Abstract
As the central organ of stress and adaptation to stressors, the brain plays a pivotal role in behavioral and physiological responses that may lead to successful adaptation or to pathophysiology and mental and physical disease. In this context, resilience can be defined as “achieving a positive outcome in the face of adversity”. Underlying this deceptively simple statement are several questions; first, to what extent is this ability limited to those environments that have shaped the individual or can it be more flexible; second, when in the life course does the brain develop capacity for flexibility for adapting positively to new challenges; and third, can such flexibility be instated in individuals where early life experiences have limited that capacity? Brain architecture continues to show plasticity throughout adult life and studies of gene expression and epigenetic regulation reveal a dynamic and ever-changing brain. The goal is to recognize those biological changes that underlie flexible adaptability, and to recognize gene pathways, epigenetic factors and structural changes that indicate lack of resilience leading to negative outcomes, particularly when the individual is challenged by new circumstances. Early life experiences determine individual differences in such capabilities via epigenetic pathways and laying down of brain architecture that determine the later capacity for flexible adaptation or the lack thereof. Reactivation of such plasticity in individuals lacking such resilience is a new challenge for research and practical application. Finally, sex differences in the plasticity of the brain are often overlooked and must be more fully investigated.
Keywords: Hippocampus, Early life stress, Epigenetics, Gene expression, Allostasis, Sex difference
1. Introduction
The brain is the central organ of stress and adaptation to stressors because it perceives what is potentially threatening and determines the behavioral and physiological responses (McEwen, 1998, McEwen and Gianaros, 2011). Moreover, the brain is a target of stress and stressful experiences change its architecture, gene expression and function through internal neurobiological mechanisms in which circulating hormones play a role (Gray et al., 2013, McEwen, 2007). In healthy young adult animals, neuroanatomical changes in response to repeated stress are largely reversible (Conrad et al., 1999, Radley et al., 2005), or so it appears, based upon the restoration of dendritic length and branching and spine density. Yet there are underlying changes that can be seen at the level of gene expression and epigenetic regulation which indicate that the brain is continually changing (Gray et al., 2013, Hunter et al., 2013, McEwen, 2007, Nasca et al., 2013). Insofar as brain architecture and associated behavioral states are restored after stressful experiences in ways that appear to be healthy and functional, does this constitute “resilience”? This review examines this question in relation to new insights from the growing topic of epigenetics and gene expression by focusing on recent work on the hippocampus, amygdala and prefrontal cortex after acute and chronic stress and treatment with antidepressant agents.
2. Definitions of resilience
Resilience means to most people “achieving a positive outcome in the face of adversity”. This can involve “bending and not breaking,” that is, recovering from a bad experience. Or it can involve an “active resistance” to adversity through coping mechanisms that operate at the time of trauma (Karatsoreos and McEwen, 2011). But this adaptation does not, by itself, indicate flexibility in successful adaptation to new challenges over the life course. The individual traits that allow the more flexible outcomes undoubtedly depend upon a foundational capacity of that individual that is built upon experiences in the life course, particularly early in life, that promote the development of healthy brain architecture supporting cognitive flexibility that allows the brain to continue to change with ongoing experiences. A healthy brain architecture provides the basis for good self-esteem, and a locus of control for effective self-regulation, not only of behavior but also of the physiological responses to stressors that are regulated by the central and peripheral nervous systems. We shall now review how the brain and body adapt to challenges, often called “stressors”.
3. How do the brain and body adapt?
3.1. Allostasis, allostatic load and health-related behaviors
The active process of responding to challenges to, and adaptive changes by, an individual is called “allostasis”. This involves multiple mediators (autonomic, cortisol, immune/inflammatory, metabolic, neuromodulators within the brain) that interact non-linearly with each other and promote adaptation in the short run as long as they are turned on efficiently when needed and turned off promptly when no longer needed. Over-use (too much stress) or dysregulation among the mediators (e.g., too much or little cortisol; too much or little inflammatory cytokines) results in cumulative change that is referred to as “allostatic load and overload” (McEwen, 1998).
As the key organ of stress and adaptation, the brain directs “health-related behaviors” (caloric intake, alcohol, smoking, sleep, exercise) that contribute to or ameliorate physiological dysregulation and thereby play a key role in exacerbating or counteracting allostatic load/overload (McEwen, 2007). Brain development and healthy or unhealthy neural function determines in part whether the response to challenges or “stressors” is efficient or dysregulated. The development of self esteem and locus of control and good self regulatory behaviors are key factors that determine whether a challenge, such as going to a new place or giving a speech, will result in “positive stress”, with a satisfying outcome, or have negative consequences. Tolerable stress refers to experiencing stressful major life events that will result in successful coping with minimal allostatic load due to good internal resources and external support. Toxic stress refers to the situation where there is unsuccessful coping due to lack of adequate internal capacities as well as poor external support that may also be based upon inadequate neural architecture to handle the stressors, and “allostatic overload” applies to those toxic stress situations where physiological dysregulation is likely to accelerate development of disease (McEwen and Wingfield, 2003).
3.2. Brain architecture responds to stressors
In the healthy brain, structural remodeling occurs after both acute and chronic stress. The discovery of receptors for glucocorticoids in the hippocampus has led to many investigations in animal models and translation to the human brain using modern imaging methods. The most striking findings from animal models have identified structural plasticity in the hippocampus, consisting of ongoing neurogenesis in the dentate gyrus (Cameron and Gould, 1996) and remodeling of dendrites and synapses in the major neurons of Ammon's horn (McEwen, 1999). Indeed, neurogenesis in the adult mammalian brain was initially described (Altman and Das, 1965, Kaplan and Bell, 1983) and then suppressed (Kaplan, 2001), only to be rediscovered in the dentate gyrus of the hippocampus (Cameron and Gould, 1994, Gould and McEwen, 1993) in the context of studies of neuron cell death and actions of adrenal steroids and excitatory amino acids in relation to stress. This was further developed to call attention to the generality of neurogenesis across vertebrates (Alvarez-Buylla and Lois, 1995), with recent evidence making it clear that the human hippocampus shows significant neurogenesis in adult life (Spalding et al., 2013). See also Box 1.
Box 1. Relevance to neural architecture of the human brain.
Studies of the human hippocampus have demonstrated shrinkage of the hippocampus not only in mild cognitive impairment and Alzheimer's disease (de Leon et al., 1997), but also in Type 2 diabetes (Gold et al., 2007), prolonged major depression (Sheline, 2003), Cushing's disease (Starkman et al., 1999) and post-traumatic stress disorder (PTSD) (Gurvits et al., 1996). Moreover, in non-disease conditions, such as chronic stress (Gianaros et al., 2007b), chronic inflammation (Marsland et al., 2008), lack of physical activity (Erickson et al., 2009) and jet lag (Cho, 2001), smaller hippocampal or temporal lobe volumes have been reported.
So far there is no indication as to whether these changes are due to volume reduction in dentate gyrus due to inhibited neuronal replacement or to dendritic shrinkage or glial cell loss, or a combination of all three. Autopsy studies on depression-suicide have indicated loss of glial cells and smaller neuron soma size (Stockmeier et al., 2004), which is indicative of a smaller dendritic tree.
With regard to Type 2 diabetes, it should be emphasized that the hippocampus has receptors for, and the ability to take up and respond to insulin, ghrelin, insulin-like growth factor-1 (IGF1) and leptin; and that IGF-1 mediates exercise-induced neurogenesis (McEwen, 2007). Thus, besides its response to glucocorticoids, the hippocampus is an important target of metabolic hormones that have a variety of adaptive actions in the healthy brain which is perturbed in metabolic disorders, such as diabetes (McEwen, 2007).
