Abstract
Resuscitation of a severely traumatised patient with the administration of crystalloids, or colloids along with blood products is a common transfusion practice in trauma patients. The determination of this review article is to update on current transfusion practices in trauma. A search of PubMed, Google Scholar, and bibliographies of published studies were conducted using a combination of key-words. Recent articles addressing the transfusion practises in trauma from 2000 to 2014 were identified and reviewed. Trauma induced consumption and dilution of clotting factors, acidosis and hypothermia in a severely injured patient commonly causes trauma-induced coagulopathy. Early infusion of blood products and early control of bleeding decreases trauma-induced coagulopathy. Hypothermia and dilutional coagulopathy are associated with infusion of large volumes of crystalloids. Hence, the predominant focus is on damage control resuscitation, which is a combination of permissive hypotension, haemorrhage control and haemostatic resuscitation. Massive transfusion protocols improve survival in severely injured patients. Early recognition that the patient will need massive blood transfusion will limit the use of crystalloids. Initially during resuscitation, fresh frozen plasma, packed red blood cells (PRBCs) and platelets should be transfused in the ratio of 1:1:1 in severely injured patients. Fresh whole blood can be an alternative in patients who need a transfusion of 1:1:1 thawed plasma, PRBCs and platelets. Close monitoring of bleeding and point of care coagulation tests are employed, to allow goal-directed plasma, PRBCs and platelets transfusions, in order to decrease the risk of transfusion-related acute lung injury.
Keywords: Blood transfusion, damage control resuscitation, massive transfusion protocol, packed red blood cells, plasma, platelets, trauma resuscitation
INTRODUCTION
The foremost reason of death among 1-40 years old is traumatic injury,[1] with an estimate of five million deaths per year globally,[2,3] among which an estimated 10-20% of deaths are preventable.[4] Uncontrolled haemorrhage within 6 h of injury is one of the prime causes of avoidable death,[1,5] which led many trauma specialists to find ways to reduce early mortality due to severe injuries. Patients who present to the emergency in hypovolemic shock and coagulopathy are more likely to receive massive blood transfusions, which increase mortality. Morbidity and mortality are four times higher in patients who develop trauma-induced coagulopathy. Early recognition of these patients remains a crucial goal of resuscitation to increase chances of survival by early aggressive treatment.[6,7]
Recent studies have suggested that only 25% of trauma patients require a blood transfusion, and only 2-3% of them receive a massive transfusion (MT).[8] A patient transfused with ten or more packed red blood cells (PRBCs) within first 24 h is said to have received a MT.[9] These patients are at high risk of early haemorrhagic death with a mortality rate of 40-70%.[2] In the last four decades, transfusion therapy has changed significantly from the use of whole blood to mainly component therapy. Most developed countries and major health centres primarily use blood component in MT. However, many developing countries and the armed forces are still using whole blood due to concerns for safety and also due to the scarce resources.[10] Unfortunately, this change occurred without any major retrospective studies or randomized controlled trials on MT. Component therapy does convey benefits in financial, logistical and inventory management. It is uncertain if component therapy is better or even comparable to whole blood transfusion in a MT patient.[2]
People did not adapt well to the component therapy, and there was confusion as to how to use it in MT. In 1970's and 1980's, this resulted in unintentional haemodilution as part of MT, which led to a vicious cycle of problems such as coagulopathy, acidosis and hypothermia, known as “lethal triad of trauma”.[11] During the same period, it became a common practice to administer significant amounts of crystalloid before admission, leading to abdominal compartment syndrome, acute respiratory distress syndrome, and multi-organ failure.[12] Clinicians in the late 1990's, realised the lethal effects of excess crystalloid administration and began restricting its use.[13]
Retrospective data from recent war experiences recommend that whole blood is clinically superior to component therapy in severely traumatised patient requiring MT.[10] Since whole blood is not easily available in major hospitals; clinicians are widely using damage control resuscitation (DCR) practices with the increased ratios of plasma: Platelets: PRBCs during transfusion.[7] This review will focus on latest transfusion practices in trauma along with DCR.
