Abstract
Background
The urine lipoarabinomannan (LAM) strip test has been suggested as a new point-of-care test for active tuberculosis (TB) among human immunodeficiency virus (HIV) infected individuals. It has been questioned if infections with nontuberculous mycobacteria (NTM) affect assay specificity. We set forth to investigate if the test detects LAM in urine from a Danish cystic fibrosis (CF) population characterized by a high NTM prevalence and negligible TB exposure.
Method
Patients followed at the Copenhagen CF Center were comprehensively screened for pulmonary NTM infection between May 2012 and December 2013. Urine samples were tested for LAM using the 2013 Determine™ TB LAM Ag strip test.
Results
Three-hundred and six patients had a total of 3,322 respiratory samples cultured for NTM and 198 had urine collected (65%). A total of 23/198 (12%) had active pulmonary NTM infection. None had active TB. The TB-LAM test had an overall positive rate of 2.5% applying a grade 2 cut-point as positivity threshold, increasing to 10.6% (21/198) if a grade 1 cut-point was applied. Among patients with NTM infection 2/23 (8.7%) had a positive LAM test result at the grade 2 cut-point and 9/23 (39.1%) at the grade 1 cut -point. Test specificity for NTM diagnosis was 98.3% and 93.1 for grade 2 and 1 cut-point respectively.
Conclusions
This is the first study to assess urine LAM detection in patients with confirmed NTM infection. The study demonstrated low cross-reactivity due to NTM infection when using the recommended grade 2 cut-point as positivity threshold. This is reassuring in regards to interpretation of the LAM test for TB diagnosis in a TB prevalent setting. The test was not found suitable for NTM detection among patients with CF.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-014-0655-4) contains supplementary material, which is available to authorized users.
Keywords: Lipoarabinomannan, LAM, NTM, Nontuberculous, Abscessus, Avium, CF
Background
The prevalence of nontuberculous mycobacteria (NTM) infection among patients with cystic fibrosis (CF) in Copenhagen rose from 7.4% in 2011 to 13.0% in 2014 [1],[2]. While NTM were previously thought to be transient colonizers of the CF lung, the two types of NTM seen in Danish CF patients; Mycobacterium abscessus complex (MABSC) and Mycobacterium avium complex (MAC) are now recognized as pathogens that can seriously affect morbidity and mortality [3]. The diagnosis of clinical significant pulmonary infection caused by NTM is a challenge and relies on culture of respiratory secretions and clinical criteria set forth by the American Thoracic Society (ATS) [4].
Lipoarabinomannan (LAM) is found in the outer cell wall of mycobacterial species, and has gained attention as a possible TB diagnostic target [5]. Antigen detection assays based on LAM are available both in the format of enzyme-linked immunosorbent assays (ELISAs) and as a point-of-care test. During mycobacterial infection, LAM is released from metabolically active or degrading mycobacterial cells into the blood stream with subsequent filtration by the kidneys, passing into urine [6],[7]. Clinical evaluation of the urine LAM test has consistently shown promising results for diagnosing TB among people living with HIV in resource constrained settings, although test sensitivity and specificity varies considerably among studies and in relation to the degree of immunodeficiency [8]-[12]. Two reviews of LAM tests have argued that false positive results could be caused by colonization of NTM and point to this as a key unresolved issue [6],[7]. No studies have looked specifically at excretion of LAM from NTM patients or addressed the extent to which NTM infection affects LAM test performance. We set forth to investigate LAM test performance in the Danish CF population characterized by a high NTM prevalence and no reported TB cases.