The mediators of brain structural plasticity include excitatory amino acids and glucocorticoids, along with a growing list of other mediators such as oxytocin, corticotrophin releasing factor, brain derived neurotrophic factor (BDNF), lipocalin-2 and tissue plasminogen activator (tPA) (McEwen, 2010). Moreover, glucocorticoid actions involve both genomic and non-genomic mechanisms that implicate mineralocorticoid, as well as glucocorticoid receptors and their translocation to mitochondria as well as cell nuclei; and, an as-yet unidentified G-protein coupled membrane receptor related to endocannabinoid production (Du et al., 2009, Hill and McEwen, 2010, Popoli et al., 2012).
The implications of stress and glucocorticoid effects in the hippocampus have led to exploration of other brain regions involved in cognition, mood and behavioral self-regulation. The amygdala shows quite different responses to acute and chronic stress compared to the hippocampus. The amygdala responds to glucocorticoids in the formation of emotionally-charged memories (Roozendaal et al., 2004), and acute stress causes a delayed formation of dendritic spines in basolateral amygdala neurons and an increase of anxiety after 10 days (Mitra et al., 2005). Chronic stress of the same type that impairs dentate gyrus neurogenesis and cause dendritic shrinkage and spine loss in Ammon's horn neurons, causes expansion of dendrites in the basolateral amygdala (Vyas et al., 2002) while causing spine down-regulation in the medial amygdala (Bennur et al., 2007). The latter is dependent on tissue plasminogen activator (tPA) while the former does not (Bennur et al., 2007). See Box 2.
Box 2. Importance of the human amygdala in responding to stressors.
Translating to the human brain, amygdala hyperactivity is reported in major depression (Sheline et al., 2001), as well as in anxiety disorders (Drevets, 2000) and enlargement of the amygdala has been reported in acute depression (Frodl et al., 2003). With respect to PTSD, a novel approach after acute trauma is the administration of glucocorticoids, based on the counter-intuitive findings that low normal glucocorticoid levels at the time of trauma predispose towards develop of PTSD symptoms (Rao et al., 2012, Zohar et al., 2011).
Increased amygdala reactivity to angry and sad faces is reported in individuals with early signs of cardiovascular disease (Gianaros et al., 2009), suggesting that the increased sympathetic activity and blood pressure reactivity may be a cause of allostatic load resulting from increased reactivity to daily experiences over time. Increased amygdala reactivity to faces has also been reported in individuals traumatized by 9/11 (Ganzel et al., 2008), as well as after sleep deprivation (Yoo et al., 2007).
The prefrontal cortex (PFC) is another, now well-studied, target of chronic stress (McEwen and Morrison, 2013). In the same chronic stress models that lead to amygdala neuronal hypertrophy and shrinkage of dendrites in hippocampus, there is shrinkage of dendrites and loss of spines throughout the medial prefrontal cortex while dendrites expand in the orbitofrontal cortex (OFC) (Liston et al., 2006). Because the OFC is involved in determining the saliency of reward or punishment (Schoenbaum and Roesch, 2005), this may reinforce the changes in the basolateral amygdala. For the medial prefrontal cortex, stress-induced impairment has been linked to poor cognitive flexibility in both animal and human studies (Dias-Ferreira et al., 2009, Liston et al., 2009, Liston et al., 2006). Moreover, circadian disruption impairs cognitive flexibility and causes shrinkage of medial prefrontal cortical dendrites (Karatsoreos et al., 2011). The mechanism for medial PFC dendritic remodeling is likely to involve the same mechanisms as those in the hippocampus, namely, excitatory amino acids and glucocorticoids (Cerqueira et al., 2005, Martin and Wellman, 2011).
The structural changes are largely reversible in healthy young animals after the termination of stress. See Box 3. When the stress is over, remodeled brain circuits recover at least in younger animals with healthy brain architecture (Bloss et al., 2010, Radley et al., 2005), but there are clues that the recovered state is not the same as the initial state. For example, in the studies of recovery from chronic stress in the medial prefrontal cortex of young adult rats, the retraction of apical dendrites during chronic stress was from distal dendrites and the re-growth of those dendrites during recovery was from the more proximal dendrites (Fig. 1) (Goldwater et al., 2009). Yet there was reversal of deficits in D1 receptor expression and recovered function in terms of dopamine enhanced LTP during recovery from chronic stress, and it is not yet clear if the differences in dendritic retraction and regrowth reflect any reorganization of neuroanatomical circuitry (Goldwater et al., 2009). This apparent reversibility hides the fact that genomic responses to stressors are dependent on the stress-history of the individual, as will be elaborated below. Moreover, there is clearly loss of reversibility in aging (Bloss et al., 2010) and also a failure to show plasticity in response to stress as a result of maternal separation stress in infancy (Eiland and McEwen, 2012) and haploinsufficiency (Magarinos et al., 2011) or overexpression (Govindarajan et al., 2006) of brain derived neurotrophic factor (BDNF).
Box 3. Many factors affect plasticity and resilience of the amygdala, hippocampus and PFC.
The young adult human prefrontal cortex reflects the effects of chronic stress by showing impaired cognitive flexibility and reduced functional connectivity that parallels the effects of stress in the young adult rat brain, including the reversibility after the end of the stressful period (Bloss et al., 2010, Liston et al., 2009, Liston et al., 2006, Radley et al., 2005). The studies of circadian disruption complement those on the hippocampus/temporal lobe noted above in flight crews suffering from chronic jet lag (Cho, 2001) and raise important questions about how the brain handles shift work, jet lag and chronic sleep deprivation. Furthermore, aging in rats is associated with failure to spontaneously reverse shrinking of medial prefrontal cortical neurons after chronic stress (Bloss et al., 2010) and this harkens back to the glucocorticoid cascade hypothesis (Sapolsky et al., 1986).
Indeed, when brain circuits remain changed there are behavioral states and cognitive impairment that also remain and some of these may be maladaptive. Amygdala over-activity is a consequence of exposure to traumatic stressors in a PTSD-like animal model that produces a delayed increase in spine density in basolateral amygdala along with a delayed increase in anxiety-like behavior (Rao et al., 2012). Amygdala overactivity is also associated with mood disorders (Drevets and Raichle, 1992) and amygdala enlargement is reported in children of chronically depressed mothers (Lupien et al., 2011). Hippocampal volume reduction in prolonged depression, Type 2 diabetes and Cushing's disease is associated with cognitive and mood impairment (Convit et al., 2003, Gold et al., 2007, Sheline, 2003, Starkman et al., 1992). These conditions require external intervention that may include use of antidepressants (Vermetten et al., 2003), surgery to reduce hypercortisolemia (Starkman et al., 1999), regular physical activity (Erickson et al., 2011) and mindfulness-based stress reduction (Holzel et al., 2010).
