Abstract
An efficient strategy for the synthesis of indolizines from readily available starting materials via oxidative C-H functionalization and 5-endo-dig cyclization in one step has been demonstrated. This protocol represents wide substrate scope, high functional group tolerance and selectivity. The structure of the product was confirmed by the X-ray crystallographic studies. The Ag2CO3 required of this tandem reaction can be recycled and reused after undergoing oxidative reaction.
Keywords: C-H functionalization, Indolizine, 5-endo-dig cyclization
The study of the synthesis of small heterocyclic compounds and natural products using transition metal-catalyzed tandem reactions is a rapidly expanding area of interest in the field of synthetic organic chemistry. In recent years, various examples of direct oxidative C-H functionalization and subsequent C-C and C-N bond formation have improved the atom economy and efficiency of multistep synthesis.1 Unfortunately, these approaches required expensive metal catalysts, such as Pd, Rh, and Ru, as well as pre-functionalized starting materials for both reactivity and selectivity. Limited progress has been made in the utilization of less expensive copper and silver salts as oxidative promoters of C-C bond formation via C-H activation. Recently, Lei et al.2 reported stoichiometric silver-mediated oxidative C-H/C-H functionalization of 1,3-dicarbonyl compounds with terminal alkynes for the synthesis of polysubstituted furans and pyrroles. Duan3 and Wu4 independently disclosed silver-promoted oxidative C-H/P-H functionalization to construct benzo[b]phosphole oxides and 3-phosphorated coumarins, respectively.
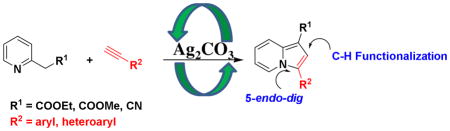
The indolizine-based scaffolds are found in many natural alkaloids and biologically active compounds (Figure 1). Such derivatives have shown utility in anticancer,5 antibacterial,6 antituberculosis,7 H3 receptor antagonist8 and antifungal 9 applications. While numerous methods for the synthesis of indolizine scaffolds are known, the direct and region-selective synthesis of this class of scaffolds from readily available starting materials has drawn considerable attention.10 Recently, we developed new synthetic methodologies for highly substituted imidazoles based on C-H functionalization.11 Herein, we communicate our discovery of a silver-mediated indolizines synthesis via one pot oxidative C-H functionalization and 5-endo-dig cyclization in one step under mild reaction conditions (Scheme 1).
Figure 1.
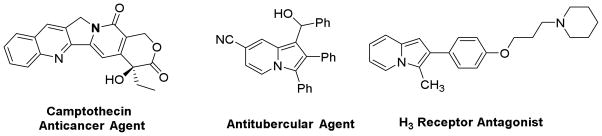
Selected examples of biologically relevant indolizines derivatives
Scheme 1.
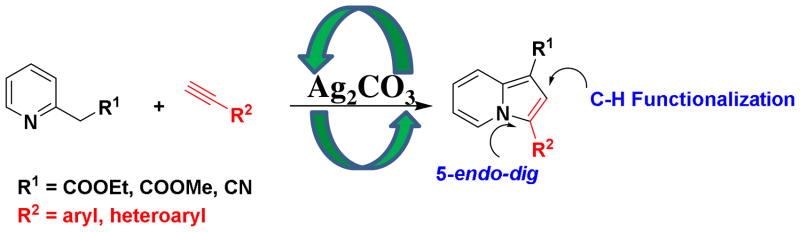
Silver-Mediated Oxidative C-H Functionalization to synthesize Indolizines
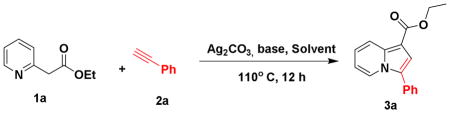
To identify suitable reaction conditions, various substrate ratios and solvent conditions were screened as summarized in Table 1. Initially, we carried out the reaction of ethyl 2-pyridylacetate (1 equiv.), phenylacetylene (1 equiv.), Ag2CO3 (1 equiv.) and KOAc (2 equiv.) in DMF solvent at room temperature (Table 1, entry 1). These conditions did not produce desired product 3a, and lack the visual markers of the reaction initiation whereupon the reaction mixture turns black. Notably, after increasing the reaction temperature to 110°C this substrate ratio did produce the desired indolizine product with excellent region-selectivity, albeit low yield (Table 1, entry 2). Changing the reaction conditions to refluxing THF along with an increased ratio of silver salt (1.5 equiv.) afforded only moderate improvement in product yield (Table 1, entry 3). Further increase in the amounts of silver salt and compound 1a to two equivalents improved the reaction outcome with KOAc base, showing the best overall yield among K2CO3 or Cs2CO3 alternatives (Table 1, entries 4–6).
