Abstract
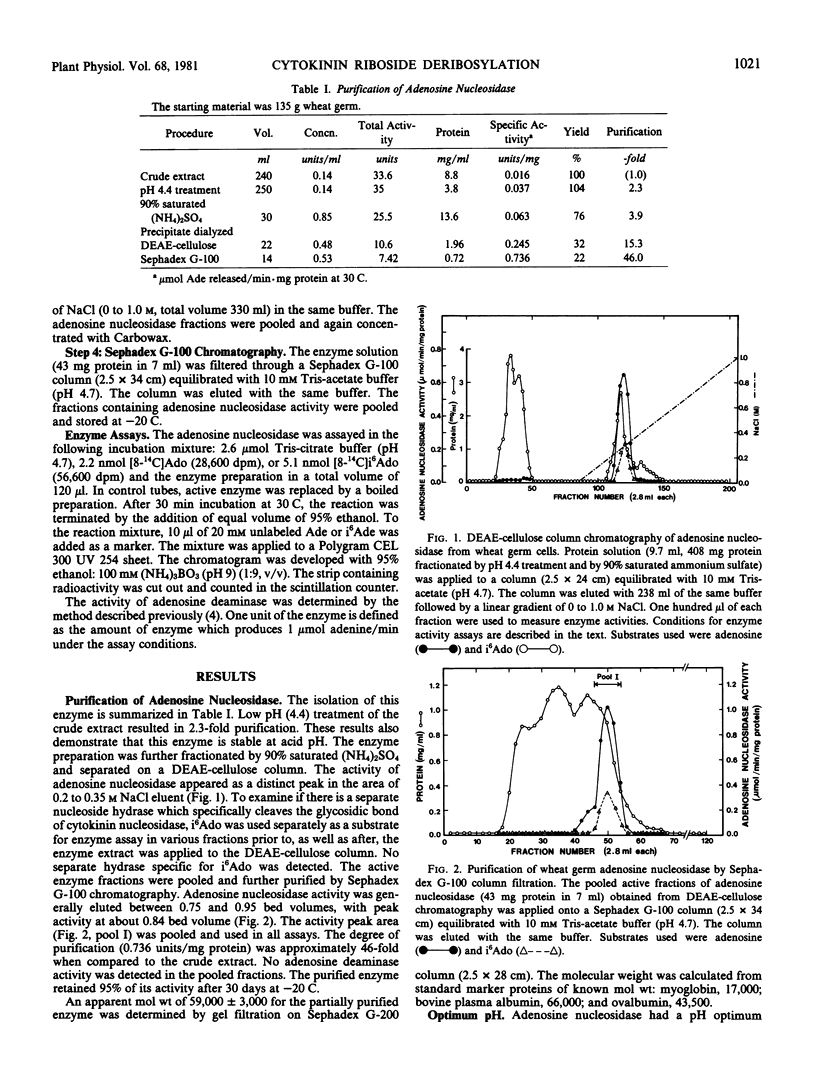
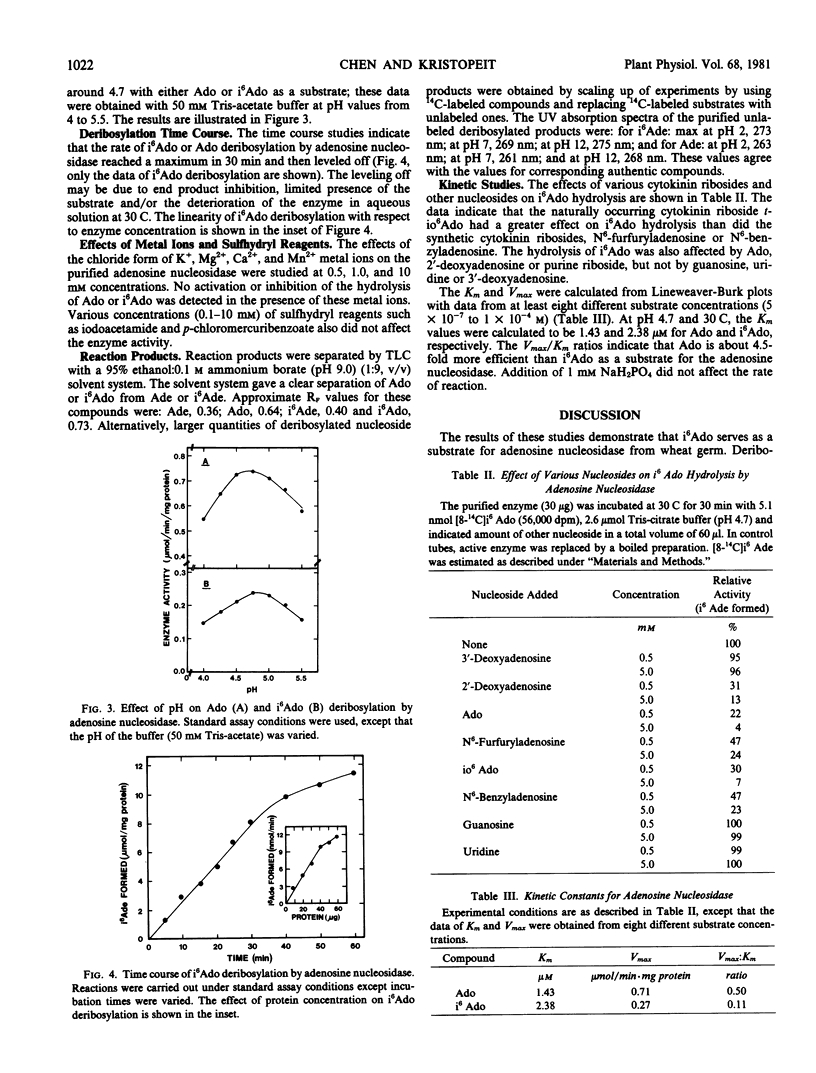
Adenosine nucleosidase (adenosine ribohydrolase, EC 3.2.2.7) which catalyzes the deribosylation of N6-(Δ2-isopentenyl)adenosine and adenosine to form the corresponding bases was partially purified from wheat germ. This enzyme (molecular weight 59,000 ± 3,000) deribosylates the ribonucleosides at an optimum pH of 4.7 Km values for the cytokinin nucleoside and adenosine are 2.38 and 1.43 micromolar, respectively, in 50 millimolar Tris-citrate buffer (pH 4.7) at 30 C. The presence of adenosine and other cytokinin nucleosides inhibited the hydrolysis of N6-(Δ2-isopentenyl)adenosine but this reaction was insensitive to guanosine, uridine, or 3′-deoxyadenosine. It is hypothesized that an adequate level of “active cytokinin” in plant cells may be provided through the deribosylation of cytokinin riboside in concert with other cytokinin metabolic enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Kristopeit S. M. Metabolism of Cytokinin : DEPHOSPHORYLATION OF CYTOKININ RIBONUCLEOTIDE BY 5'-NUCLEOTIDASES FROM WHEAT GERM CYTOSOL. Plant Physiol. 1981 Mar;67(3):494–498. doi: 10.1104/pp.67.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Melitz D. K. Cytokinin biosynthesis in a cell-free system from cytokinin-autotrophic tobacco tissue cultures. FEBS Lett. 1979 Nov 1;107(1):15–20. doi: 10.1016/0014-5793(79)80452-4. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Petschow B. Cytokinin biosynthesis in cultured rootless tobacco plants. Plant Physiol. 1978 Dec;62(6):861–865. doi: 10.1104/pp.62.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Petschow B. Metabolism of cytokinin: ribosylation of cytokinin bases by adenosine phosphorylase from wheat germ. Plant Physiol. 1978 Dec;62(6):871–874. doi: 10.1104/pp.62.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Melitz D. K., Petschow B., Eckert R. L. Isolation of cytokinin-binding protein from plant tissues by affinity chromatography. Eur J Biochem. 1980 Jul;108(2):379–387. doi: 10.1111/j.1432-1033.1980.tb04733.x. [DOI] [PubMed] [Google Scholar]