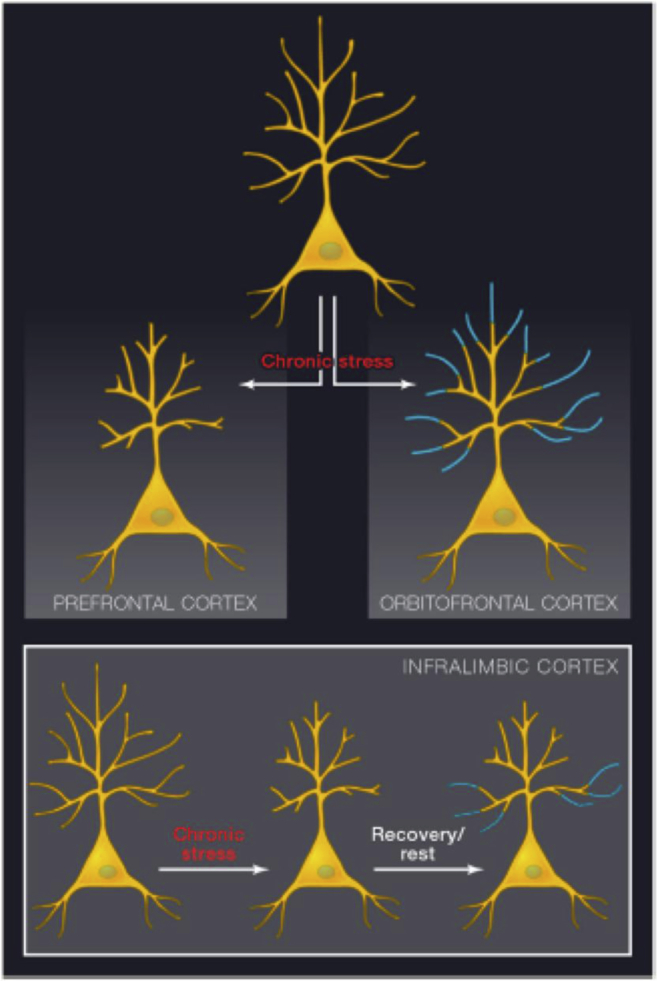
Fig. 1.
Schematic diagrams depicting dendritic shrinkage and expansion in response to chronic stress and recovery. Top: chronic stress leads to dendritic shrinkage in layer 3 pyramidal neurons in the prelimbic and anterior cingulate cortex, whereas it causes dendritic expansion in the corresponding neurons within orbitofrontal cortex. Both effects are seen primarily in the distal apical dendritic tree. Bottom: while shrinkage and recovery both affect distal dendrites in neurons depicted in the top panel, layer 5 neurons in infralimbic cortex lose distal dendritic branches in response to stress, yet recovery occurs primarily in proximal dendrites, shifting the dendritic architecture (see (Goldwater et al., 2009)). Reprinted from (McEwen and Morrison, 2013) by permission.
4. Sex differences and what this means for the rest of the brain
All of the animal model studies of stress effects summarized above and below were carried out on male rodents. Thus, it is very important to note before proceeding further by discussing sex differences in how the brain responds to stressors. Indeed, female rodents do not show the same pattern of neural remodeling after chronic stress as do males. The first realization of this was for the hippocampus, in which the remodeling of CA3 dendrites did not occur in females after CRS, even though all the measures of stress hormones indicated that the females were experiencing the stress as much as males (Galea et al., 1997). Females and males also differ in the cognitive consequences of repeated stress, with males showing impairment of hippocampal dependent memory, whereas females do not (Bowman et al., 2001, Luine et al., 1994, Luine et al., 2007).
In contrast, acute tail shock stress during classical eyeblink conditioning improves performance in males, but suppresses it in females (Wood and Shors, 1998) by mechanisms influenced by gonadal hormones in development and in adult life (Shors and Miesegaes, 2002, Wood et al., 2001). However, giving male and female rats control over the shock abolishes both the stress effects and the sex differences (Leuner et al., 2004). These findings suggest that sex differences involve brain systems that mediate how males and females interpret stressful stimuli and that a sense of control is paramount to coping with those stimuli.
More recently, in collaboration with John Morrison, Becca Shansky showed that female rats fail to show the mPFC dendritic remodeling seen in males after CRS in those neurons that do not project to amygdala. Instead, they show an expansion of the dendritic tree in the subset of neurons that project to the basolateral amygala (Shansky et al., 2010). Moreover, ovariectomy prevented these CRS effects on dendritic length and branching. Furthermore, estradiol treatment of OVX females increased spine density in mPFC neurons, irrespective of where they were projecting (Shansky et al., 2010).
Taken together with the fact that estrogen, as well as androgen, effects are widespread in the central nervous system, these findings indicate that there are likely to be many more examples of sex × stress interactions related to many brain regions and multiple functions, as well as developmentally programmed sex differences that affect how the brain responds to stress, e.g., in the locus ceruleus (Bangasser et al., 2010, Bangasser et al., 2011). Clearly, the impact of sex and sex differences has undergone a revolution and much more is to come (Cahill, 2006, Laje et al., 2007, McEwen, 2009, McEwen and Lasley, 2005, Meites, 1992), including insights into X and Y chromosome contributions to brain sex differences (Carruth et al., 2002). In men and women, neural activation patterns to the same tasks are quite different between the sexes even when performance is similar (Derntl et al., 2010). This leads to the concept that men and women often use different strategies to approach and deal with issues in their daily lives, in part because of the subtle differences in brain architecture. Nevertheless, from the standpoint of gene expression and epigenetic effects, the principles of what we have learned in animal models regarding plasticity, damage and resilience are likely to apply to both males and females.
5. Lessons from gene expression
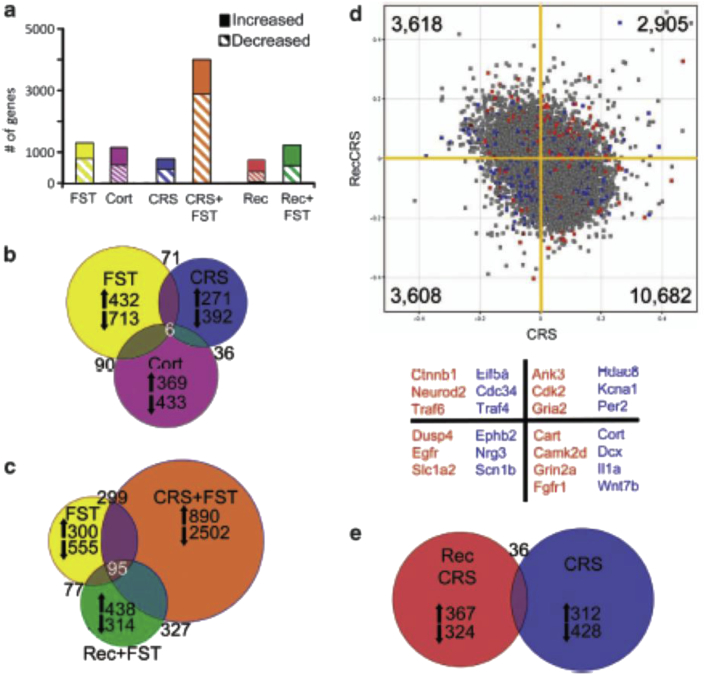
We have noted that resilience means to most people achieving a positive outcome in the face of adversity. Even when the healthy brain and associated behavior appears to have recovered from a stressful challenge, studies of gene expression have revealed that the brain is not the same, just as the morphology after recovery appears to be somewhat different from what it was before stress (Goldwater et al., 2009). See Fig. 1. Transcriptional profiling of the mouse hippocampus has revealed that after a recovery period from chronic stress, which is equivalent to the duration of the stressor (21d) and is sufficient to restore anxiety-like behaviors to pre-stress baselines, the expression levels of numerous genes remained distinct from the stress naïve controls (Gray et al., 2013). See Fig. 2. Further, exposure to a novel swim stress 24 h after chronic stress or after a 21d recovery period from the chronic stress, produced distinct gene expression profiles from mice that experienced a swim stress but had no history of chronic stress. Together, these findings suggest that gene expression patterns after recovery from stress do not reflect a return to the stress naïve baseline (even when the behaviors have recovered) and chronic stress alters reactivity to future stressors. Studies examining longer recovery periods, as well as how intermittent stress during recovery might alter gene expression will be necessary to answer whether these seemingly lasting changes might eventually reverse or if additional stressors can compound certain changes. These changes in transcriptome reactivity represent one molecular signature for resilience that are themselves likely to be driven by epigenetic changes discussed in the next section.