Methods
Patients and setting
The Copenhagen CF Center cares for 100 children and 216 adults with cystic fibrosis, accounting for 70% of the Danish CF population [13]. No TB case has ever been reported in this population despite extensive monitoring since the mid 1980ies. Patients are seen for clinical exams in an outpatient clinic every 4 weeks their entire lives. All patients have either chest radiograph or high-resolution computer tomography (HRCT) scans performed once a year. Since 2011, all CF patients are screened systematically once a year for mycobacteria with acid-fast microscopy and mycobacterial culture performed at the National Reference Laboratory of Mycobacteriology at Statens Serum Institute. Additionally from 2012, supplementary in-house mycobacterial culture is performed on all sputum samples, collected every four weeks. The CF Center used ATS criteria to classify NTM patients [4]. The criteria for NTM infection are: Pulmonary symptoms, nodular or cavitatory opacities on chest radiograph, or a HRCT scan that shows multifocal bronchiectasis with multiple small nodules after appropriate exclusion of other diagnoses. In addition, positive culture results from at least 2 separate sputum samples or positive culture results from at least 1 bronchial lavage. Subjects were included prospectively; all patients attending the CF center were eligible for inclusion if they had both respiratory samples and urine available for testing.
Respiratory samples and isolates
All respiratory samples were prospectively collected and consisted of expectorated sputa, laryngeal aspirates or bronchial lavage fluids. Samples were pre-treated with NaOH-N-acetyl cysteine to prevent bacterial overgrowth and inoculation was done on solid (Löwenstein-Jensen slants, SSI Diagnostica, Hilleroed, Denmark) and liquid culture media (MGIT 960, Becton Dickinson Microbiology Systems, Sparks, MD, USA) and incubated at 35°C to 37°C for 8 weeks. Locally, Burkholderia cepacia selective agar BCSA (Biomérieux) was used as growth medium for 14 days at 37 degrees Celsius with CO2. In case of a recent history of Aspergillus colonization, samples were pre-treated with 100 μL of amphotericin B. In case of growth of acid-fast bacilli, colonies were subcultured on 10% Blood Agar Base for identified by MALDI-TOF and 16S rRNA sequencing locally. Positive cultures were further identified to the species-level by a PCR based technique targeting the 23S rRNA gene followed by reverse hybridization and line probe assay (Hain Lifescience, Germany) and/or growth ability on Löwenstein-Jensen slants with 5% NaCl.
LAM testing
For the purpose of the study, urine was collected in sterile containers during outpatient visits and frozen within 60 minutes to -20°C and within 24 hours transferred to a -80°C freezer. Frozen urine samples were thawed to ambient temperature before applying The Determine™ TB LAM Ag test (Lot # 130103). Each sample was placed in a vortex briefly to ensure a homogenous mixture, from which 60 μL was aspirated and placed on the bottom of the sample pad. Between 25 and 35 minutes later the test was read and scored by two individuals (readers), who were blinded to their counterpart’s observations and to patient identity as well as the outcome of sputum analysis, through the use of anonymous study ID’s.
Interpretation of the LAM test
LAM test were performed and graded by study staff with previous experience in LAM testing and interpretation. Both readers visually inspected the strip and graded the intensity of any visualized test band by comparing test band color intensity to the color intensity of the series of bands on a manufacturer-supplied paper reference card. For this study, we made use of the original 2013 reference card provided with the commercial assay of the Determine TB-LAM test. The reference scale consists of 5 color intensity grades. The test band was graded as zero if no visual band appeared and graded 1 through 5 for a visualized band of equal intensity as those on the reference card. The mean values of reader 1 and 2’s recorded test band grade were used for data analysis except for analysis of inter-reader agreement. While we report results for both grade 2 and 1 cut-point, defined as a band of equal or greater intensity to the grade 2 and 1 cut-point respectively, we acknowledge that a positive result is defined as a band of equal or greater intensity to the grade 2 cut-point in a recent consensus paper on LAM test [14].
Clinical data collection
Clinical data including renal clearance measurements and MBL genotypes were extracted from patient files and the Danish CF registry. Data on NTM specific IgG antibody level, were captured from an ongoing study previously reported [15].
