Abstract
In addition to eradication of Helicobacter pylori, chemotherapy with anticancer agents, and radiation therapy, the treatment with molecular target drugs including rituximab, a CD20 antagonist, is one of the promising new regimens. The mucosa-associated lymphoid tissue (MALT) lymphoma is histologically characterized by rich distribution of the microvascular network consisting of the immature capillaries, lymphatics and venules, and this microvascular network could be the target of the new pharmacotherapy in addition to the direct action on the accumulated B lymphocytes. We have established the animal model of the gastric MALT lymphoma by the Helicobacter heilmannii (H. heilmannii) peroral infection of C57BL/6 mice. The disease induced by this model is very similar to the human counterpart, because of the lymphoepithelial lesion characteristic of the human MALT lymphoma as well as the rich vascularization and localization of vascular endothelial growth factor (VEGF) and its receptors, Flt-1, Flk-1 and Flt-4. By administering VEGF receptor antibodies or celecoxib, one of the cyclooxygenase 2 inhibitors, we were able to induce a significant decrease in the size of the tumor and the apoptotic changes of the endothelial cells of the microvascular network. These antiangiogenic strategies were suggested to be candidates for the new pharmacological treatment of gastric MALT lymphoma, when other treatments are not effective.
Keywords: Gastric MALT lymphoma, angiogenesis, lymphangiogenesis, VEGF, Flt-1, Flk-1, Flt-4, celecoxib.
INTRODUCTION
For the treatment of low-grade mucosa-associated lymphoid tissue (MALT)-type gastric B-cell lymphoma (MALT lymphoma), eradication of Helicobacter pylori (H. pylori) is the gold standard, and chemotherapy with anticancer agents and radiation therapy have been used as adjuvant treatments, but about 10% of the cases cannot be cured with these treatments. The monoclonal antibody against CD20, rituximab alone or rituximab with a combination of chemotherapy agents or radiotherapy has been reported to be very promising [1], though cases resistant to rituximab have still been reported and new treatments are awaited [2]. In our research, we have used several antiangiogenic agents for the treatment of Helicobacter heilmannii (H. heilmannii)-induced mouse gastric MALT lymphoma.
In this paper, we discuss the present putative pathogenesis, pathological characteristics and possible new pharmacotherapy of gastric MALT lymphoma.
Pathogenesis of the Gastric MALT Lymphoma
The pathophysiology of gastric MALT lymphoma is one of the most important points yet to be clarified in the study of Helicobacter-related upper gastrointestinal diseases. Many reports have pointed out the strong relation of H. pylori to the pathogenesis of MALT lymphoma. The predominantly antral localization of gastric MALT lymphoma is the result of the distribution of reactive MALT in response to H. pylori infection [3], which provides an antigenic drive for the growth of these tumors, but the precise mechanism for gastric MALT lymphoma growth is still under investigation.
Stolte et al. [4] pointed out the stronger presence of H. heilmannii than H. pylori in human MALT lymphoma cases based on the histological study. This bacillus exhibits a zoonotic infection pattern and is sometimes detected in domestic animals, such as cats, dogs, cows and pigs, as well as in humans. We also detected high H. heilmannii positivity in human cases of gastric MALT lymphoma by PCR analysis [5]. In addition, our recent study has revealed that oral infection of H. heilmannii from cynomolgus monkeys induced gastric low-grade MALT lymphoma in almost all C57BL/6 mice after a period of six months [6], suggesting the significance of H. heilmannii as well as H. pylori in the formation of gastric MALT lymphoma.
Pathological Characteristics of Gastric MALT Lymphoma
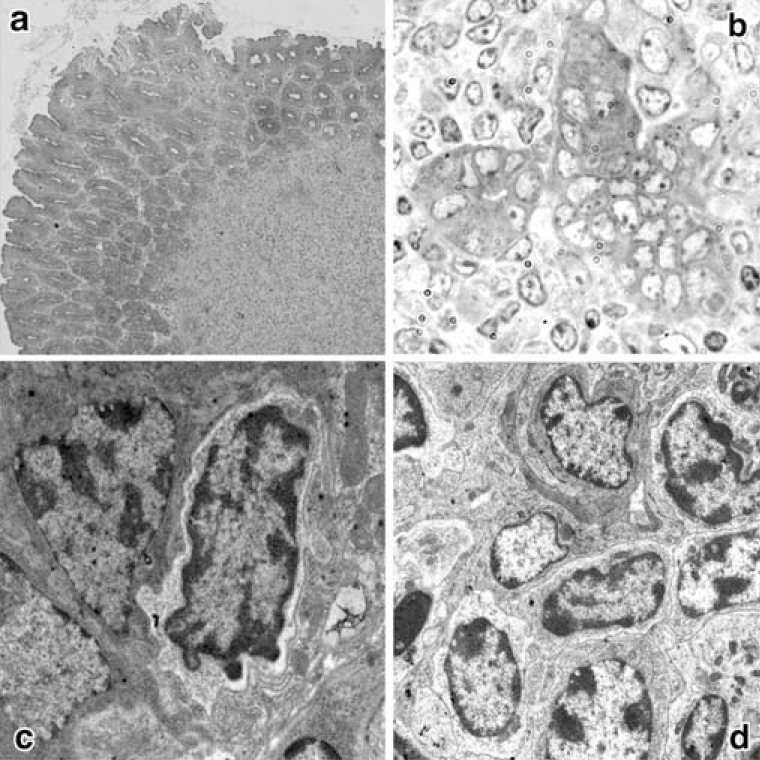
Gastric MALT lymphoma is characterized by an accumulation of B lymphocytes along with the destruction of glandular elements and the presence of lymphoepithelial lesions (Fig. 1) [6]. Electron microscopic observation revealed numerous H. heilmannii bacilli mostly in the fundic glandular lumen as well as in the intracellular canaliculi, and the cytoplasm of intact and damaged parietal cells (Fig. 2).