Fig. 2.
Gene expression changes in hippocampus in response to stress depend on the prior stress history of the subject. (A) Solid bars represent the number of significantly increased genes and hatched bars represent significantly decreased genes identified by microarray comparisons of each stress group with age-matched controls (t-test, P < 0.05, n = 4 mice per group) (yellow = naive forced swim test (FST), purple = Corticosterone (Cort) vs vehicle injected, blue = chronic restraint stress (CRS), orange = CRS + FST, red = recovery from CRS, green = recovery from CRS + FST). (B) Proportional Venn diagram illustrating the genes significantly altered by both the acute stress (FST = yellow), chronic stress (CRS = blue), and Cort injection (Cort = purple) conditions. The numbers of genes unique to each comparison that were increased or decreased are listed next to arrows indicating the direction of change. (C) Venn diagram of genes altered by each FST condition reveals a core of 95 genes that were always changed by this stressor. The large number of unique gene expression changes in each condition shows that the response to FST is altered by the stress history of the group (yellow = naive, orange = CRS + FST, green = Recovery from CRS + FST), with the vast majority of changes occurring when the animal is exposed to a novel stressor immediately after a chronic stress exposure (orange circle). (D) Scatter plot of normalized expression values for each microarray probe comparing CRS (x axis) with recovery from CRS (y axis). The majority of genes are increased by CRS, but decreased after recovery (10 682, lower right quadrant); however, there are a number of probes that are increased by CRS that remain elevated after recovery (2905, top right quadrant) or are suppressed by CRS and remain low in recovery (3608 probes, lower left quadrant). Highlighted probes are those that reached significance when compared with age-matched controls (blue = CRS, red = recovery from CRS, gray = not significant). Several examples of the highlighted genes are listed below the scatter plot by color designation and quadrant. For example, blue points in the lower left quadrant, such as Nrg3 and Scn1b, represent genes that are significantly changed by CRS when compared with unstressed controls and are also decreased after recovery from CRS. Whereas red points in the upper right quadrant, such as Cdk2 and Gria2, are genes that remained significantly different from controls after recovery from CRS, and were also increased immediately following CRS. (E) Venn diagram illustrating that the number of genes significantly different from controls after recovery from CRS (red) are mostly unique from those significantly altered by CRS (blue). Reprinted from (Gray et al., 2013) by permission. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Importantly, recent evidence has suggested that the in vivo transcriptional changes in response to stress represent a synthesis of multiple cellular pathways, not simply CORT activation of GR-dependent transcription. Chronic stress increases inflammatory tone and this release of cytokines can activate other signaling pathways, such as NF-kB-dependent transcription. Microarray studies have found that glucocorticoid injections produce distinct gene expression profiles from naïve acute stress (Fig. 2B) and that the gene expression response to a glucocorticoid injection changes after exposure to chronic stress (Datson et al., 2013; (Gray et al., 2013). In support of these findings, in vitro studies have demonstrated that simultaneous activation of GR and NF-kB-dependent transcription results in a unique pattern of gene expression that is distinct from the predicted sum of either pathway activated alone (Rao et al., 2011). These findings illustrate that gene expression changes in response to stress are not solely the product of glucocorticoid activity. Increasingly, research into stress resilience is looking beyond GR-dependent transcription in order to capture the complexity of the cellular response to stress.
6. Lessons from epigenetic influences
Functional insights into the ever-changing brain come from studies of epigenetic regulation. The term “epigenetics” now extends beyond its original definition (Waddington, 1942) to include the continuous, seamless interaction between genes and the factors which regulate gene expression over the life course. The core of the genomic response to those environmental factors such as hormones, cytokines and chemokines and other neuromodulators involves modification of histones (Maze et al., 2013), methylation of cytosine residues on DNA, non-coding RNA's that modify expression of mRNA molecules, and retrotransposon DNA elements (Mehler, 2008).
In our studies of stress neurobiology, acute restraint stress was shown to increase expression in the dentate gyrus of a repressive histone mark, H3K9me3, and this was accompanied by the repression of certain retrotransposon elements of as yet unidentified function; this repressive response habituates with repeated stress raising the possibility of increased genomic instability. Such instability may manifest itself in terms of genomic activity that is no longer responsive to environmental influences or lead to genomic activity that is increased as a result of chronic stress, as in accelerated aging (Hunter et al., 2013, Hunter et al., 2012). Loss of reversal of stress induced structural plasticity, as seen in aging rats (Bloss et al., 2010) is one example; and increased expression of inflammatory mediators together with loss of cholinergic and dopaminergic function (Bloss et al., 2008) is another.
In contrast, there are examples of epigenetic activation of neural activity. Indeed, acute swim stress as well as novelty exposure induce an activational histone mark in dentate gyrus, namely, acetylation of lysine residue 14 and phosphorylation of the serine residue on histone H3, which is dependent on both GR and NMDA activation and is associated with c-fos induction among other genes (Reul and Chandramohan, 2007). Acetylation of another lysine residue, K27 on histone H3, is associated with increased expression of metabotropic glutamate receptor, mGlu2, in hippocampus of Flinders Sensitive Line (FSL) rats as shown by chromatin immunoprecipitation (Nasca et al., 2013). mGlu2 is known to exert an inhibitory tone on glutamate release from synapses. The acetylating agent l-acetylcarnitine (LAC), a naturally occurring substance, behaves as an antidepressant, at least in part by the epigenetic up-regulation of mGlu2 receptors via this epigenetic mechanism. LAC caused a rapid and long-lasting antidepressant effect in both FSL rats and in mice exposed to chronic unpredictable stress, which, respectively, model genetic and environmentally induced depression. Beyond the epigenetic action on the acetylated H3K27 bound to the Grm2 promoter, LAC also increased acetylation of NF-ĸB-p65 subunit, thereby enhancing the transcription of Grm2 gene encoding for the mGlu2 receptor in hippocampus and prefrontal cortex. The involvement of NF-ĸB in LAC antidepressant-like effects supports a growing literature that shows depression may be associated with a chronic inflammatory response (Dantzer et al., 2008). Importantly, LAC reduced the immobility time in the forced swim test and increased sucrose preference as early as 3 d of treatment, whereas 14 d of treatment were needed for the antidepressant effect of chlorimipramine (Nasca et al., 2013). This suggests LAC is important for stress resilience.