Statistical methods
We compared urine LAM results to patients with culture positive pulmonary NTM infection and patients with no evidence of NTM infection. Sensitivities, specificities and likelihood ratios (positive and negative) were calculated with 95% CIs. Receiver operator characteristic (ROC) analysis was performed to evaluate LAM test sensitivity and specificity based on the two different band intensity thresholds for LAM positivity. Inter-reader agreement was determined using Cohen's kappa coefficient. Baseline data were calculated as medians and interquartile ranges (IQR) for continuous variables, and percentages for categorical variables. Group comparisons were made using analysis of variance or Kruskal Wallis non-parametric tests. SPSS version 19.0 (SPSS Inc, Chicago, IL) was used for data analysis.
Ethical considerations
All participants received written and oral information about the purpose of the study and gave oral consent before clinical samples were taken. For children below 18 years informed consent was obtained from their parent or legal guardian. Exemption from the requirement of written consent was given by the Committee on Health Research Ethics in the Capital Region of Denmark, who approved the study (H-3-2012-098). Data handling was approved by the Danish Data Protection Agency (2007-58-0015).
Results
A total of 3,322 NTM cultures were performed on samples from 306 subjects, accounting for 97% of all patients at the Copenhagen CF Center, between May 2012 and December 2013. Of the 306 NTM screened CF patients, 198 (65%) had a urine sample collected and were included in the study. The average number of NTM cultures per patient was 9.9 (range: 1-23). A study outline including overall results is shown in Figure 1.
Figure 1.
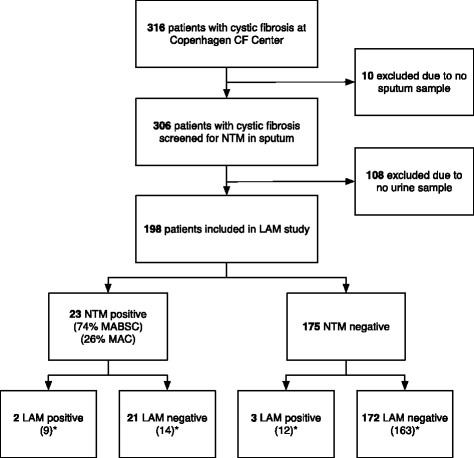
Flow chart of cystic fibrosis patients included in the LAM study. LAM = Lipoarabinomannan, NTM = nontuberculous mycobacteria, MABSC = Mycobacterium abscessus complex, MAC = Mycobacterium avium complex. (n) *Number of LAM positive patients if the grade 1 cut-point was used.
Characteristics of CF population
Among the 198 patients included in the study, 23 patients (12%) had at least one positive NTM culture within 12 months prior to LAM testing. Of these, 19 (83%) fulfilled ATS criteria for NTM disease and had been NTM infected a median of 3 years (IQR: 2 – 4 years) with a total of 276 positive NTM cultures combined. Fifteen patients (65%) received NTM treatment at the time of LAM testing. MABSC regiments typically consisted of moxifloxacin combined with a macrolide and either inhaled or intravenous amikacin, and for MAC: Myambutol, clarithromycin and rifampicin. No CF patient had positive cultures for Mycobacterium tuberculosis complex.
Distribution of determine TB-LAM test results in the CF population
All LAM test had a valid test result with a control bar appearing. The distribution of LAM test grading was: No band (grade 0), 177/198 (89.4%); grade 1, 18/198 (8.1%); grade 2, 1/198 (0.5%); grade 3, 2/198 (1.0%); grade 4, 2/198 (1.0%); grade 5, 0/198 (0%). As such, a total of 5 CF patients (2.5%) were LAM test positive when using a grade 2 cut-point. When lowering the positivity threshold to cut-point 1 we identified 21 LAM positive cases, increasing the positive rate to 10.6%. As there were no past or present cases of TB, all LAM positive results can be considered false positive in evaluation of the test performance for TB diagnosis.
Inter-rater agreement between reader 1 and 2 was 1.0 (SE 0.07) and 0.7 (SE 0.07) for grade 2 and grade 1 cut-point, respectively, calculated with kappa coefficient (Figure 2).
Figure 2.