TRAUMA-INDUCED COAGULOPATHY
Trauma-induced coagulopathy is a condition in which various elements like acidosis, hypothermia, haemodilution and consumption of clotting factors from transfusion of crystalloids and PRBCs play a crucial role.[14] It is a significant predictor of blood utilisation and trauma-related mortality and is iatrogenic.[15]
This iatrogenic coagulopathy is preceded by early trauma-induced coagulopathy (ETIC) which presents with prolonged PT on admission. One theory suggests that ETIC is caused due to the release of tissue factor from the actual injury, which subsequently causes thrombin and fibrin production and utilisation causing disseminated intravascular coagulation.[16] Alternate theory suggests that ETIC may be due to hyper-perfusion and ischemia, known to be allied with trauma, which promotes the release of activated protein C, leading to depletion of plasminogen activator inhibitor and inhibition of the systemic anti-coagulation, clotting cascade and hyper-fibrinolysis.[17]
Shaz et al. in a recent case-control study of ETIC patients found no difference in thrombin or fibrin generation between the cases and the control had no difference in the amount of fibrinolysis.[18] Patients with ETIC were administered more crystalloids in the prehospital phase and concluded that ETIC is secondary to trauma-induced coagulopathy that occurs early before patient reaches hospital. Coagulopathy of trauma (both ETIC and trauma-induced coagulopathy) are concomitant with a significant risk of bleeding and high mortality. Care ought to be taken to decrease this risk that can be achieved by significantly reducing the quantity of crystalloids that are initially administered.[18]
Ley et al. found out that receiving intravenous crystalloid >1.5 litres in the emergency department (ED) is an independent risk factor for mortality.[19] Other major factors like Glasgow coma scale <8, injury severity scores >16, age >80 years and hypotension, were also found to be the causes of increased mortality in trauma patients. Elderly patients receiving a higher volume of crystalloids (>3 L) are associated with higher mortality rates.[19]
James et al. in a recent study regarding the use of either crystalloid or colloids in early trauma patients in South Africa, observed that initial resuscitation with colloids instead of crystalloids, after a penetrating injury, had less renal injury and decreased lactate levels.[20]
FIBRINOGENS
Thousand milligrams (mg) of fibrinogen are present in a unit of whole blood, so the loss of one unit of whole blood, dissipates 1000 mg of fibrinogen. This loss is usually, restored with transfusion of one unit of both PRBC and fresh frozen plasma (FFP), where 1 unit of FFP contains only 500 mg of fibrinogen in it. So in later stages, it is necessary to add more fibrinogen to restore the deficit. Cryoprecipitate derived from cold-thawed human plasma is transfused to restore the fibrinogen deficit. At the end of an MT, ten units of cryoprecipitates are transfused to restore the fibrinogen deficit as ten units of cryoprecipitates contains 2.5 g of fibrinogens.[2]
Stinger et al. has shown that a high fibrinogen: PRBC ratio (>0.2 g of fibrinogen: PRBC) was concomitant with survival to discharge after an injury.[21] One unit of cryoprecipitate contains 0.25 g of fibrinogen, and this ratio can be obtained by transfusing cryoprecipitate: PRBC in a 1:1 ratio. In clinical practice in the ED, for every ten units of PRBCs transfused, transfuse ten units of cryoprecipitates. Shaz et al., has shown transfusion of cryoprecipitate and PRBCs in the ratio of 1:1 has higher 24-hour, and 30-day survival after MT in trauma.[22]
PLATELETS
Perkins et al. focused on platelet to PRBCs ratios in military trauma, and observed improved 24-h and 30-days survival rates in a high-ratio group which received approximately 1:1 platelets to PRBCs in comparison to other groups.[23] Shaz et al. also has shown increased 24-h and 30-days survival rates in patients who received higher platelet: PRBC ratios, 1:1 during MT secondary to civilian trauma.[22] The current resuscitation approach is to use 1:1:1 FFP: PRBCs: Platelets in resuscitation for all casualties who are expected to receive an MT.