Fig. (1).
Light and electron microscopic observations of lymphoid follicles and lymphoepithelial lesions.
a: Lymphoid follicle (L) observed in the fundic mucosa of a mouse infected for 6 months. Toluidine blue-stained Epon (x100).
b: Lymphoepithelial lesion in the stomach of a mouse infected for 6 months. Toluidine blue-stained Epon (x600).
c: Invasion of a lymphocyte between fundic glandular cells in a mouse infected for 6 months (x3000).
d: Accumulation of centrocyte-like cells in a lymphoid follicle (x2000).
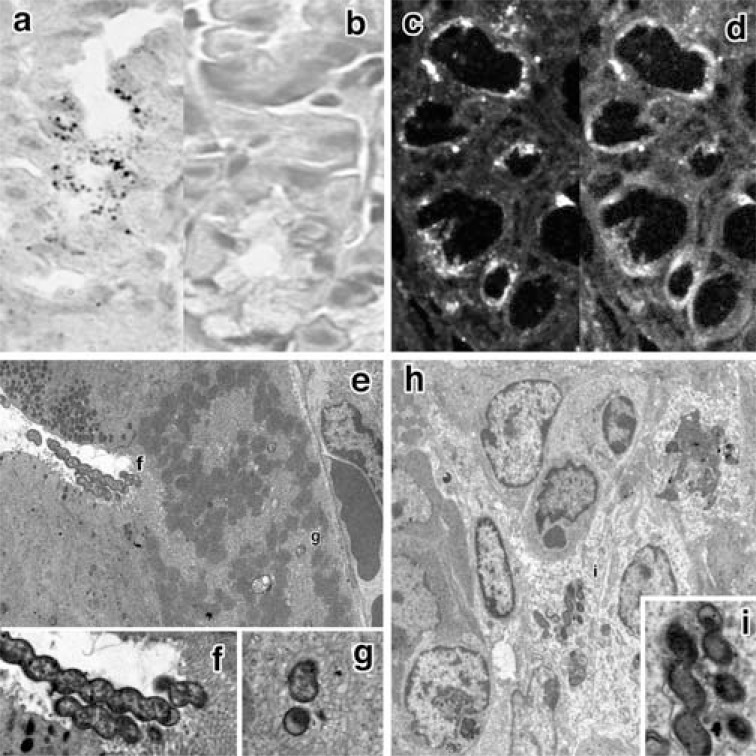
Fig. (2).
The localization of H. heilmannii by in situ hybridization, immunohistochemistry and electron microscopic cytochemistry.
a, b: Many reactive bacilli were recognized by in situ hybridization at the luminal side of the body of the fundic gland (a). No reaction was detected with the use of a sense probe (b). (x800).
c, d: Indirect fluorescent immunohistochemistry using anti-Hp polyclonal antibody revealed immunoreactive bacilli at the luminal side of the body of the fundic gland (c). Alexa-phalloidin fluorescence (d) revealed that the localization of bacilli coincided approximately with that of f-actin-rich parietal cells (x800).
e, f, g: Electron microscopy revealed the presence of extremely numerous bacilli near (e, f) and in (g) the intracellular canaliculi (e: x2000; f, g: x6000).
h, i: Some bacilli-disrupted epithelial cells. An adjacent parietal cell was destroyed (arrowhead) (h: x2000; i: x6000).
Angiogenesis and Lymphangiogenesis of Gastric MALT Lymphoma
Markedly enhanced angiogenesis is another pathological characteristic of this tumor. Immunohistochemical methods using the vascular endothelial antibody CD31 and anti-lymphatic endothelial antibodies prox-1 and podoplanin (Figs. 3, 4, 5) have shown that this tumor has an irregular microvascular network [7, 8]. VEGF-A, VEGF-C and related receptors Flt-1, Flk-1 and Flt-4 were found to be richly distributed in and near the MALT lymphoma [9]. In this respect, the relation of the MALT lymphoma to lymphangiogenesis as shown by the localization of Flt-4, prox-1, podoplanin and VEGF-C is also very important, because it influences the metastasis of the tumors and prognosis of the bearing person or animal, and this could be one of the targets of the pharmacotherapy for gastric MALT lymphoma.