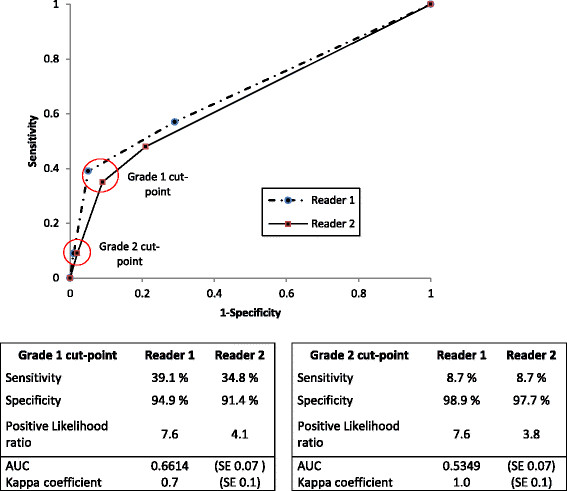
Receiver operator characteristic (ROC) curve for urine LAM test graded by two independent readers. Sensitivity, specificity, positive likelihood ratios, AUC and Kappa coefficient for grade 1 and grade 2 cut-point. LAM = Lipoarabinomannan.
TB LAM operating characteristics and NTM cross-reactivity
Receiver operating characteristic curve analysis showed that a positivity threshold of grade 2 cut-point minimized sensitivity, increased specificity and had better inter-rater agreement (Figure 2). Among patients with NTM infection 2/23 (8.7%, 95% CI: 1.3 – 28.1) had a positive LAM test result at the grade 2 cut-point and 9/23 (39.1%, 95% CI: 19.7 – 61.5) at the grade 1 cut -point. Test specificity for NTM diagnosis was 98.3% (95% CI: 95.1 - 99.6) and 93.1% (95% CI: 88.3 – 96.4) for grade 2 and 1 cut-point respectively. The positive likelihood ratio (LR+) was 5.1 (95% CI: 0.9 – 28.8) for grade 2 cut-point and statistically insignificant. The LR+ for grade 1 cut-point was 5.7 (95% CI: 2.7-12-0). TB-LAM test properties for NTM diagnosis (sensitivity, specificity and likelihood ratios) are shown in Table 1.
Table 1.
Performance of urine LAM test to detect NTM in 198 cystic fibrosis patients in Denmark
| Sensitivity | Specificity | PLR | NLR | |||||
|---|---|---|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | (95% CI) | (95% CI) | |||
| Grade 1 cut-point | 9/23 | 39.1 (19.7-61.5) | 163/175 | 93.1 (88.3-96.4) | 5.7 (2.7-12.0) | 0.7 (0.5-0.9) | ||
| Grade 2 cut-point | 2/23 | 8.7 (1.3-28.1) | 172/175 | 98.3 (95.1-99.6) | 5.1 (0.9-28.8) | 0.9 (0.8-1.1) | ||
PLR = Positive Likelihood Ratio, NLR = Negative Likelihood Ratio.
Factors associated with a positive LAM test among patients with verified NTM infection
Two NTM species were observed among the 23 culture positive patients, MABSC (74%) and MAC (26%). When using the cut-point 2 only two patients with NTM were LAM positive. These two were female, 20 and 12 years old, one was newly infected with MABSC and not yet in NTM treatment at the time of urine sampling; the other had received extensive NTM treatment for an unusually aggressive MABSC infection with consistently high bacterial loads in respiratory samples. Both had significant clinical and radiological deterioration as result of their infection. When stratifying NTM patients according to the cut-point 1 for LAM positivity, nine patients were positive and 14 were negative (Table 2). The proportion of female patients was higher among LAM positive than LAM negative NTM patients (80% vs. 30%, p = 0.04). Likewise the proportion of patients carrying a defective MBL gene was higher among LAM positive than LAM negative patients (56% vs. 7%, p = 0.01) despite a similar distribution of MBL alleles when comparing the 23 NTM patients to the background CF population (data not shown). No other statistically significant differences between LAM positive and LAM negative NTM patients were observed, including rate of chronic renal disease (p = 0.70), treatment rate (p = 0.58) or NTM specific IgG antibody levels (p = 0.08, data not shown), measured by a previously described ELISA method [15].