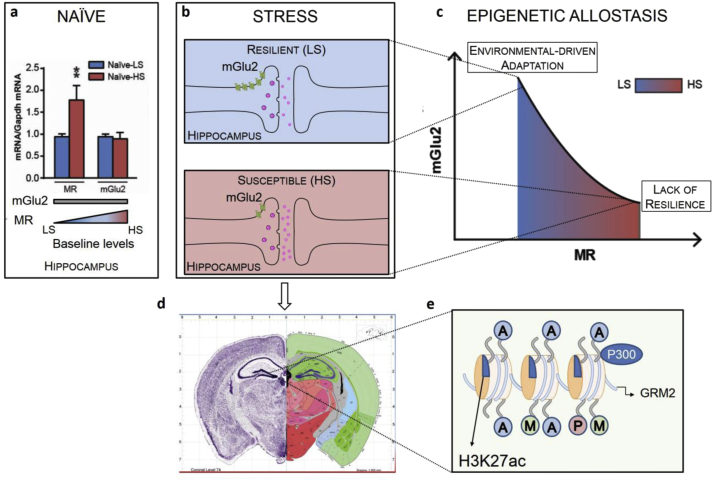
A recent study from our laboratory has shown that hippocampal expression of mGlu2, is also a marker of individual susceptibility to mood disorders. Interestingly, mGlu2 is the same receptor regulating inhibitory glutamate tone that has been shown to be elevated by treatment with LAC in FSL rats to reverse depressive-like behavior (Nasca et al., 2013). Using a novel and acute approach for rapidly screening an inbred population of laboratory animals, it has been shown that both chronic unpredictable stress and acute restraint stress results in individual behavioral and molecular differences in wild-type mice that are more (HS) or less (LS) susceptible to stress-induced mood abnormalities. At the molecular level, HS and LS mice differ in the ability of stress to induce a decrease of mGlu2 receptor expression in hippocampus. Mapping the steps of this intricate dance that allow some individuals to face adverse life experience, the HS subset of mice was associated with higher baseline levels of MR genes than the LS subset, showing an MR-dependent down-regulation of mGlu2 receptors in hippocampus. These findings led to the introduction of the epigenetic allostasis model, which incorporates an epigenetic core into the allostasis–allostatic load model of stress and adaptation to emphasize the gene–environment interactions. In particular, the epigenetic allostasis model suggests that a non-shared experience early in life may epigenetically set each individual, via expression of MR genes, to a somewhat different trajectory of development as far as responses to subsequent stressful life experiences (Nasca et al., September 2014). In agreement, juvenile stress was associated with increased hippocampal MR mRNA levels and anxiety-like behavior in adulthood (Brydges et al., 2014). See Fig. 3.
Fig. 3.
Central role of hippocampal mineralocorticoid (MR) receptor in the Epigenetic Allostasis model and MR-driven down-regulation of glutamatergic tone in response to stress. (a) Based on the different susceptibility to stress assessed by a novel and acute screening method, naïve c57bl/6N mice show different MR mRNA transcript levels and no difference in mGlu2 mRNA transcripts, suggesting an effect of earlier “non-shared” experiences (e.g. differential exposure to maternal care). (b) The two subpopulations designated as LS and HS show different responses to stress. The susceptible subpopulation is characterized by a MR activation that mediates an inhibition of mGlu2 transcription, leading to an increased transcription of mGlu2 receptors and, consequently, to glutamate overflow. (c) The epigenetic allostasis model points out the MR-driven regulation of mGlu2 expression: MR and mGlu2 experimental data fit a quadratic MR-dependent curve that show how epigenetic changes induced by early life experiences bias each subject to responses to future stressors that may be adaptive under some circumstances (e.g., anxiety and vigilance in a dangerous environment with higher MR) or maladaptive (proneness to develop anxiety or depressive disorders with higher MR) under others. (d) The most striking findings from animal models are identifying epigenetic mechanisms in the hippocampus. Image credit: Allen Institute for Brain Science (e) Chromatin immunoprecipitation assay show an epigenetic regulation of mGlu2 promoter gene through the acetylation of the histone H3K27. This functional insight in the control of glutamate tone paved the possibility of intervention with acetylating agents, such as acetyl-L-carnitine (LAC), which may play a role in resilience. (a) and (c) re-adapted from (Nasca et al., September 2014) by permission.
7. The life course and the epigenetics of individual differences
The individual traits that allow these adaptive or maladaptive outcomes depend upon the unique neurological capacity of each individual, which is built upon experiences in the life course, particularly those early in life. These influences can result in healthy or unhealthy brain architecture and in epigenetic regulation that either promotes or fails to promote gene expression responses to new challenges. Genetically similar or identical individuals differ in many ways ranging from length of dendrites in the prefrontal cortex (Miller et al., 2012) to differences in MR levels in hippocampus (Nasca et al., September 2014), locomotor activity and neurogenesis rates (Freund et al., 2013) and the influences that lead to those differences begin early in life. For example, identical twins diverge over the life course in patterns of CpG methylation of their DNA reflecting the influence of “non-shared” experiences (Fraga et al., 2005).
Early life events related to maternal care in animals, as well as parental care in humans, play a powerful role in later mental and physical health, as demonstrated by the adverse childhood experiences (ACE) studies (Felitti et al., 1998) and recent work that will be noted below. See Box 4. Animal models have contributed enormously to our understanding of how the brain and body are affected, starting with the “neonatal handling” studies of Levine and Denenberg (Levine et al., 1967) and the recent, elegant work of Meaney, Syzf and colleagues involving methylation of CpG residues in DNA (Meaney and Szyf, 2005). Such epigenetic, transgenerational effects transmitted by maternal care are central to these findings. Besides the amount of maternal care, the consistency over time of that care and the exposure to novelty are also very important not only in rodents (Akers et al., 2008, Tang et al., 2006), but also in monkey models (Parker et al., 2006). Prenatal stress impairs hippocampal development in rats, as does stress in adolescence (Isgor et al., 2004). Insufficient maternal care in rodents (e.g., (Rice et al., 2008)) and the surprising attachment shown by infant rats to their less-attentive mothers appears to involve an immature amygdala (Moriceau and Sullivan, 2006), activation of which by glucocorticoids causes an aversive conditioning response to emerge. Maternal anxiety in the variable foraging demand (VFD) model in rhesus monkeys leads to chronic anxiety in the offspring, as well as signs of metabolic syndrome (Coplan et al., 2001, Kaufman et al., 2005).
Box 4. When things go wrong early in life.
In studies of adverse childhood experiences (ACE) in human populations (Felitti et al., 1998), there are reports of increased inflammatory tone, not only in children, but also in young adults related to early life abuse, that includes chronic harsh language, as well as physical and sexual abuse (Danese et al., 2009, Miller and Chen, 2010). It should be noted that the ACE study was carried out in a middle class population (Anda et al., 2010), indicating that poverty and low socioeconomic status (SES) are not the only source of early life stressors.
Nevertheless, low SES does increase the likelihood of stressors in the home and neighborhood, including also toxic chemical agents such as lead and air pollution (McEwen and Tucker, 2011), and chaos in the home is associated with development of poor self-regulatory behaviors, as well as obesity (Evans et al., 2005). Moreover, low SES children are found to be more likely to be deficient in language skills, as well as self-regulatory behaviors and also in certain types of memory that are likely to be reflections of impaired development of parasylvian gyrus language centers, prefrontal cortical systems and temporal lobe memory systems (Farah et al., 2006, Hart and Risley, 1995). Low SES is reported to correlate with smaller hippocampal volumes (Hanson et al., 2011), and lower subjective SES, an important index of objective SES, is associated with reduction in prefrontal cortical gray matter (Gianaros et al., 2007a). Moreover, having grown up in lower SES environment is accompanied by greater amygdala reactivity to angry and sad faces (Gianaros et al., 2008), which, as noted above, may be a predisposing factor for early cardiovascular disease that is known to be more prevalent at lower SES levels (Adler et al., 1993). Finally, depression is often associated with low SES, and children of depressed mothers, followed longitudinally, have shown increased amygdala volume while hippocampal volume was not affected (Lupien et al., 2011).
On the positive side, there are the “reactive alleles.” Genes that in nurturing environments facilitate beneficial outcomes when compared to less reactive alleles, even though those same alleles can enhance adverse outcomes in a stressful early life environment (Boyce and Ellis, 2005, Caspi et al., 2003, Obradovic et al., 2010, Suomi, 2006). Regarding adverse outcomes and good and bad ”environments”, it must be recognized that allostatic processes are adjusted via epigenetic influences to optimize the individuals adaptation to, and resulting fitness for, a particular environment, whether more or less threatening or nurturing (Del Giudice et al., 2011). Yet, there are “trade-offs” in terms of physical and mental health that, on the one hand, may increase the likelihood of passing on one's genes by improving coping with adversity and enhancing mental health and overall reproductive success, but, on the other hand, may impair later health, e.g., by eating of “comfort foods” (see for example (Jackson et al., 2010)).