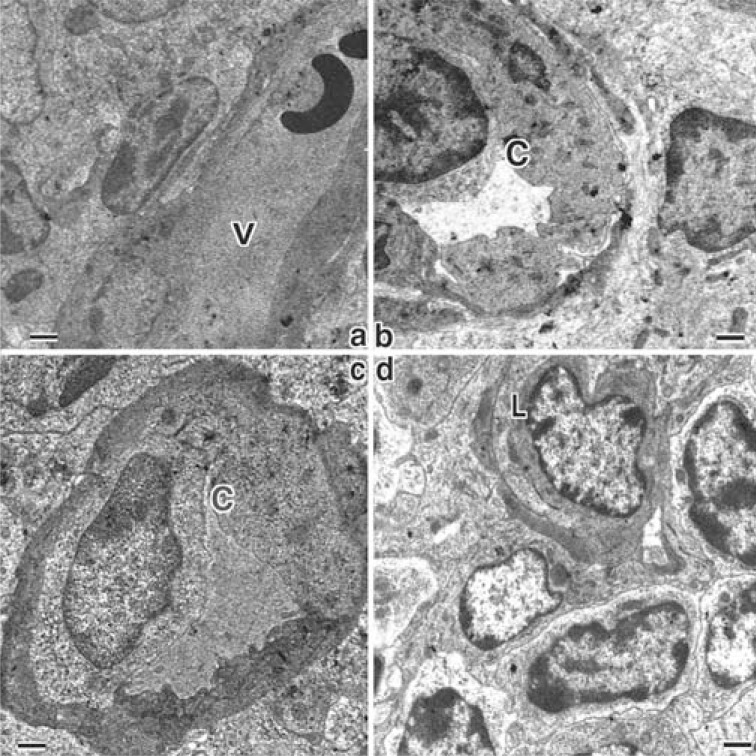
Fig. (3).
Electron micrographs showing the microvessels in and near the MALT lymphoma in H. heilmannii-administered mice.
a: A venule (V) within the MALT lymphoma (x3000).
b: A capillary (C) with irregular endothelial cells with rich cytoplasm (x3000).
c: A capillary (C) surrounded with pericyte with electron-dense cytoplasm (x4000).
d: A lymph capillary (L) within the MALT lymphoma (x4000).
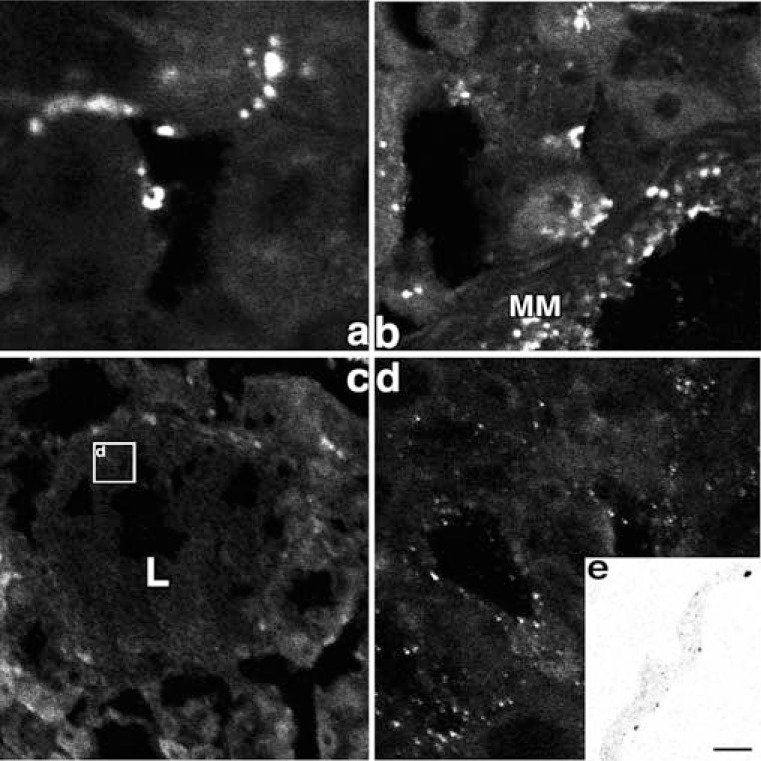
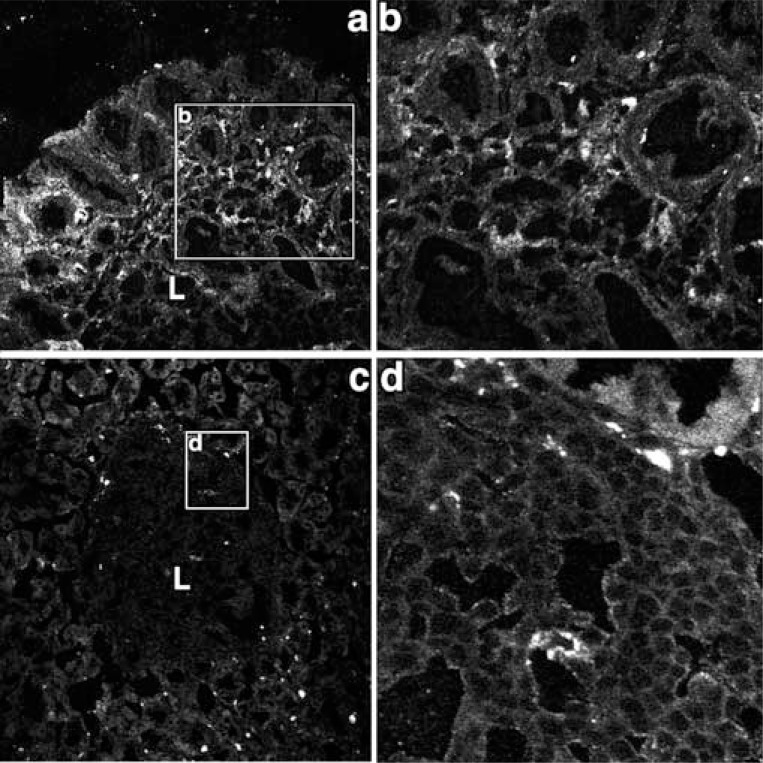
Fig. (4).