Table 2.
Characteristics of NTM culture positive patients stratified by LAM result at grade 1 cut-point
| Characteristic at time of first positive NTM culture | LAM positive ( n = 9) | LAM negative ( n = 14) | p -value |
|---|---|---|---|
| Median age (IQR), y | 19.7 (13.7 – 25.6) | 16.1 (11.9 – 24.0) | 0.41 |
| Female, % | 88.9 | 28.6 | 0.01 |
| Homozygote for Delta 508, % | 78.8 | 85.7 | 0.62 |
| Median FEV1% of pred. (IQR) | 80.0 (70.3 – 84.8) | 70.0 (66.3 – 83.5) | 0.59 |
| Diabetes mellitus, % | 11.1 | 28.6 | 0.32 |
| Structurally defective MBL allele*, % | 55.6 | 7.1 | 0.01 |
| Other chronic infection, % | 77.8 | 50.0 | 0.18 |
| Received NTM treatment, % | 55.6 | 57.1 | 0.94 |
| Number of positive NTM cultures | 11.0 (3.5 – 19.5) | 6.0 (1.8 – 14.5) | 0.68 |
| MABSC, % | 88.9 | 64.3 | 0.19 |
| Fulfilled ATS criteria for NTM, % | 88.9 | 78.6 | 0.52 |
| Median time positive for NTM (IQR), y | 2.5 (1.0 – 4.0) | 3.0 (1.0 – 5.0) | 1.00 |
| Chronic renal disease**, % | 0 | 14.3 | 0.24 |
MABSC = M. abscessus complex, MAC = M. avium complex, NTM = nontuberculous mycobacteria, IQR = Interquartile range, FEV1% = forced expiratory volume in 1 second as percent of predicted for age, height and sex, ATS = American Thoracic Society.
*XA/B (n = 3) or YA/B (n = 3) genotype.
**Defined as renal-clearance below 107 ml/min for adult men and 87 ml/min for adult women or in case of missing renal-clearance measurement, a mean serum creatinine at time of LAM testing over 105 or 90 μmol/L for adult men and women respectively or > 35, 40, 50, 55, 65 or 70 μmol/L for age groups 3-5 y, 6-7 y, 8-9 y, 10-11 y, 12-13 y and 14-15 y respectively.
Discussion
This is the first study to investigate urine LAM strip test performance in Danish CF patients infected with pulmonary NTM. At the grade 2 cut-point (“2013 reference card”) the LAM test had a sensitivity of 8.7% and specificity of 98.3% to detect NTM infection, which is below the acceptable performance level for NTM diagnosis among CF patients. However, the primary clinical utility of LAM point-of-care testing is not to detect NTM infection, but TB in HIV positive patients from high TB burden settings [6],[7],[16]. In this context pulmonary NTM infection has previously been suspected of contributing to false positive LAM tests results, and the potential impact of NTM infections on LAM assay specificity questioned [7]. Our results can inform this debate – although carried out in a very different population. It is important to note that the TB-LAM manufacturers changed the reference card in 2014 omitting the weakest color grade (grade 1 cut-point) such that grade 1 cut-point for the new 2014 reference card corresponds to the grade 2 cut-point at the original 2013 reference card. The modification was based on previous LAM test studies [12],[17] and the recommendations in a consensus paper by Lawn et al. [14]. To increase the evidence base we have reported results for both grade 1 and grade 2 cut-points. At the recommended grade 2 cut-point a low sensitivity (8.7%) and insignificant positive likelihood ratio demonstrate a limited rate of LAM test cross-reactivity of pulmonary NTM infection. At the grade 1 cut-point we found a higher sensitivity of 39.1% and a positive likelihood ratio that demonstrated statistically convincing evidence of cross-reactivity of NTM infection. The results implies that NTM infection is likely to be one of the factors contributing to the variation in LAM test specificity as suggested in a review of the LAM test to diagnose TB in HIV infected individuals [7]. Furthermore, LAM test cross-reactivity of NTM infection could partially explain the increase in specificity reported when switching from a grade 1 cut-point to grade 2 cut-point in recent evaluations of the LAM test for TB diagnosis [12],[17]. LAM is a component of both the TB and NTM cell wall and structural differences in LAM across mycobacterial species are well described [18]. This diversity of LAM molecules makes it likely, that different mycobacteria have different thresholds for test positivity in a LAM assay. Our study provides evidence for excretion of LAM from pulmonary NTM patients, although this excretion is apparently at lower concentrations, than that seen for active TB. The reason for this might be a lower bacterial burden in patients with NTM infection or diversity of LAM molecules. Indeed an in-vitro analysis of a LAM ELISA prototype showed that LAM could be detected from patients colonized with NTM, albeit only at significantly higher mycobacterial concentrations than TB [19].