Table 1.
Survey of reaction conditions for silver-mediated indolizine formation
| ||||
|---|---|---|---|---|
| entry | base | solvent | 1a:2a:Ag2CO3 | yields (%)c |
| 1a | KOAc | DMF | 1:1:1 | 0 |
| 2 | KOAc | DMF | 1:1:1 | 36 |
| 3b | KOAc | THF | 1:1:1.5 | 57 |
| 4 | K2CO3 | DMF | 2:1:2 | 69 |
| 5 | Cs2CO3 | DMF | 2:1:2 | 72 |
| 6 | KOAc | DMF | 2:1:2 | 86 |
Reaction carried out at room temperature.
Reaction carried out at 80° C.
Isolated Yields.
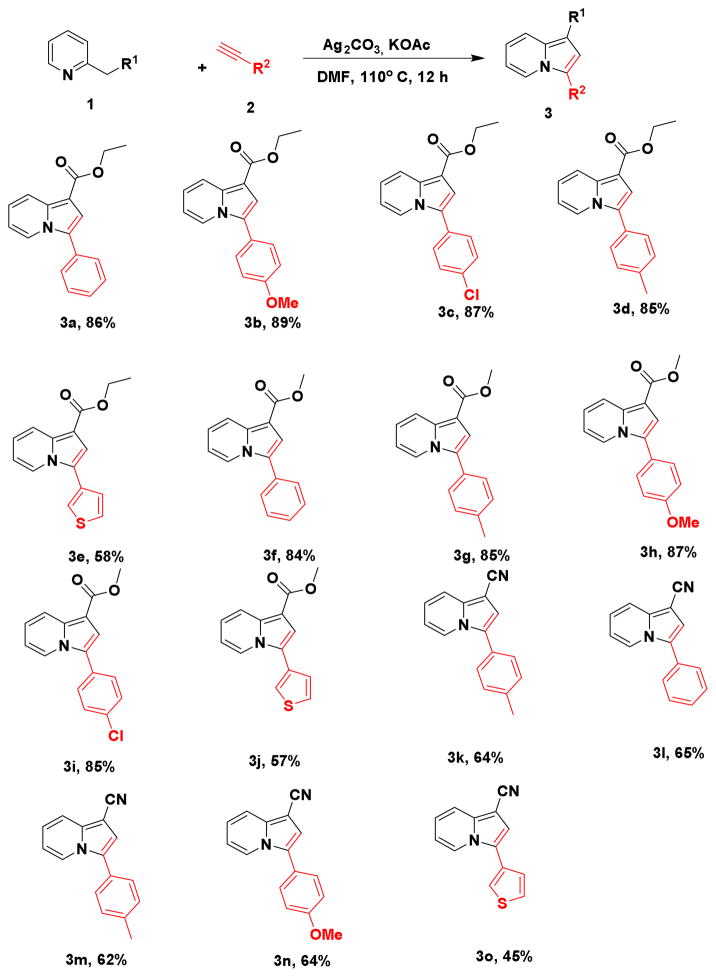
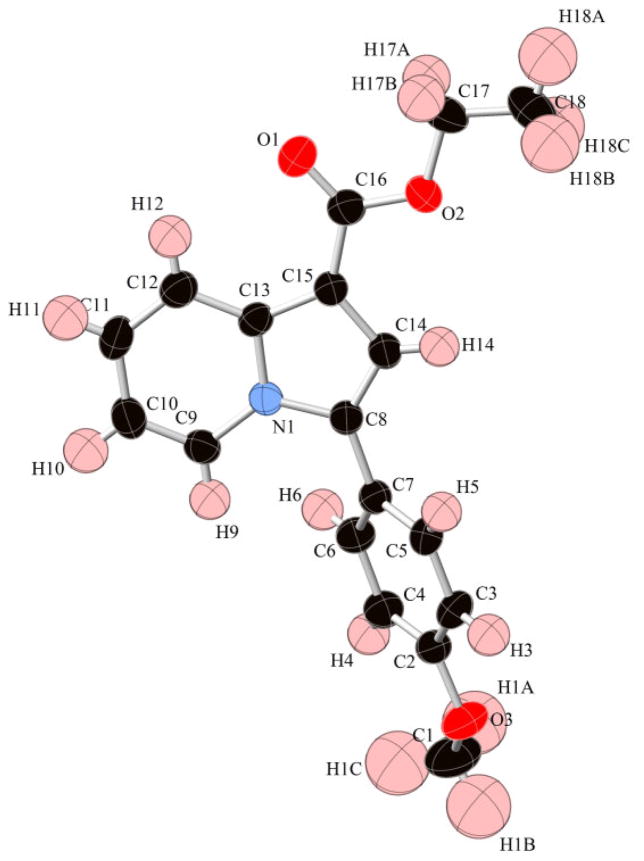
After completing the standardization of the reaction conditions, we examined the scope of the reaction towards pyridine and alkyne substitution, as shown in scheme 2. This included CH2COOEt, CH2COOMe, CH2CN groups substituted at the C2 position of pyridine and diverse electron withdrawing and donating aryl groups on the alkyne. Good overall tolerance was demonstrated by these reaction conditions, resulting in the formation of diverse products with excellent regio-selectivity in medium-to-high yield (3a-3o). The presence of strong electron-withdrawing groups (COOEt, COOMe) at the C2 position of pyridine (R3) provided higher yields relative to the less electron-withdrawing cyanide group (Scheme 2, 3a-3j vs 3k-3o). In contrast, no significant differences in yields were observed when aryl alkyne reactant substitution was varied with both electron-withdrawing (-Cl) and electron-donating substituents (-OMe, Me) affording similar outcomes. The evaporation of compound 3b from dichloromethane gave a single crystal suitable for X-ray analysis. As illustrated in Figure 2, this proves unambiguously the heterocyclic and regio-isomeric identity of the reaction product being an indolizine ring substituted at the 2- and 4-positions. Furthermore, the structures were confirmed by 1D and 2D NMR spectrometry (Supporting Information).
Scheme 2.
Substrate scope for the silver mediated synthesis of indolizines
Figure 2.
X-ray crystal structure of indolizine 3b
Because two equivalents of Ag2CO3 are necessary to achieve high yields, this significantly increases the cost and chemical waste produced by this reaction. To address this issue, we examined whether Ag2CO3 could be regenerated from the reaction waste and successfully reused for this transformation. Upon completion of the reaction, the precipitated silver salts were separated by filtration, then treated with nitric acid followed by Na2CO3 at room temperature to provide Ag2CO3.12 This regenerated Ag2CO3 was shown to mediate C-H functionalization for synthesizing indolizines with analogous activity to commercial Ag2CO3 salts (Scheme 3).
Scheme 3.
