Abstract
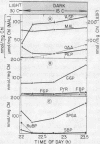
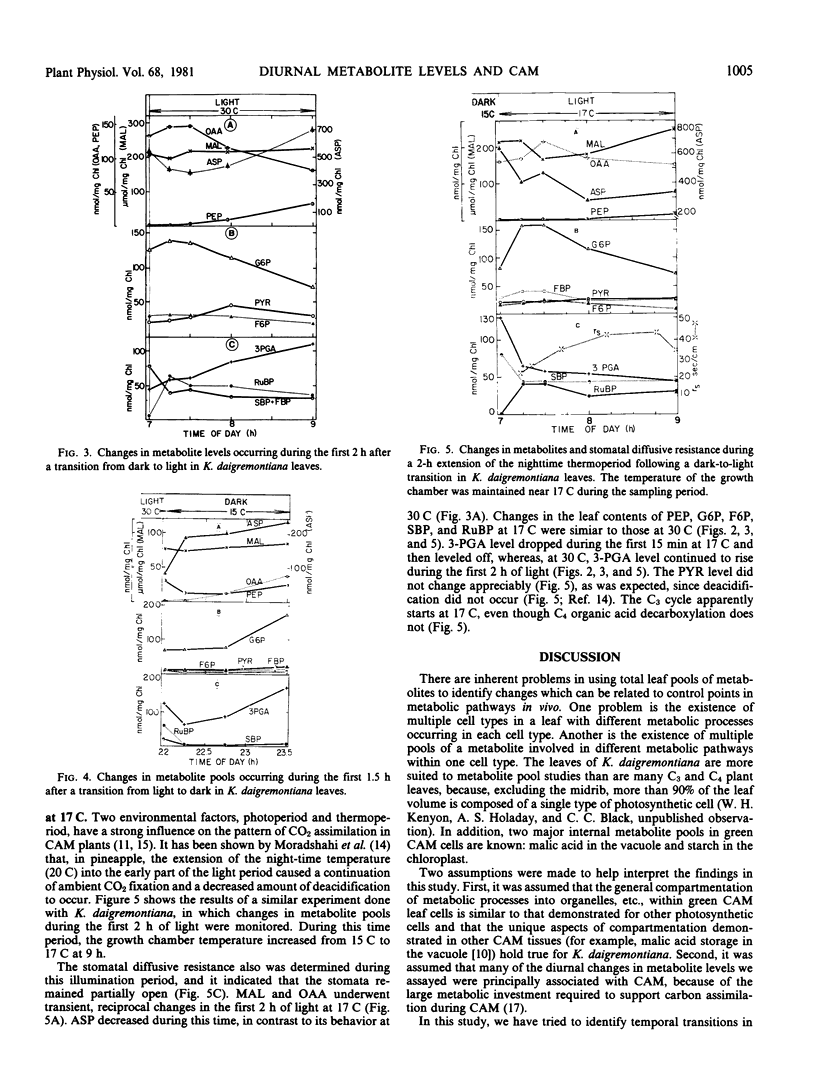
Diurnal changes in levels of selected metabolites associated with glycolysis, the C3 cycle, C4-organic acids, and storage carbohydrates were analyzed in active Kalanchoë daigremontiana Crassulacean acid metabolism leaves. Three metabolic transition periods occurred each day. During the first two hours of light, nearly all of the metabolite pools underwent transient changes. Beginning at daylight, stomata opened transiently and closed again within 30 minutes; malate synthesis continued for about 1 hour into the light; C3 photosynthesis began within 30 minutes; and net quantities of starch and glucan began to accumulate after 2 hours, continuing linearly throughout the rest of the day.
The second transition occurred in midafternoon: stomata reopened; malate decarboxylation nearly terminated; and the assimilation of ambient CO2 occurred primarily via the C3 cycle. The third transition occurred at dark: stomata transiently closed before opening again; the C3 cycle stopped; malate synthesis started in about 1 hour; starch and glucan degradation began within 1 hour; and the bulk of carbon flow was through glycolysis leading to the synthesis and accumulation of malate throughout the night. At night, the levels of metabolites involved in acidification and glycolysis (except for phosphoenolpyruvate) generally accumulated. Phosphoenolpyruvate levels peaked near midday and were minimal at night. The ribulose 1,5-bisphosphate pool was depleted at night, while sedoheptulose 1,7-bisphosphate, fructose 1,6-bisphosphate, glucose 6-phosphate, and fructose 6-phosphate accumulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., McAulay A. Changes in Metabolite Levels in Kalanchoë daigremontiana and the Regulation of Malic Acid Accumulation in Crassulacean Acid Metabolism. Plant Physiol. 1977 Mar;59(3):455–458. doi: 10.1104/pp.59.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W. Relationships between Stomatal Behavior and Internal Carbon Dioxide Concentration in Crassulacean Acid Metabolism Plants. Plant Physiol. 1979 Jun;63(6):1029–1032. doi: 10.1104/pp.63.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich P. Nicotinamide Adenine Dinucleotide-specific "Malic" Enzyme in Kalanchoë daigremontiana and Other Plants Exhibiting Crassulacean Acid Metabolism. Plant Physiol. 1976 Feb;57(2):310–314. doi: 10.1104/pp.57.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. Mechanism of C4 photosynthesis in Chloris gayana: pool sizes and kinetics of 14CO2 incorporation into 4-carbon and 3-carbon intermediates. Arch Biochem Biophys. 1979 Apr 15;194(1):117–127. doi: 10.1016/0003-9861(79)90601-5. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Measurement of the intermediates of the photosynthetic carbon reduction cycle, using enzymatic methods. Methods Enzymol. 1972;24:261–268. doi: 10.1016/0076-6879(72)24073-3. [DOI] [PubMed] [Google Scholar]
- Moradshahi A., Vines H. M., Black C. C. Carbon Dioxide Exchange and Acidity Levels in Detached Pineapple, Ananas comosus (L.), Merr., Leaves during the Day at Various Temperatures, Oxygen and Carbon Dioxide Concentrations. Plant Physiol. 1977 Feb;59(2):274–278. doi: 10.1104/pp.59.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucher G. W., Vickery H. B., Abrahams M. D., Leavenworth C. S. STUDIES IN THE METABOLISM OF CRASSULACEAN PLANTS: DIURNAL VARIATION OF ORGANIC ACIDS AND STARCH IN EXCISED LEAVES OF BRYOPHYLLUM CALYCINUM. Plant Physiol. 1949 Oct;24(4):610–620. doi: 10.1104/pp.24.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]