WARM FRESH WHOLE BLOOD
Spinel et al. compared component therapy use to warm fresh whole blood (WFWB), using the US Army Institute of Surgical Research transfusion database, among all patients receiving >1 unit of RBC transfusion.[24] Patients who received on average, 70% component therapy and 30% WFWB, had a better survival rate in comparison to those who received only component therapy.[24]
Warm fresh whole blood (WFWB) transfusion of 500 ml carries a decent haematocrit level and no storage deficits. WFWB is healthier and more beneficial to patients as they contain an optimum amount of clotting factors; platelets and fibrinogen. A 1:1:1 ratio of plasma, PRBCs and platelets component therapy does not contain equivalent amounts of clotting factors, platelets or fibrinogen as WFWB does.[25]
CRYSTALLOIDS AND COLLOIDS
Crystalloids instead of being the primary resuscitation fluid, in many trauma centres, have become a carrier fluid for medications and blood products. Synthetic colloids are initially favoured until blood products are available and where speed of resuscitation is paramount like acute major trauma or massive haemorrhage. They provide more rapid restoration of circulating volume with a smaller infused volume than crystalloids. Although studies comparing synthetic colloids to crystalloids confirmed that the colloid was a better volume expander and maintained or restored serum protein levels, however also confirmed that colloids had no significant reduction in organ dysfunction or mortality.[20,26,27] Given the much higher costs of colloids and the greater risk of renal injury and death with them, resuscitation should focus on crystalloid solutions.
OTHER DRUG THERAPIES
Usage of Recombinant Factor VII (rFVII) in the injured has decreased in recent years and is controversial. Tranexamic acid is a cheaper alternative to Factor VII and reduces the risk of death from haemorrhage significantly without any thromboembolic impediments (Crash-2 trail).[28] Tranexamic acid is administered initially as a bolus loading dose of 10 mg/kg intravenously, trailed by an infusion dose of 1 mg/kg/h. The bolus and infusion dose of tranexamic acid produces sufficient concentrations of plasma in order to inhibit fibrinolysis.[29,30] Tranexamic acid use should be deliberated in all patients requiring an MT.
DAMAGE CONTROL RESUSCITATION
An evolution of opinion, encouraged by new facts from combat casualties in recent conflicts, is happening in anaesthesia, emergency medicine, trauma, and transfusion medicine communities, about the best resuscitative approach in response to haemorrhagic shock.[2] Borgman et al. observed an improved survival in 252 MT combat casualties who were resuscitated with FFP: PRBC in the ratio of 1:1.[31] Similar results were noticed in a study by Holcomb et al. in 466 MT civilian trauma patients.[32]
In 2007, Holcomb et al., while addressing early coagulopathy in trauma, advocated DCR,[7]
Early definitive haemorrhage control
Early and increased use of 1:1:1 FFP, PRBC and platelets, and minimising crystalloids
Avoiding hypothermia, acidosis and coagulopathy
Hypotensive resuscitation strategies
Use of other products like Ca2+, rFVII, tranexamic acid, and tris-hydroxymethyl aminomethane should be deliberated.