8. Can we reverse adverse changes?
What can be done to remediate the effects of chronic stress, as well the biological embedding associated with early life adversity? Epigenetics in its original meaning (Waddington, 1942) refers to the emergence at each stage of development of features of the organism not present before or even predictable from the prior state through cellular differentiation. As discussed above, genetic factors interact seamlessly with environmental influences not only during development but also in adult life, leading to the newer meaning of “epigenetics”. Thus at each stage of development there is no “going back” and a new set of possibilities emerges that offer opportunities for epigenetic influences. Interventions will not, therefore, “reverse” developmental events but rather produce compensatory mechanisms (Caldji et al., 1998). Indeed, development never ends and adolescents, young adults, mature and aging individuals continue to show the results of experiences, including opportunities for redirection of unhealthy tendencies through a variety of interventions. One of the most interesting interventions in animal models is the use of an “enriched environment” to reverse effects of early life maternal separation on HPA and behavioral responses (Francis et al., 2002), indicating the potential power in humans of psychosocial interventions after the early life trauma.
Interventions to foster compensatory mechanisms may involve pharmaceutical, as well as behavioral, or “top-down” interventions (i.e., interventions that involve integrated CNS activity). These include cognitive-behavioral therapy, physical activity and programs that promote social support, social integration, and developing meaning and purpose in life (Ganzel and Morris, 2011, McEwen and Gianaros, 2011). More targeted interventions for emotional and cognitive dysfunction may arise from fundamental studies of such developmental processes as the reversal of amblyopia and other conditions by “releasing the brakes” that retard structural and functional plasticity (Vetencourt et al., 2008). It should be noted that many of these interventions that are intended to promote plasticity and slow decline with age, such as physical activity and positive social interactions that give meaning and purpose, are also useful for promoting “positive health” and “eudaimonia” (Ryff and Singer, 1998, Singer et al., 2005) independently of any notable disorder and within the range of normal behavior and physiology (Ryff, 2014).
Moreover, interventions directed towards changing physiology and brain function may be useful when adaptation to a particular environment has resulted in an individual who then chooses, or is forced to adapt to a different, e.g. more or less threatening or nurturing, environment. A powerful “top down” therapy (i.e., an activity, usually voluntary, involving activation of integrated nervous system activity, as opposed to pharmacologic therapy which has a more limited target) is regular physical activity, which has actions that improve prefrontal and parietal cortex blood flow and enhance executive function (Colcombe et al., 2004). Moreover, regular physical activity, consisting of walking an hour a day, 5 out of 7 days a week, increases hippocampal volume in previously sedentary adults (Erickson et al., 2011). This finding complements work showing that fit individuals have larger hippocampal volumes than sedentary adults of the same age-range (Erickson et al., 2009). It is also well known that regular physical activity is an effective antidepressant and protects against cardiovascular disease, diabetes and dementia (Babyak et al., 2000, Snyder et al., 2010). Moreover, intensive learning has also been shown to increase volume of the human hippocampus (Draganski et al., 2006). Furthermore, the evidence that the novel antidepressant candidate, LAC, exerts fast antidepressant-like effects in a genetic animal model where a LAC deficiency was found in the hippocampus and prefrontal cortex, prompts investigation of how lifestyle as well as diet, vitamin intake or depletion, oxidative stress and the aging process will determine epigenetic states in ways yet unidentified (Denu, 2007, Nasca et al., 2013).
Social integration, social support and finding meaning and purpose in life are known to be protective against allostatic load (Seeman et al., 2002) and dementia (Boyle et al., 2010). Programs such as the Experience Corps, which promotes both cognitive adaptations along with increased physical activity, have been shown to slow the decline of physical and mental health and to improve prefrontal cortical blood flow in a similar manner to regular physical activity (Carlson et al., 2009, Fried et al., 2004).
Depression and anxiety disorders are examples of a loss of resilience, in the sense that changes in brain circuitry and function, caused by the stressors that precipitate the disorder, become “locked” in a particular state and thus need external intervention. Indeed, prolonged depression is associated with shrinkage of the hippocampus (Sheline, 1996, Sheline, 2003) and prefrontal cortex (Drevets et al., 1997). While there appears to be no neuronal loss, there is evidence for glial cell loss and smaller neuronal cell nuclei (Rajkowska, 2000, Stockmeier et al., 2004), which is consistent with a shrinking of the dendritic tree described above after chronic stress. Indeed, a few studies indicate that pharmacological treatment may reverse the decreased hippocampal volume in unipolar (Vythilingam et al., 2004) and bipolar (Moore et al., 2000) depression, but the possible influence of concurrent cognitive-behavioral therapy in these studies is unclear.
Depression is more prevalent in individuals who have had adverse early life experiences (Anda et al., 2010). BDNF may be a key feature of the depressive state and elevation of BDNF by diverse treatments ranging from antidepressant drugs to regular physical activity may be a key feature of treatment (Duman and Monteggia, 2006). Yet, there are other potential applications, such as the recently reported ability of fluoxetine to enhance recovery from stroke (Chollet et al., 2011). However, a key aspect of this new view (Castren and Rantamaki, 2010) is that the drug is opening a “window of opportunity” that may be capitalized by a positive behavioral intervention, e.g., behavioral therapy in the case of depression or the intensive physiotherapy to promote neuroplasticity to counteract the effects of a stroke.
This is consistent with animal model work that shows that ocular dominance imbalance from early monocular deprivation can be reversed by patterned light exposure in adulthood that can be facilitated by fluoxetine, on the one hand (Vetencourt et al., 2008) and food restriction, on the other hand (Spolidoro et al., 2011). Investigations of underlying mechanisms for the re-establishment of a new window of plasticity are focusing on the balance between excitatory and inhibitory transmission and removing molecules that put the “brakes” on such plasticity (Bavelier et al., 2010).
It is important to reiterate that successful behavioral therapy, which is tailored to individual needs, can produce volumetric changes in both prefrontal cortex in the case of chronic fatigue (de Lange et al., 2008), and in amygdala, in the case of chronic anxiety (Holzel et al., 2010). This reinforces two important messages: i. that plasticity-facilitating treatments should be given within the framework of a positive behavioral or physical therapy intervention; and ii. that negative experiences during the window may even make matters worse (Castren and Rantamaki, 2010). In that vein, it should be noted that excess BDNF also has the ability to promote pathophysiology, such as seizures in some instances (Heinrich et al., 2011, Kokaia et al., 1995, Scharfman, 1997).
9. Conclusion and future directions
Beyond recognizing resilience as “achieving a positive outcome in the face of adversity”, the flexibility of the brain based upon healthy architecture emerges as a primary consideration. We have seen that brain architecture continues to show plasticity throughout adult life and studies of gene expression and epigenetic regulation reveal a dynamic and ever-changing brain. Ongoing work is identifying those biological changes that underlie flexible adaptability, as well as recognizing gene pathways, epigenetic factors and structural changes that indicate lack of resilience and which may lead to negative outcomes, particularly when the individual is challenged by new circumstances. We have seen that early life experiences determine individual differences in such capabilities via epigenetic pathways and the laying down of brain architecture that determines the later capacity for flexible adaptation or the lack thereof. Reactivation of such plasticity in individuals lacking such resilience is a new challenge for research and practical application and top-down interventions such as physical activity, social support, behavioral therapies including mindfulness and mediation and finding meaning and purpose are emerging as important new directions where pharmaceutical agents will not by themselves be effective but may be useful in combination with the more holistic interventions. And, finally and most importantly, even though the principles of epigenetic neurobiology apply to both genders, determining how the processes involved in resilience differ between men and women constitutes an important challenge for future research and practical application.