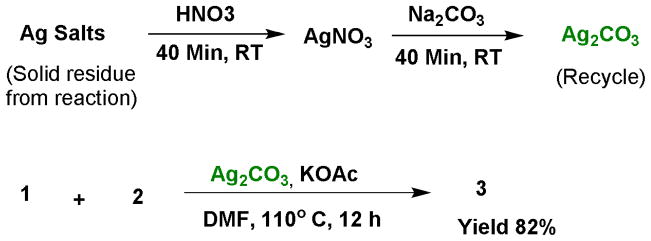
Regeneration Ag2CO3 from reaction mixture
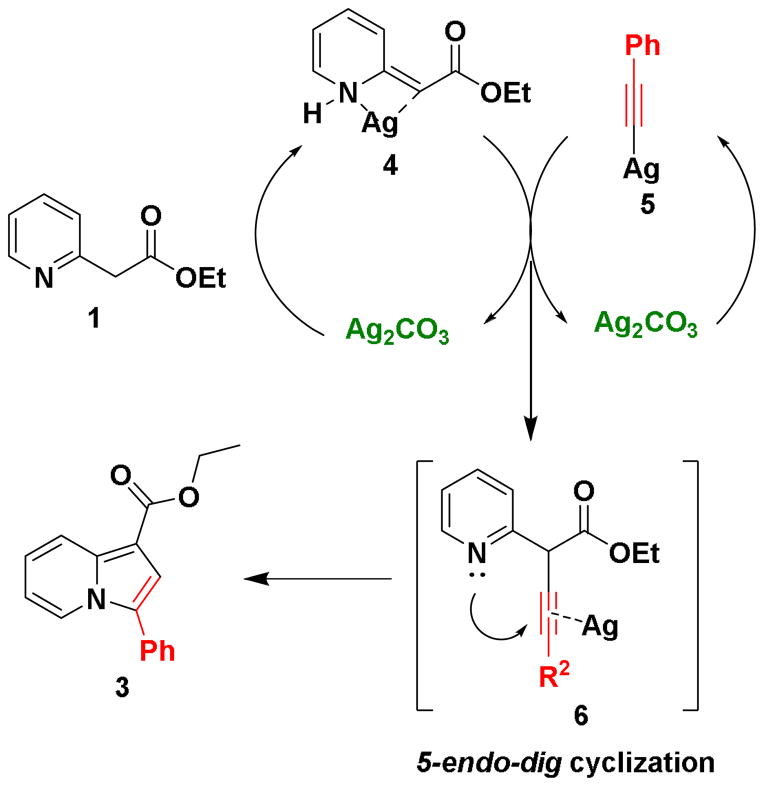
A plausible mechanism of this transformation is shown in scheme 4. One equivalent of Ag2CO3 activates ethyl 2-pyridylacetate by chelation to form 4.13 While a second equivalent of Ag2CO3 reacts with phenylacetylene to form silver phenylacetylide 2 5. Subsequent coupling of these two intermediates results in the formation of intermediate 6, which undergo 5-endo-dig cyclization to form the final product 3.14
Scheme 4.
Plausible reaction mechanism
In summary, we have successfully developed a facile and highly selective synthesis of indolizines via silver-mediated oxidative C-H functionalization and 5-endo-dig cyclization from readily available starting materials. This methodology provides a simple and direct way to access derivatives of the biologically important heterocycles. These reaction conditions display a wide range of functional group tolerance, including those that allow for further scaffold decoration. From an environmental point of view, this protocol represents good atom economy in that the expended Ag2CO3 can be recycled and reused to mediate this reaction. Molecular biology studies involving derivatives of this scaffold are currently underway in our laboratory.
Supplementary Material
Acknowledgments
This work was supported by research grants R01HL104516 and R01HL120659 from the National Institutes of Health, USA to DK Agrawal.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessary represent the official views of the NIH.