The current US military resuscitation practice,[33] established by the Army Surgeon General, is to use DCR approach as the primary resuscitation method in severely traumatised casualties, using 1:1:1 FFP: PRBCs: Platelets. This clinical policy was developed based on a study by Perkins et al. concentrating on platelet ratios in casualties during recent conflicts, which evidently defined the survival benefits.[23]
Lower volume or hypotensive resuscitation stratagem is employed in DCR, prior to haemorrhage control, in order to avoid ‘popping the clot’ as the blood pressure rises rapidly with resuscitation. Instead of large amounts of crystalloids and PRBCs very early in trauma resuscitation, DCR methodology, replaces the lost blood with PRBCs, plasma and platelets in the ratio of 1:1:1, in order to minimise the exacerbating multifactorial trauma-induced coagulopathy. DCR is quickly becoming the primary resuscitation technique in many trauma centres in resuscitating traumatised patients.[2]
MASSIVE TRANSFUSION PROTOCOLS
Many hospitals implemented massive transfusion protocol (MTPs), as mortality improved with changes in blood products,[34] but the principle remains the same, even though it varies between institutions practicing MTPs. The blood bank delivers several ‘rounds’ of 1:1:1 PRBCs, FFP and platelets to the patients once MTP is activated and will continue to do so until deactivated.[11]
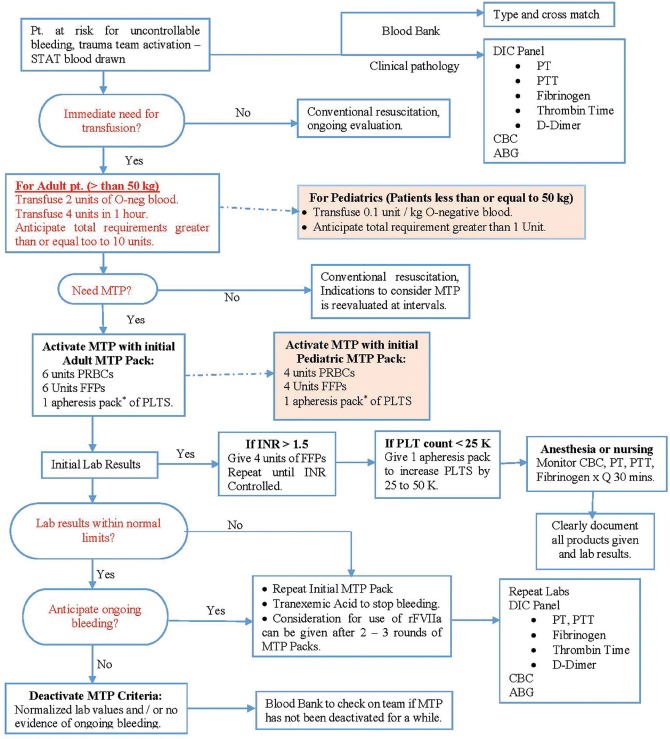
Riskin et al. observed that with the implementation of MTP, deaths from trauma have decreased significantly.[35] Expeditious availability of blood products due to MTP, lead to early transfusion of PRBCs, which decreased the time for first plasma and platelet transfusion, and increased survival of trauma patients [Figure 1].[35] Enhanced communication and organisation within MTP, empowering prompt delivery of blood packs from the blood bank was fundamental to the success of the protocol.[11] Upon arrival to the ED, an MTP should enable emergency physician or trauma surgeon immediately to administer 1:1:1:1 ratio of PRBCs, plasma, platelets and cryoprecipitates, instead of administering PRBCs first and at a later stage giving plasma.[11] This means that in the ED, thawed plasma has to be readily available for administration in the first round of the MTP.[36]
Figure 1.