Acknowledgments
Research is supported by RO1 MH41256 from NIH, by the Hope for Depression Research Foundation and the American Foundation for Suicide Prevention. Dr. McEwen wishes to acknowledge the contributions of his colleagues in the National Scientific Council on the Developing Child (http://developingchild.harvard.edu/activities/council/) and Frameworks Institute (http://www.frameworksinstitute.org) to concepts of resilience discussed in this article.
References
- Adler N.E., Boyce T.W., Chesney M.A., Folkman S., Syme L. Socioeconomic inequalities in health. J. Am. Med. Assoc. 1993;269:3140–3145. [PubMed] [Google Scholar]
- Akers K.G., Yang Z., DelVecchio D.P., Reeb B.C., Romeo R.D. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS One. 2008;3(7):e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Butchart A., Felitti V.J., Brown D.W. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am. J. Prev. Med. 2010;39:93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Babyak M., Blumenthal J.A., Herman S., Khatri P., Doraiswamy M. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom. Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry. 2010;15(877):96–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Zhang X., Garachh V., Hanhauser E., Valentino R.J. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol. Behav. 2011;103:342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D., Levi D.M., Li R.W., Dan Y., Hensch T.K. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S., Shankaranarayana Rao B.S., Pawlak R., Strickland S., McEwen B.S., Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Bloss E.B., Hunter R.G., Waters E.M., Munoz C., Bernard K., McEwen B.S. Behavioral and biological effects of chronic S18986, a positive AMPA receptor modulator, during aging. Exp. Neurol. 2008;210:109–117. doi: 10.1016/j.expneurol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bloss E.B., Janssen W.G., McEwen B.S., Morrison J.H. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J. Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman R.E., Zrull M.C., Luine V.N. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Boyce W.T., Ellis B.J. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyle P.A., Buchman A.S., Barnes L.L., Bennett D.A. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch. Gen. Psychiatry. 2010;67:304–310. doi: 10.1001/archgenpsychiatry.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges N.M., Wood E.R., Holmes M.C., Hall J. Prepubertal stress and hippocampal function: sex-specific effects. Hippocampus. 2014 Jun;24(6):684–692. doi: 10.1002/hipo.22259. PubMed PMID: 24677338. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P.M., Meaney M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H.A., Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron H.A., Gould E. The control of neuronal birth and survival. In: Shaw C., editor. Receptor Dynamics in Neural Development. CRC Press; Boca Raton, FL: 1996. pp. 141–157. [Google Scholar]
- Carlson M.C., Erickson K.I., Kramer A.F., Voss M.W., Bolea N. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruth L.L., Reisert I., Arnold A.P. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castren E., Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev. Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Cerqueira J.J., Pego J.M., Taipa R., Bessa J.M., Almeida O.F.X., Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Chollet F., Tardy J., Albucher J.F., Thalamas C., Berard E. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- Colcombe S.J., Kramer A.F., Erickson K.I., Scalf P., McAuley E. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D., Magarinos A.M., LeDoux J.E., McEwen B.S. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Convit A., Wolf O.T., Tarshish C., de Leon M.J. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan J.D., Smith E.L.P., Altemus M., Scharf B.A., Owens M.J. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol. Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Moffitt T.E., Harrington H., Milne B.J., Polanczyk G. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson N.A., van den Oever J.M., Korobko O.B., Magarinos A.M., de Kloet E.R., McEwen B. Prior history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology. 2013 Apr 30 doi: 10.1210/en.2012-2233. PubMed PMID: 23633533. Epub 2013/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange F.P., Koers A., Kalkman J.S., Bleijenberg G., Hagoort P. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- de Leon M.J., George A.E., Golomb J., Tarshish C., Convit A. Frequency of hippocampus atrophy in normal elderly and Alzheimer's disease patients. Neurobiol. Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Del Giudice M., Ellis B.J., Shirtcliff E.A. The adaptive calibration model of stress responsivity. Neurosci. Biobehav Rev. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu J.M. Vitamins and aging: pathways to NAD+ synthesis. Cell. 2007;129:453–454. doi: 10.1016/j.cell.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Eickhoff S., Kellermann T., Falkenberg D.I. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E., Sousa J.C., Melo I., Morgado P., Mesquita A.R. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Kempermann G., Kuhn H.G., Winkler J. Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Raichle M.E. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol. Bull. 1992;28:261–274. [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Simpson J.R., Jr., Todd R.D., Reich T. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging studies of mood disorders. Biol. Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Du J., McEwen B.S., Manji H.K. Glucocorticoid receptors modulate mitochondrial function. Commun. Integr. Biol. 2009;2:1–3. doi: 10.4161/cib.2.4.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eiland L., McEwen B.S. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22:82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Hu L. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W., Gonnella C., Marcynyszyn L.A., Gentile L., Salpekar N. The role of chaos in poverty and children's socioemotional adjustment. Psychol. Sci. 2005;16:560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Shera D.M., Savage J.H., Betancourt L., Giannetta J.M. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fraga M.F., Ballestar E., Paz M.F., Ropero S., Setien F. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D.D., Diorio J., Plotsky P.M., Meaney M.J. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J., Brandmaier A.M., Lewejohann L., Kirste I., Kritzler M. Emergence of individuality in genetically identical mice. Science. 2013;340:756–759. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- Fried L.P., Carlson M.C., Freedman M., Frick K.D., Glass T.A. A social model for health promotion for an aging population: initial evidence on the experience corps model. J. Urban Health: Bull. N. Y. Acad. Med. 2004;81:64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Zetzsche T., Born C., Jager M. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Galea L.A.M., McEwen B.S., Tanapat P., Deak T., Spencer R.L., Dhabhar F.S. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Ganzel B.L., Morris P.A. Allostasis and the developing human brain: explicit consideration of implicit models. Dev. Psychopathol. 2011;23:955–974. doi: 10.1017/S0954579411000447. [DOI] [PubMed] [Google Scholar]
- Ganzel B.L., Kim P., Glover G.H., Temple E. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage. 2008;40:788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Horenstein J.A., Cohen S., Matthews K.A., Brown S.M. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc. Cogn. Affect Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Jennings J.R., Sheu L.K., Greer P.J., Kuller L.H., Matthews K.A. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Horenstein J.A., Hariri A.R., Sheu L.K., Manuck S.B. Potential neural embedding of parental social standing. Soc. Cogn. Affect Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Hariri A.R., Sheu L.K., Muldoon M.F., Sutton-Tyrrell K., Manuck S.B. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol. Psychiatry. 2009;65:943–950. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S.M., Dziobek I., Sweat V., Tirsi A., Rogers K. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Goldwater D.S., Pavlides C., Hunter R.G., Bloss E.B., Hof P.R. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., McEwen B.S. Neuronal birth and death. Curr. Opin. Neurobiol. 1993;3:676–682. doi: 10.1016/0959-4388(93)90138-o. [DOI] [PubMed] [Google Scholar]
- Govindarajan A., Rao B.S.S., Nair D., Trinh M., Mawjee N. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.D., Rubin T.G., Hunter R.G., McEwen B.S. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol. Psychiatry. 2013 doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits T.V., Shenton M.E., Hokama H., Ohta H., Lasko N.B. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol. Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PLoS One. 2011;6:e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B., Risley T.R. Brookes Publishing Company; Baltimore, MD: 1995. Meaningful Differences in the Everyday Experience of Young American Children. p. 304. [Google Scholar]