The minimized risk of cross-reactivity from NTM infection observed in our study setting when using grade 2 cut-point (corresponding to the new grade 1 cut-point) is reassuring for the future use of LAM testing for TB diagnosis using the 2014 reference card. However, the need remains to assess the association between detection of urine LAM and NTM infection among the appropriate target population i.e. HIV positive patients from high TB burden settings. Four TB studies have reported a total of 10 cases of LAM positivity in HIV-positive patients with NTM infection instead of TB [8],[11],[20],[21], but information on NTM species was not reported. One of the papers, a clinical study on 291 patients from Tanzania, found LAM reactivity in four TB suspected patients, who were later found culture positive for NTM. The authors regarded the finding as background false-positivity [21], but our findings challenge this conclusion and instead suggest that genuine cross-reactivity to NTM was a likely explanation.
The impact of NTM infection on LAM test accuracy also depends on NTM prevalence among HIV-positive patients. The burden of TB is the leading cause of morbidity and mortality in HIV patients [22],[23]. A recent autopsy study among HIV positive individuals in South Africa found M. tuberculosis in 26/39 (67%) of the mycobacterial cases and comparatively 2/39 (5%) with NTM [24]. This confirms findings in a previous meta-analysis of all autopsy studies of HIV patients from Sub-Saharan Africa were TB was considered a cause of death in 32-45% of 593 autopsied adults [25]. However, there are reports that NTM disease could be underreported in some settings [26],[27] and that misclassification of NTM as TB might occur [28]-[30]. A study from Nigeria recently found a NTM prevalence of 15% among patients with presumptive TB [31]. Unlike TB, NTM are environmental pathogens with surprising disparity in incidence in different geographical regions [4]. The reasons for this heterogeneity in NTM pulmonary disease distribution is not well understood and even less is known about these factors in Sub-Saharan Africa.
The question of whether the LAM point-of-care test could be used to screen for pulmonary NTM infection in a resource constrained setting remains open. This would certainly require supplementary species discrimination, but given the manufacturers change in reference card and the low sensitivity at the grade 2 cut-point, the test has no immediate applicability for this purpose. Additionally, in a Western European setting, LAM testing for the purpose of NTM diagnosis is inferior to the readily available gold standard: mycobacterial culture, microscopy and PCR.
Why NTM patients who were LAM positive using cut-point 1 were statistically more likely to carry a MBL variant allele and were more likely to be female remains unknown. It is well described that female patients have worse clinical outcomes in CF [32], but there were no statistically significant differences in lung function, renal clearance or CF mutations in the present comparison to further explain this association. MBL insufficiency in CF patients is associated with a poorer prognosis, specifically earlier acquisition of chronic Gram-negative infection and progression to end stage lung disease [33]-[35]. Previous TB studies have suggested that carriers of MBL variant alleles might be either more [36],[37] or less [38],[39] susceptible to TB infection, but to our knowledge this has not previously been studied for NTM. The NTM patients in the current study had the same distribution of MBL alleles as the background CF population.