Massive Transfusion Protocol (Figure 1. Stanford Massive Transfusion Protocol.[35] Reprinted from the Journal of the American College of Surgeons, 209 (2), Daniel J. Riskin, Thomas C. Tsai, Loren Riskin, et al., Massive Transfusion Protocols: The Role of Aggressive Resuscitation Versus Product Ratio in Mortality Reduction, 198-205, Copyright (2009), with permission from Elsevier). ABG – Arterial blood gases; CBC – Complete blood count; DIC – Disseminated intravascular coagulation; FFP – Fresh frozen plasma; INR – Internationalized normalized ration; MTP – Massive transfution protocol; PRBCs – Packed red blood cells; PT – Prothrombin time; PTT – Partial thromboplastin time; PLT – Platelets and rFVIIa – recombinant factor VII a
Thawed plasma or FFP is fresh plasma, taken from a unit of whole blood, frozen within 8 h of collection, and stored at 4°C for 5 days after thawing.[36] In the ED refrigerator, thawed AB plasma is stored alongside emergency release type O blood, which allows both products (PRBCs and plasma) to be used immediately and even concurrently when a MTP is activated. A MTP improves patient survival and also reduce clinician stress.[11] In order for a MTP to be activated, to avert early haemorrhagic death, it is imperative to envisage who will need an MT. A vast majority of trauma patients do not require a MTP to be activated as only 2% of civilian trauma patients need an MT.[8] Overuse of a MTP can lead to blood products wastage and also cause TRALI when administering blood components, mainly plasma.[37]
It is cumbersome to predict who will require a MTP and when to be activated. Dente et al. in early predictors of MT study reported 27% overtriage rate in whom a MTP was activated, but MT was not administered.[38] The study observed that all trauma patients with a mulitcavity or a transpelvic gunshot wound required MT. In trauma patients with isolated abdomen or thorax gunshot injuries, many patients did not require MT. Conversely, a hypotensive patient with a systolic blood pressure <90 mmHg and a patient in acidosis with a base deficit more than ten units are robust prognosticators for MT.[38]
Due to practice variation at different trauma centres, a universal criteria to activate MTP may not be possible, and the ultimate decision to activate a MTP relies on the judgment and experience of the Emergency physician or trauma surgeon, who heads the trauma team.[38]
FUTURE
Point of care coagulation testing
A smart substitute to formula-driven methodology to blood transfusion in trauma is point of care (POC) coagulation testing. POC haemoglobin, platelet count, fibrinogen level, prothrombin time, thromboelastometry and thromboelastography are currently available and used in some trauma centres.[11] In majority of patients having severe traumatic injury, but not enough to activate a MTP, POC coagulation testing is now used. In trauma patients, POC coagulation testing may become a substitute to formula-driven MTPs as progress is made in the speed and accuracy of these technologies.[11] POC testing is not yet a gold standard diagnostic test and is not used in most hospitals. Further studies are required to demonstrate decreased use of blood products and improved patient survival by using POC coagulation tests instead of formula-driven transfusion protocols.
SUMMARY
In a trauma patient with life-threatening injuries, early identification of coagulopathy and treating it in 1:1:1 ratio, with thawed plasma, PRBCs, and platelets, limited use of crystalloids and rapid haemorrhage control will improve their survival. These principles of DCR should only be utilised in resuscitation of patients with haemorrhagic shock and life-threatening injuries, and one should be cautious not to overuse DCR principles. Accurate models to predict which trauma patient will benefit from DCR and who require MT is the need of the hour and is an area where future research is required with more prospective randomized trials.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, et al. Causes of death in U.S. special operations forces in the global war on terrorism: 2001-2004. Ann Surg. 2007;245:986–91. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb JB. Optimal use of blood products in severely injured trauma patients. Hematology Am Soc Hematol Educ Program 2010. 2010:465–9. doi: 10.1182/asheducation-2010.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: A comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–11. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 4.Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: Lessons learned from 2,594 deaths. Ann Surg. 2006;244:371–80. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito TJ, Sanddal TL, Reynolds SA, Sanddal ND. Effect of a voluntary trauma system on preventable death and inappropriate care in a rural state. J Trauma. 2003;54:663–9. doi: 10.1097/01.TA.0000058124.78958.6B. [DOI] [PubMed] [Google Scholar]