Prox-1 immunoreactivity and 5’ nucleotidase enzyme cytochemistry in the control and H. heilmannii-treated mouse fundic mucosa.
a, b: In the control mouse fundic mucosa, prox-1 immunoreactivity was detected near the muscularis mucosae (MM) and in the lamina propria mucosae between the fundic glands. (a: x400, b: x200)
c, d: In the H. heilmannii-administered mouse fundic mucosa, prox-1 immunoreactivity was recognized within the lymphoma. (c: x 100, d: x1000)
e: By electron microscopic enzyme cytochemistry of 5’ nucleotidase, the reaction products were recognized on the abluminal surface of the endothelial cells. (x4000)
Fig. (5).
VEGF-C and Flt-4 Immunoreactivity in the H. heilmannii-treated mouse fundic mucosa.
a, b: VEGF-C immunoreactivity is recognized in the marginal area of the lymphoma. (a: x200, b: x400)
c, d: Flt-1 immunoreactivity is found mostly in the marginal area of the lymphoma. (c: x200, d: x 1000)
Regarding the localization of the lymphatics in the fundic mucosa, Sugito et al. [10] reported that lymphatic vessels existed only in the base of the mucosa, submucosa and muscular layer as a plexus and not in the upper or middle portion of the mucosa. In our experiment, the formation of MALT lymphoma was accompanied by lymphatic vessel formation with Flt-4 immunoreactivity in the marginal area of the MALT lymphoma. As to the origin of these newly formed lymphatic vessels, the extension from the preexisting lymphatic vessels in the base of the mucosa is most probable. On the other hand, some reports have pointed out that the sprouting of lymphatic endothelial cells with strong Flt-4 activity was recognized from the venules [11].
New Treatment of Gastric MALT Lymphoma
At present, the first choice of gastric MALT lymphoma treatment is the eradication by triple therapy applied to H. pylori, i.e., the combination of two kinds of antibiotics and proton pump inhibitors for several days, but this regimen generally shows a less than 70% response rate, due to H. pylori resistance to antibiotics, especially clarithromycin, chromosomal aberration and the presence of perigastric lymph nodes [12]. In our experiment, some strains of H. heilmannii were also found to be resistant to standard triple therapy [13]. As mentioned above, the most promising molecular target agent for the treatment of MALT lymphoma is rituximab, the monoclonal antibody against CD20, but several resistant cases against rituximab have already been reported [2]. Based on our histochemical analysis, the antiangiogenic therapy could be effective. The antiangiogenic agents available clinically or experimentally could be classified as shown in Table 1. We selected two agents, VEGF-receptor antibodies [14] and a COX-2 inhibitor, celecoxib [9], for the treatment of mouse gastric MALT lymphoma.
Table 1.
