be treated with purified fibrinogen concentrate or cryoprecipitate with a goal to keep fibrinogen level above 1 g/l. A dose of 3 g would raise plasma fibrinogen by around 1 g/l. This can be given as approximately 4 units of FFP, 2 cryoprecipitate pools (10 donor units) or as 3 g of a fibrinogen concentrate.[26] The aim was to bring PT and aPTT to <1.5 times the normal. Packed red cells may be given to maintain the haemoglobin >8 g/dl to ensure adequate oxygen delivery to the tissues. The response to therapy should be closely monitored clinically and by repeated tests. Role of recombinant factor VIIa in patient with DIC and life-threatening bleeding is doubtful.[26]
Anti-coagulant therapy with heparin is still debatable. It is generally accepted that heparin is advantageous in DIC due to metastasized malignancies, purpura fulminans and major vascular abnormalities.[28] It is also effective for the treatment of thrombosis or embolic complications in patient with low grade sustained DIC. The guideline also advocates the administration of low molecular weight heparin prophylaxis for the prevention of venous thrombosis in intensive care unit (ICU) patients.[26] Recently, a recombinant human soluble thrombomodulin is found to be a promising drug in DIC.[29] Role of anti-thrombin concentrate is yet to be proved and the role of activated protein C is gaining importance as it is found to be beneficial in patients with severe sepsis and organ failures. Tranexamic acid (1 g every 8 h) may be useful in patients with DIC having hyper-fibrinolysis with severe bleeding.
SUMMARY
Though a complex clinical syndrome affecting the circulatory system, DIC can be diagnosed now with the help of laboratory tests and the international scoring system which makes it simple with strong prognostic power. Irrespective of the cause, pathways involved in DIC are the tissue factor mediated thrombin generation; impaired natural anti-coagulant system and inhibition of endogenous fibrinolysis. Treatment is aimed at management of the underlying cause; correction of coagulation abnormalities and other supportive measures in an ICU. Newer insight into the pathophysiology of DIC has resulted in its management by inhibiting the initiation or propagation of fibrin formation or regulation of coagulation activation. Sophisticated assays on coagulation factors or molecular biomarkers of DIC may help in early detection but the availability is limited to higher laboratories or centres and needs more clinical evaluation for their use. Early recognition of the causative factor, specific treatment aimed against it, repeated laboratory tests and monitoring of specific treatment is the backbone in the management of DIC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–92. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 2.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. [PubMed] [Google Scholar]
- 3.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–14. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 4.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost. 2001;27:585–92. doi: 10.1055/s-2001-18864. [DOI] [PubMed] [Google Scholar]
- 5.Levi M. Cancer and DIC. Haemostasis. 2001;31(Suppl 1):47–8. [PubMed] [Google Scholar]
- 6.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 7.Levi M, ten Cate H, Bauer KA, van der Poll T, Edgington TS, Büller HR, et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994;93:114–20. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimura M, Wada H, Wakita Y, Nakase T, Hiyoyama K, Nagaya S, et al. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol. 1997;55:169–74. doi: 10.1002/(sici)1096-8652(199707)55:4<169::aid-ajh1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Mesters RM, Mannucci PM, Coppola R, Keller T, Ostermann H, Kienast J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood. 1996;88:881–6. [PubMed] [Google Scholar]
- 10.Esmon CT. Role of coagulation inhibitors in inflammation. Thromb Haemost. 2001;86:51–6. [PubMed] [Google Scholar]
- 11.Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29:S90–4. doi: 10.1097/00003246-200107001-00028. [DOI] [PubMed] [Google Scholar]
- 12.Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345:408–16. doi: 10.1056/NEJM200108093450603. [DOI] [PubMed] [Google Scholar]
- 13.de Jonge E, Dekkers PE, Creasey AA, Hack CE, Paulson SK, Karim A, et al. Tissue factor pathway inhibitor dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood. 2000;95:1124–9. [PubMed] [Google Scholar]
- 14.Levi M, de Jonge E, van der Poll T, ten Cate H. Disseminated intravascular coagulation. Thromb Haemost. 1999;82:695–705. [PubMed] [Google Scholar]
- 15.Toh CH, Dennis M. Disseminated intravascular coagulation: Old disease, new hope. BMJ. 2003;327:974–7. doi: 10.1136/bmj.327.7421.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde JT, Kitchen S, Kinsey S, Greaves M, Preston FE. Plasma D-dimer levels and their relationship to serum fibrinogen/fibrin degradation products in hypercoagulable states. Br J Haematol. 1989;71:65–70. doi: 10.1111/j.1365-2141.1989.tb06276.x. [DOI] [PubMed] [Google Scholar]
- 17.Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30:753–6. doi: 10.1097/00003246-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Tomura S, Tachibana K, Chida Y, Marumo F. Enhanced fibrinolytic activity during the course of hemodialysis. Clin Nephrol. 1992;38:90–6. [PubMed] [Google Scholar]
- 19.Asakura H, Ontachi Y, Mizutani T, Kato M, Ito T, Saito M, et al. Decreased plasma activity of antithrombin or protein C is not due to consumption coagulopathy in septic patients with disseminated intravascular coagulation. Eur J Haematol. 2001;67:170–5. doi: 10.1034/j.1600-0609.2001.5790508.x. [DOI] [PubMed] [Google Scholar]
- 20.Downey C, Kazmi R, Toh CH. Early identification and prognostic implications in disseminated intravascular coagulation through transmittance waveform analysis. Thromb Haemost. 1998;80:65–9. [PubMed] [Google Scholar]
- 21.Matsumoto T, Wada H, Nishioka Y, Nishio M, Abe Y, Nishioka J, et al. Frequency of abnormal biphasic aPTT clot waveforms in patients with underlying disorders associated with disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2006;12:185–92. doi: 10.1177/107602960601200206. [DOI] [PubMed] [Google Scholar]
- 22.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2416–21. doi: 10.1097/01.ccm.0000147769.07699.e3. [DOI] [PubMed] [Google Scholar]
- 23.Sivula M, Tallgren M, Pettilä V. Modified score for disseminated intravascular coagulation in the critically ill. Intensive Care Med. 2005;31:1209–14. doi: 10.1007/s00134-005-2685-2. [DOI] [PubMed] [Google Scholar]
- 24.Cauchie P, Cauchie Ch, Boudjeltia KZ, Carlier E, Deschepper N, Govaerts D, et al. Diagnosis and prognosis of overt disseminated intravascular coagulation in a general hospital – Meaning of the ISTH score system, fibrin monomers, and lipoprotein-C-reactive protein complex formation. Am J Hematol. 2006;81:414–9. doi: 10.1002/ajh.20597. [DOI] [PubMed] [Google Scholar]
- 25.Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: A useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction scores. Crit Care Med. 2006;34:314–20. doi: 10.1097/01.ccm.0000196832.27501.b2. [DOI] [PubMed] [Google Scholar]
- 26.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 27.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi M. Diagnosis and treatment of disseminated intravascular coagulation. Int J Lab Hematol. 2014;36:228–36. doi: 10.1111/ijlh.12221. [DOI] [PubMed] [Google Scholar]
- 29.Levi M, Van Der Poll T. Thrombomodulin in sepsis. Minerva Anestesiol. 2013;79:294–8. [PubMed] [Google Scholar]