- 6.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. Early coagulopathy in multiple injury: An analysis from the German trauma registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: Directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 8.Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–13. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 9.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–6. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 10.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66:S69–76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller TE. New evidence in trauma resuscitation-is 1:1:1 the answer? Perioper Med (Lond) 2013;2:13. doi: 10.1186/2047-0525-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363:1988–96. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- 13.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–21. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 14.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 15.Eddy VA, Morris JA, Jr, Cullinane DC. Hypothermia, coagulopathy, and acidosis. Surg Clin North Am. 2000;80:845–54. doi: 10.1016/s0039-6109(05)70099-2. [DOI] [PubMed] [Google Scholar]
- 16.New York, NY, USA: Copyright© 2001 by Thieme Medical Publishers, Inc; 2001. Disseminated intravascular coagulation in trauma patients. Seminars in Thrombosis and Haemostasis. [DOI] [PubMed] [Google Scholar]
- 17.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: Hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–7. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 18.Shaz BH, Winkler AM, James AB, Hillyer CD, MacLeod JB. Pathophysiology of early trauma-induced coagulopathy: Emerging evidence for hemodilution and coagulation factor depletion. J Trauma. 2011;70:1401–7. doi: 10.1097/TA.0b013e31821266e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley EJ, Clond MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, et al. Emergency department crystalloid resuscitation of 1.5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011;70:398–400. doi: 10.1097/TA.0b013e318208f99b. [DOI] [PubMed] [Google Scholar]
- 20.James MF, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: The FIRST trial (Fluids in Resuscitation of Severe Trauma) Br J Anaesth. 2011;107:693–702. doi: 10.1093/bja/aer229. [DOI] [PubMed] [Google Scholar]
- 21.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 22.Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50:493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 23.Perkins JG, Cap AP, Spinella PC, Blackbourne LH, Grathwohl KW, Repine TB, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66:S77–84. doi: 10.1097/TA.0b013e31819d8936. [DOI] [PubMed] [Google Scholar]
- 24.Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit Care Med. 2008;36:S340–5. doi: 10.1097/CCM.0b013e31817e2ef9. [DOI] [PubMed] [Google Scholar]
- 25.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–31. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 26.Marik PE, Iglesias J, Maini B. Gastric intramucosal pH changes after volume replacement with hydroxyethyl starch or crystalloid in patients undergoing elective abdominal aortic aneurysm repair. J Crit Care. 1997;12:51–5. doi: 10.1016/s0883-9441(97)90001-0. [DOI] [PubMed] [Google Scholar]
- 27.Shatney CH, Deepika K, Militello PR, Majerus TC, Dawson RB. Efficacy of hetastarch in the resuscitation of patients with multisystem trauma and shock. Arch Surg. 1983;118:804–9. doi: 10.1001/archsurg.1983.01390070016004. [DOI] [PubMed] [Google Scholar]
- 28.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 29.Fiechtner BK, Nuttall GA, Johnson ME, Dong Y, Sujirattanawimol N, Oliver WC, Jr, et al. Plasma tranexamic acid concentrations during cardiopulmonary bypass. Anesth Analg. 2001;92:1131–6. doi: 10.1097/00000539-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995;82:383–92. doi: 10.1097/00000542-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 32.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 33.Impact of policy change on US army combat transfusion practices. [Last accessed on 2014 July 01]. Available from: http://www.usaisr.amedd.army.mil/cpgs/DmgCntrlResus0903.pdf . [DOI] [PubMed]
- 34.Schuster KM, Davis KA, Lui FY, Maerz LL, Kaplan LJ. The status of massive transfusion protocols in United States trauma centers: Massive transfusion or massive confusion? Transfusion. 2010;50:1545–51. doi: 10.1111/j.1537-2995.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 35.Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, et al. Massive transfusion protocols: The role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209:198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, et al. The effect of plasma transfusion on morbidity and mortality: A systematic review and meta-analysis. Transfusion. 2010;50:1370–83. doi: 10.1111/j.1537-2995.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y, Saw CL, Hannach B, Goldman M. Transfusion-related acute lung injury prevention measures and their impact at Canadian Blood Services. Transfusion. 2012;52:567–74. doi: 10.1111/j.1537-2995.2011.03330.x. [DOI] [PubMed] [Google Scholar]
- 38.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Ficke BW, et al. Early predictors of massive transfusion in patients sustaining torso gunshot wounds in a civilian level I trauma center. J Trauma. 2010;68:298–304. doi: 10.1097/TA.0b013e3181cf7f2a. [DOI] [PubMed] [Google Scholar]