| Classes of inhibitors | Kinds | Examples of Drugs |
|---|---|---|
| VEGF antagonists | anti-VEGF-mAb | Bebacizumab |
| VEGF trap | s-Flt-1, s-Flk-1 | |
| other VEGF inhibitors | NM-3 | |
| Broad GF inhibitors | Inhibitors of heparin-GF binding | Suraimn |
| VEGF receptor antagonists | anti-VEGF-R2 mAbs | IMC-1C11 |
| VEGF-R2 inhibitor | sumaxanib | |
| VEGF-R mRNA ribozyme | Angiozyme | |
| PDGF receptor antagonists | PDGF-R inhibitors | Gleevec |
| Endothelin R (lectin) inhibitors | GBC-590 | |
| Integrin angatonists | anti-alphaVbeta3 mAbs | LM-609 |
| alpha5beta1 antagonist | Endostatin | |
| Signaling inhitibitors | PKC inhibitors | PKC-412 |
| PKA inhibitors | AS PKAI | |
| MAPK ihbibitors | PD98039 | |
| mTOR inhibitors | Rapamycin | |
| Anti-inflammatory drugs | NSAIDs | Indomethacin |
| COX-2 inhibitors | celecoxib, rofecoxib, NS398 | |
| Inhibitor of Mf activation | thalidomide | |
| Proteinase inhibitors | MMP inhibitors | Batimastat |
| PA inhibitors | PAI-1 | |
| Heparinase | PI-88 | |
| Cototoxic agents | Pro-apoptotic factors | TNF |
| Tubulin-binding agents | combretastatin | |
| HSP inhibitors | HSP90 inhibitor | 17-AAG |
Effect of VEGF Receptor Antibodies on Gastric MALT Lymphoma
The H. heilmannii-infected mice were divided into the following four groups: phosphate-buffered saline group, Flt-4 group, Flt-4 and Flt-1group, and Flt-4 and Flk-1 group. Rabbit polyclonal antibodies against Flt-1, Flk-1and Flt-4 (50 mg/mouse) (R&D Systems, MN, USA) were intraperitoneally administered. Stereomicroscopic study revealed that the administration of the Flt-4 and Flt-1 antibodies significantly reduced the occupied surface area of the lymphoma in the fundic portion of the mouse stomach as well as the average size of tumors (Fig. 6). In double staining with caspase-3 and prox-1, some of the apoptotic cells were shown to be lymphatic endothelial cells (Fig. 7).
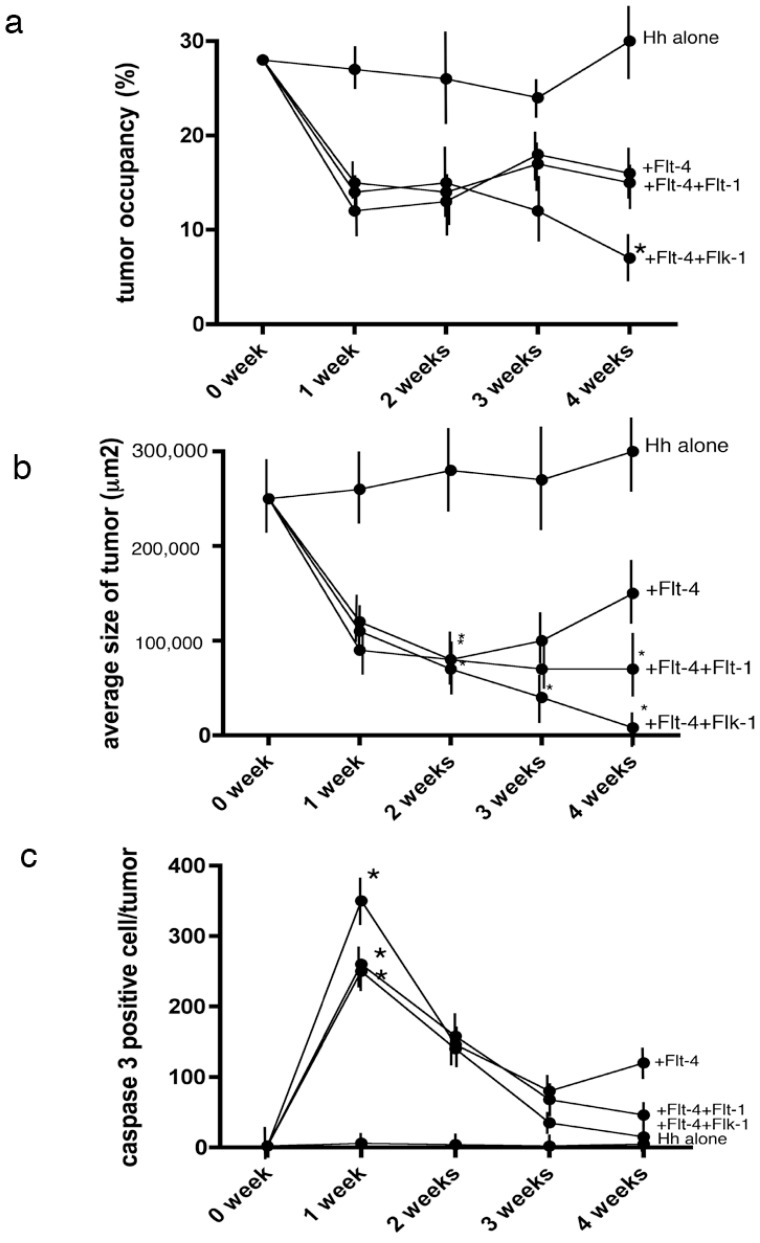
Fig. (6).