- Erion J. L., Fox J. E. Purification and Properties of a Protein Which Binds Cytokinin-active 6-Substituted Purines. Plant Physiol. 1981 Jan;67(1):156–162. doi: 10.1104/pp.67.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A., Schneider Z. Purification and characterization of adenosine nucleosidase from barley leaves. Biochim Biophys Acta. 1977 May 12;482(1):145–158. doi: 10.1016/0005-2744(77)90362-x. [DOI] [PubMed] [Google Scholar]
- Hall R. H. N6-(delta 2-isopentenyl)adenosine: chemical reactions, biosynthesis, metabolism, and significance to the structure and function of tRNA. Prog Nucleic Acid Res Mol Biol. 1970;10:57–86. doi: 10.1016/s0079-6603(08)60561-9. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Frye R. B., Werner D., Hawrelak S. D., Skoog F., Schmitz R. Y. On the "activation" of cytokinins. J Biol Chem. 1975 Sep 25;250(18):7343–7351. [PubMed] [Google Scholar]
- Laloue M., Terrine C., Guern J. Cytokinins: Metabolism and Biological Activity of N-(Delta-Isopentenyl)adenosine and N-(Delta-Isopentenyl)adenine in Tobacco Cells and Callus. Plant Physiol. 1977 Mar;59(3):478–483. doi: 10.1104/pp.59.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZELIS M., CREVELING R. K. AN ADENOSINE HYDROLASE FROM BRUSSELS SPROUTS. J Biol Chem. 1963 Oct;238:3358–3361. [PubMed] [Google Scholar]
- Moore F. H. A Cytokinin-binding Protein from Wheat Germ: Isolation by Affinity Chromatography and Properties. Plant Physiol. 1979 Oct;64(4):594–599. doi: 10.1104/pp.64.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAGI Y., HORECKER B. L. Purification and properties of a bacterial riboside hydrolase. J Biol Chem. 1957 Mar;225(1):77–86. [PubMed] [Google Scholar]