- Heinrich C., Lahteinen S., Suzuki F., Anne-Marie L., Huber S. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2011;42:35–47. doi: 10.1016/j.nbd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McEwen B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel B.K., Carmody J., Evans K.C., Hoge E.A., Dusek J.A. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R.G., Murakami G., Dewell S., Seligsohn M., Baker M.E. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17657–17662. doi: 10.1073/pnas.1215810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R.G., McEwen B.S., Pfaff D.W. Environmental stress and transposon transcription in the mammalian brain. Mob. Genet. Elem. 2013;3:e24555. doi: 10.4161/mge.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C., Kabbaj M., Akil H., Watson S.J. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jackson J.S., Knight K.M., Rafferty J.A. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am. J. Public Health. 2010;100:933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.S., Bell D.H. Neuronal proliferation in the 9-month-old rodent-radioautographic study of granule cells in the hippocampus. Exp. Brain Res. 1983;52:1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- Kaplan M.S. Environment complexity stimulates visual cortex neurogenesis: death of a dogma and a research career. Trends Neurosci. 2001;24:617–620. doi: 10.1016/s0166-2236(00)01967-6. [DOI] [PubMed] [Google Scholar]
- Karatsoreos I.N., McEwen B.S. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn. Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Karatsoreos I.N., Bhagat S., Bloss E.B., Morrison J.H., McEwen B.S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D., Smith E.L.P., Gohil B.C., Banerji M.A., Coplan J.D. Early appearance of the metabolic syndrome in socially reared bonnet macaques. J. Clin. Endocrinol. Metab. 2005;90:404–408. doi: 10.1210/jc.2004-0452. [DOI] [PubMed] [Google Scholar]
- Kokaia M., Ernfors P., Kokaia Z., Elmer E., Jaenisch R., Lindvall O. Suppressed epileptogenesis in BDNF mutant mice. Exp. Neurol. 1995;133:215–224. doi: 10.1006/exnr.1995.1024. [DOI] [PubMed] [Google Scholar]
- Laje G., Paddock S., Manji H., Rush A.J., Wilson A.F. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am. J. Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- Leuner B., Mendolia-loffredo S., Shors T.J. Males and females respond differently to controllability and antidepressant treatment. Biol. Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Haltmeyer G., Kara G., Denenberg V. Physiological and behavioral effects of infantile stimulation. Physiol. Behav. 1967;2:55–59. [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. U. S. A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V., Villegas M., Martinez C., McEwen B.S. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V.N., Beck K.D., Bowman R.E., Frankfurt M., MacLusky N.J. Chronic stress and neural function: accounting for sex and age. J. Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., Parent S., Evans A.C., Tremblay R.E., Zelazo P.D. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos A.M., Li C.J., Gal Toth J., Bath K.G., Jing D. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A.L., Gianaros P.J., Abramowitch S.M., Manuck S.B., Hariri A.R. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol. Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.P., Wellman C.L. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb. Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I., Noh K.M., Allis C.D. Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacology. 2013;38:3–22. doi: 10.1038/npp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Stress- and allostasis-induced brain plasticity. Annu Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Lasley E.N. Cerebrum. The Dana Forum on Brain Science. Dana Press; 2005. The end of sex as we know it. [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am. J. Public Health. 2011;101(Suppl. 1):S131–S139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Introduction: the end of sex as we once knew it. Physiol. Behav. 2009;97:143–145. doi: 10.1016/j.physbeh.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann. N. Y. Acad. Sci. 2010;1204(Suppl. l.):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J., Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler M.F. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog. Neurobiol. 2008;86:305–341. doi: 10.1016/j.pneurobio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J. Short history of neuroendocrinology and the International Society of Neuroendocrinology. Neuroendocrinology. 1992;56:1–10. doi: 10.1159/000126201. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.M., Morrison J.H., McEwen B.S. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav. Brain Res. 2012;229:280–288. doi: 10.1016/j.bbr.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Mitra R., Jadhav S., McEwen B.S., Vyas A., Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G.J., Bebehuk J.M., Wilds I.B., Chen G., Manji H.K. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;8:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Xenos D., Barone Y., Caruso A., Scaccianoce S. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4804. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Bigio B., Zelli D., Nicoletti F., McEwen B.S. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol. Psychiatry. September 2014;2 doi: 10.1038/mp.2014.96. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic J., Bush N.R., Stamperdahl J., Adler N.E., Boyce W.T. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child. Dev. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K.J., Buckmaster C.L., Sundlass K., Schatzberg A.F., Lyons D.M. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Janssen W.G.M., Hof P.R., McEwen B.S., Morrison J.H. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rao N.A., McCalman M.T., Moulos P., Francoijs K.J., Chatziioannou A. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res. 2011;21:1404–1416. doi: 10.1101/gr.118042.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.P., Anilkumar S., McEwen B.S., Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol. Psychiatry. 2012;72:466–475. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M.H.M., Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32:S21–S25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., Hahn E.L., Nathan S.V., de Quervain D.J.-F., McGaugh J.L. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J. Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff C.D., Singer B. The contours of positive human health. Psychol. Inq. 1998;9:1–28. [Google Scholar]
- Ryff C.D. Psychological well-being revisited: advances in the science and practice of eudaimonia. Psychother. Psychosom. 2014;83:10–28. doi: 10.1159/000353263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M., Krey L.C., McEwen B.S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Scharfman H.E. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J. Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T.E., Singer B.H., Ryff C.D., Dienberg G., Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom. Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Hamo C., Hof P.R., Lou W., McEwen B.S., Morrison J.H. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb. Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Donnelly J.M., Ollinger J.M., Snyder A.Z., Mintun M.A. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I. Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol. Psychiatry. 1996;1:298–299. [PubMed] [Google Scholar]
- Sheline Y.I. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shors T.J., Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13955–13960. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Friedman E., Seeman T., Fava G.A., Ryff C.D. Protective environments and health status: cross-talk between human and animal studies. Neurobiol. Aging. 2005;26S:S113–S118. doi: 10.1016/j.neurobiolaging.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Snyder M.A., Smejkalova T., Forlano P.M., Woolley C.S. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J. Neurosci. Methods. 2010;188:226–234. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidoro M., Baroncelli L., Putignano E., Maya-Vetencourt J.F., Viegi A., Maffei L. Food restriction enhances visual cortex plasticity in adulthood. Nat. Commun. 2011;2:320. doi: 10.1038/ncomms1323. [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Gebarski S.S., Berent S., Schteingart D.E. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol. Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Giordani B., Gebrski S.S., Berent S., Schork M.A., Schteingart D.E. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol. Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Stockmeier C.A., Mahajan G.J., Konick L.C., Overholser J.C., Jurjus G.J. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi S.J. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann. N. Y. Acad. Sci. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Tang A.C., Akers K.G., Reeb B.C., Romeo R.D., McEwen B.S. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E., Vythilingam M., Southwick S.M., Charney Ds, Bremner J.D. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetencourt J.F.M., Sale A., Viegi A., Baroncelli L., De Pasquale R. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Rao B.S.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M., Vermetten E., Anderson G.M., Luckenbaugh D., Anderson E.R. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol. Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Waddington C.H. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- Wood G.E., Shors T.J. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G.E., Shors T.J., Beylin A.V. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav. Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Yoo S.-S., Gujar N., Hu P., Jolesz F.A., Walker M.P. The human emotional brain without sleep – a prefronta amygdala disconnect. Curr. Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zohar J., Yahalom H., Kozlovsky N., Cwikel-Hamzany S., Matar M.A. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur. Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]