Statistical analysis of the effect of Flt-4 on MALT lymphoma.
a: Time course of the change of surface area occupied by MALT lymphoma through stereomicroscopic observation. Mixed administration of Flt-4 and Flk-1 significantly decreased the occupied area in four weeks. A P value less than 0.05 denoted the presence of a statistically significant difference.
b: Time course of the size of MALT lymphoma by light microscopic observation.
The tumor showed a tendency to shrink, especially following the mixed administration of Flt-4 and Flk-1.
c: Time course of the caspase-3-immunoreactive cells within the tumor by confocal laser microscopy.
The number of caspase-3-immunoreactive cells in MALT lymphoma was greatest one week after in all Flt-1 administered group.
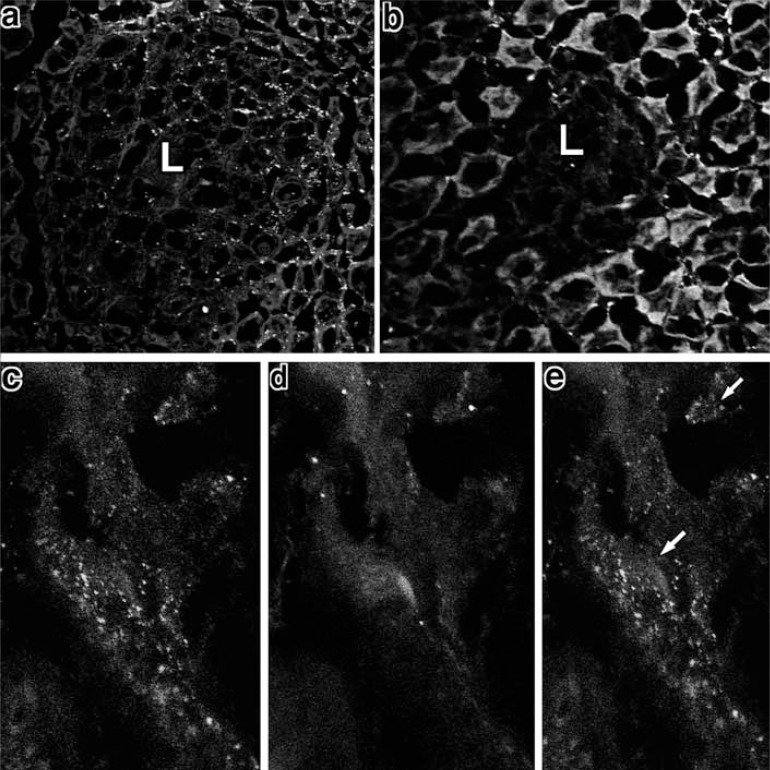
Fig. (7).
Caspase-3 and prox-1 immunoreactivities in H. heilmannii-infected and Flt-4- and Flk-1-administered mice.
a: One week after the administration, many cells within the MALT lymphoma showed positive immunoreactivity against caspase-3.(x200)
b: Three weeks after the administration, the lymphoma became very small and few caspase-3-immunoreactive cells were observed in the lymphoma. (x400)
c-e: In double staining with caspase-3 and prox-1, some of the prox-1-positive cells were found to be caspase-3 immunoreactive (arrow).
c: Prox-1 immunoreactivity, d: caspase-3 immunoreactivity, e: merged view of c and d. (x1000)
Clinically, bevacizumab [15] and aflibercept [16] have been reported to be very promising in the therapy for cancer and neovascular age-related muscular degeneration. Bevacizumab is a humanized anti-human VEGF-A monoclonal antibody; as it has been shown to be ineffective against murine VEGF-A, it could not be applied to our study.
In summary, a VEGF-mediated mechanism was shown to play an important role in the expansion of MALT lymphoma tissue through angiogenesis and lymphangiogenesis, and its inhibition by a receptor antibody showed a significant effect.
Effect of Celecoxib, a COX-2 Antagonist, on Gastric MALT Lymphoma
Selective cyclooxygenase-2 (COX-2) inhibitors have been reported to suppress tumor expansion through anti-angiogenic activity in various kinds of malignancy [17]. In gastric MALT lymphoma, COX-2 and microsomal prostaglandin E synthase-1 (mPGES-1), the rate-limiting enzyme of prostaglandin E synthase, were observed by indirect immunohistochemical methods and confocal laser microscopy. Both the COX-2 and mPGES-1 immunoreactivities were markedly recognized on the apical region of the MALT lymphoma (Fig. 8).
Fig. (8).
COX-2 and mPGES-1 immunohistochemistry in gastric MALT lymphoma. The immunoreactivities of COX-2 (a) and mPGES-1 (b) were mark-edly recognized in the interstitial cells in the lamina propria mucosae surrounding the MALT lymphoma tissues (L). (x200)
We also investigated the effect of celecoxib, one of the selective COX-2 inhibitors, on tumor expansion. Hh-infected mice were
divided into the vehicle and celecoxib-administered groups. In the celecoxib-administered group, 30 or 100 mg/kg of the agents were administered twice a day through oral intubation throughout the experiment. One, two and four weeks after the start of the administration, the area in the fundic region occupied by the tumor was estimated. The addition of celecoxib decreased the tumor occupant area in the fundic mucosa as well as the B220 mRNA (Figs. 9, 10). The immunoreactivity of the CD31 and Prox-1 declined markedly. VEGF-A and VEGF-C content in the fundic mucosa was significantly decreased four weeks after the start of administration. These data show the the effect of celecoxib was exerted, at least partly, through its angiogenic activity.