General experimental procedures, mass and NMR spectral data for compounds are provided in supporting information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Girard SA, Knauber T, Li CJ. Angew Chem, Int Ed. 2014;53:74. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]; (b) Yamaguchi J, Yamaguchi AD, Itami K. Angew Chem, Int Ed. 2012;51:8960. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; (c) Song G, Wang F, Li X. Chem Soc Rev. 2012;41:3651. doi: 10.1039/c2cs15281a. [DOI] [PubMed] [Google Scholar]; (d) Li BJ, Shi ZJ. Chem Soc Rev. 2012;41:5588. doi: 10.1039/c2cs35096c. [DOI] [PubMed] [Google Scholar]; (e) Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Angew Chem, Int Ed. 2012;51:10236. doi: 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]; (f) Yeung CS, Hsieh TH, Dong VM. Chem Sci. 2011;2:544. [Google Scholar]; (g) Cho SH, Kim JY, Kwak J, Chang S. Chem Soc Rev. 2011;40:5068. doi: 10.1039/c1cs15082k. [DOI] [PubMed] [Google Scholar]; (h) Bugaut X, Glorius F. Angew Chem, Int Ed. 2011;50:7479. doi: 10.1002/anie.201103106. [DOI] [PubMed] [Google Scholar]; (i) Rossi R, Bellina F, Lessi M. Synthesis. 2010:4131. [Google Scholar]
- 2.He C, Guo S, Ke J, Hao J, Xu H, Chen H, Lei A. J Am Chem Soc. 2012;134:5766. doi: 10.1021/ja301153k. [DOI] [PubMed] [Google Scholar]
- 3.Chen YR, Duan WL. J Am Chem Soc. 2013;135:16754. doi: 10.1021/ja407373g. [DOI] [PubMed] [Google Scholar]
- 4.Mi X, Wang C, Huang M, Zhang J, Wu Y, Wu Y. Org lett. 2014;16:3356. doi: 10.1021/ol5013839. [DOI] [PubMed] [Google Scholar]
- 5.Shen YM, Lv PC, Chen W, Liu PG, Zhang MZ, Zhu HL. Eur J Med Chem. 2010;45:3184. doi: 10.1016/j.ejmech.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 6.Hazra A, Mondal S, Maity A, Naskar S, Saha P, Paira R, Sahu KB, Paira P, Ghosh S, Sinha C. Eur J Med Chem. 2011;46:2132. doi: 10.1016/j.ejmech.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 7.Gundersen LL, Negussie AH, Rise F, Oestby OB. Arch der Pharm. 2003;336:191. doi: 10.1002/ardp.200390019. [DOI] [PubMed] [Google Scholar]
- 8.Chai W, Breitenbucher JG, Kwok A, Li X, Wong V, Carruthers NI, Lovenberg TW, Mazur C, Wilson SJ, Axe FU. Bioorg Med Chem lett. 2003;13:1767. doi: 10.1016/s0960-894x(03)00299-3. [DOI] [PubMed] [Google Scholar]
- 9.Weide T, Arve L, Prinz H, Waldmann H, Kessler H. Bioorg Med Chem lett. 2006;16:59. doi: 10.1016/j.bmcl.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B. Org Biomol Chem. 2012;10:7108. doi: 10.1039/c2ob25643f. [DOI] [PubMed] [Google Scholar]
- 11.Pandya AN, Agrawal DK. Tetrahedron lett. 2014;55:1835. doi: 10.1016/j.tetlet.2014.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang P, Ritter T. Tetrahedron Lett. 2011;67:4449. doi: 10.1016/j.tet.2011.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Li S-y, Pan Y-m, Wang H-s, Liang H, Chen Z-f, Qin X-h. Org lett. 2013;16:580. doi: 10.1021/ol4034513. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal T, Kumar S, Dhaked DK, Tiwari RK, Bharatam PV, Verma AK. J Org Chem. 2012;77:8562. doi: 10.1021/jo3015374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.