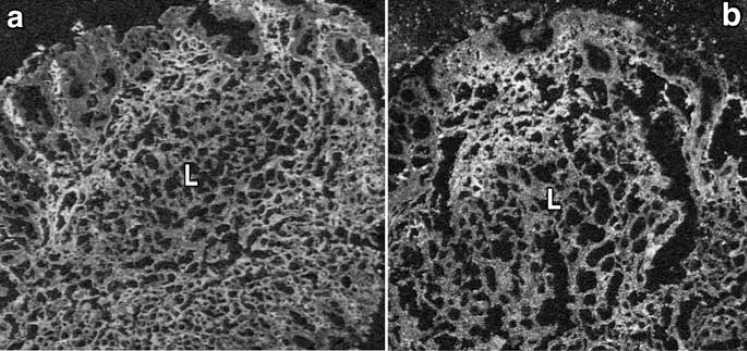
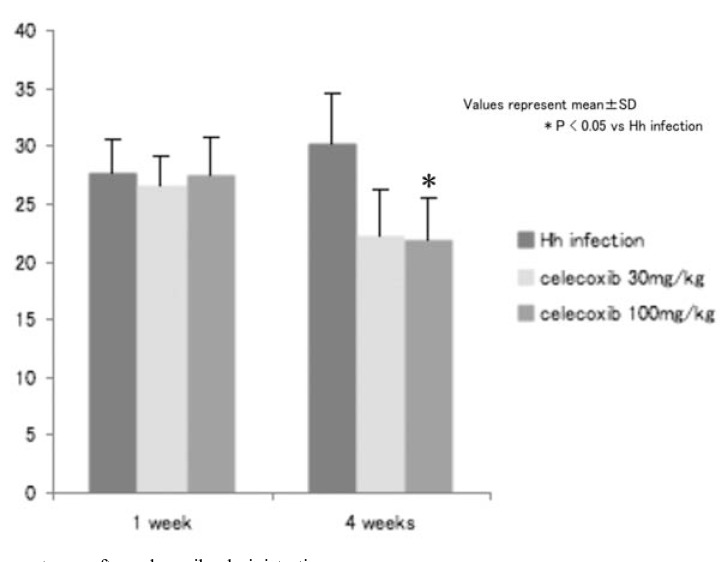
Fig. (9).
Alteration of the tumor occupant area after celecoxib administration.
Four weeks after celecoxib administration (100 mg/kg b.w.), the tumor occupant area was significantly decreased.
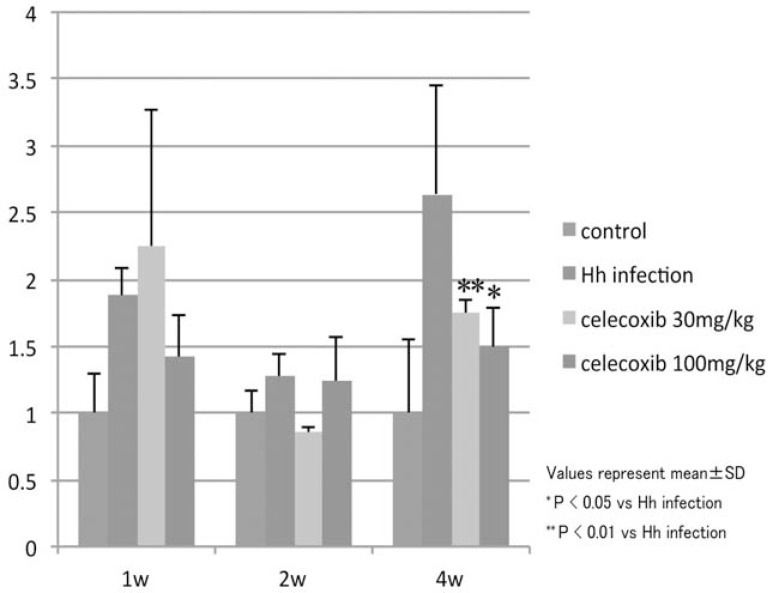
Fig. (10).
Alteration of the B220 mRNA level after celecoxib administration
Four weeks after celecoxib administration, the B220 mRNA level was significantly decreased.
New Agents for MALT Lymphoma Suppression
Recently, many factors generally thought to be irrelevant to angiogenesis have been found instead to be closely related to angiogenesis. In interleukin (IL)-10 knockout mice, the induction of MALT lymphoma was not observed, and this phenomenon was related to the suppression of angiogenesis. Saito et al recently reported the relation of microRNA to the formation of gastric MALT lymphoma [18]. In that study, miR-142-5p and miR-155 were found to be overexpressed in MALT lymphoma, and these microRNAs are related to suppression of the proapoptotic gene TP53INP1. This suggests the possibility of other anti-microRNA-related compounds as chemotherapeutic agents against gastric MALT lymphoma.
The reevaluation of pharmacological therapy from the viewpoint of angiogenesis is an important subject for future study.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
This work was supported by JSPS KAKENHI Grant Numbers 22590690, 23790155 and 21590491.
REFERENCES
- 1.Troch M, Kiesewetter B, Willenbacher W, et al. Rituximab plus subcutaneous cladribine in patients with extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue-Lymphoma a phase II study by the Arbeitsgemeinschaft Medikamentose Tumortherapie. Haematologica. 2012 doi: 10.3324/haematol.2012.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract. Res Clin Haematol. 2011;24(2):203–16. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genta RM, Huberman RM, Graham DY. The gastric cardia in Helicobacter pylori infection. Hum Path. 1994;25:915–9. doi: 10.1016/0046-8177(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 4.Morgner A, Lehn N, Andersen LP, et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–8. doi: 10.1016/s0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, Tsuchimoto K, Matsui H. Relation of Helicobacter heilmannii to gastric mucosal damage as a model of zoonosis between men and pets. Nihon Rinsho. 2005;63(Suppl 11 ):605–8. [PubMed] [Google Scholar]
- 6.Nakamura M, Murayama SY, Serizawa H, et al. Candidatus Helicobacter heilmannii from cynomolgus monkey brought about gastric low grade MALT lymphoma in C57BL/6 mice. Infect Immun. 2007;75:1214–22. doi: 10.1128/IAI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura M, Matsui H, Murayama SY, et al. Interaction of VEGF to gastric low grade MALT lymphoma by Helicobacter heilmannii infection in C57BL/6 mice. Inflammopharmacology. 2007;15:115–8. doi: 10.1007/s10787-007-1549-5. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa K, Nakamura M, Takahashi S, et al. Increased apoptosis and angiogenesis in gastric low-grade mucosa-associated lymphoid tissue-type lymphoma by Helicobacter heilmannii infection in C57/BL6 mice. FEMS Immunol Med Microbiol. 2007;50:268–272. doi: 10.1111/j.1574-695X.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Takahashi S, Matsui H, et al. Microcirculatory alteration in low-grade mucosa-associated lymphoma by Helicobacter heilmannii infection its relation to vascular endothelial growth factor and cyclooxygenase-2. J Gastroenterol Hepatol. 2008;23 (Suppl. 2 ):S157–S160. doi: 10.1111/j.1440-1746.2008.05554.x. [DOI] [PubMed] [Google Scholar]
- 10.Sugito M, Araki K, Ogata T. Three-dimensional organization of lymphatics in the dog stomach a scanning electron microscopic study of corrosion casts. Arch Histol Cytol. 1996;59:61–70. doi: 10.1679/aohc.59.61. [DOI] [PubMed] [Google Scholar]
- 11.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–60. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 12.Chelly I, Adouni O, Chouaib S, et al. Gastric MALT lymphoma: anatomo-clinical data and outcome of early stage H.pylori-associated gastric MALT-lymphoma resistant to treatment. La Tunisie m dicale. 2011;89:910–15. [PubMed] [Google Scholar]
- 13.Matsui H, Aikawa C, Sekiya Y, Takahashi S, Murayama SY, Nakamura M. Evaluation of antibiotic therapy for eradication of Candidatus Helicobacter heilmannii. Antimicrobial Agent and Chemotherapy. 2008;52:2988–9. doi: 10.1128/AAC.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura M, Matsui H, Takahashi T, et al. Suppression of lymphangiogenesis induced by Flt-4 antibody in gastric low-grade mucosa-associated lymphoid tissue lymphoma by Helicobacter heilmannii infection. J. Gastroenterol. Hepatol. 2010;25 (Suppl 1 ):S1–6. doi: 10.1111/j.1440-1746.2010.06230.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu L, Wu X, Cheng Z, et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008;49:522–7. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 16.Rudge JS, Holash J, Hylton D, et al. Inaugural article VEGF trap complex formation measures production rates of VEGF providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci USA. 2007;104:18363–70. doi: 10.1073/pnas.0708865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawaoka H, Tsuji S, Tsujii M, et al. Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab Invest. 1999;79:1469–77. [PubMed] [Google Scholar]
- 18.Saito Y, Suzuki H, Tsugawa H, et al. Overexpression of miR-142-5p and miR-155 in gastric mucosa-associated lymphoid tissue (MALT) lymphoma resistant to Helicobacter pylori eradication. PLoS One. 2012;7:e47396. doi: 10.1371/journal.pone.0047396. [DOI] [PMC free article] [PubMed] [Google Scholar]