It has been proposed that excretion of LAM in urine may depend on the kidneys ability to retain immune-bound LAM [40]. In our study we could not confirm any link between test sensitivity and chronic renal disease as measured by renal clearance rates. Nor was there any association between NTM specific IgG levels and LAM positivity, as might be expected if immune-complexes influenced the level of antigenuria. Although not statistically significant, a higher proportion of patients who were positive using cut-point 1 had a concomitant, chronic Gram-negative pulmonary infection, most commonly Pseudomonas aeruginosa followed by Stenotrophomonas maltophilia. While Nocardia species have been shown to cause cross-reaction to LAM-test, this has never been shown for Gram-negative bacteria [9]. Norcardia are seen among CF patients, but no LAM cases in the present study had a history of Norcadia infection. The reasons for the background false positivity seen at both cut-points could thus not be answered.
Study limitations include that 35% of the patient population were excluded due to lack of urine. However, no selection bias could be identified as patients were consecutively enrolled during routine visits independently of clinical or microbiological characteristics. A majority of NTM patients received NTM specific treatment at the time of urine collection, which might affect LAM test performance by reducing the mycobacterial burden.
The primary strength of the study was the prospective design in a well-described homogenous population with unparalleled visit-frequency, negligible TB exposure and a mean of 10 mycobacterial cultures per included individual.
Conclusion
This study of a comprehensively screened CF population demonstrated a low level (8.7%) of LAM excretion from patients with NTM infection when using the recommended grade 2 cut-point as positivity threshold, but high level (39.1.%) at the outdated grade 1 cut-point. This is reassuring for the intended utility of the revised “2014” LAM test to diagnose TB among patients infected with HIV in resource constrained settings, where access to mycobacterial cultures is limited. The LAM strip test was not suitable for NTM diagnostic use in cystic fibrosis patients due to superior alternatives.
Acknowledgements
The authors wish to thank the staff at the Copenhagen CF Center for helping in sample collection and a special thanks to Lena Nørregaard and Ulla Rydahl Johansen for technical assistance.
Abbreviations
- LAM
Lipoarabinomannan
- TB
Tuberculosis
- HIV
Human immune deficiency virus
- CF
Cystic fibrosis
- NTM
Nontuberculous mycobacteria
- MABSC
Mycobacterium abscessus complex
- MAC
Mycobacterium avium complex
- ELISA
Enzyme-linked immunosorbent assay
- BSCA
Burkholderia cepacia selective agar
- HRCT
High resolution computer tomography
- ATS
American thoracic society
- IQR
Interquartile range
- CI
Confidence interval
- SE
Standard error
- FPR
False positive rate
- FEV1%
forced expiratory volume in 1 second as percent of predicted for age, height and sex
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
SB and IJ conceptualized the study. TQ, IJ and SB created the study design and carried out the LAM testing. NH carried out the microbiological data collection and TK and TP collected clinical data. Data analysis and statistical analysis was performed by TQ, SB and AA. The manuscript was drafted with the help of all authors, who also approved the final manuscript.
Contributor Information
Tavs Qvist, Email: tavs.qvist@gmail.com.
Isik S Johansen, Email: isik@dadlnet.dk.
Tania Pressler, Email: tania.pressler@gmail.com.
Niels Høiby, Email: hoiby@dadlnet.dk.
Aase B Andersen, Email: bengaard@dadlnet.dk.
Terese L Katzenstein, Email: terese.katzenstein@regionh.dk.
Stephanie Bjerrum, Email: steph@medicinsk.dk.
References
- 1.Qvist T, Pressler T, Thomsen VO, Skov M, Iversen M, Katzenstein TL. Nontuberculous mycobacterial disease is not a contraindication to lung transplantation in patients with cystic fibrosis: a retrospective analysis in a Danish Patient Population. Transplant Proc. 2012;45(1873–2623):342–345. doi: 10.1016/j.transproceed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 2.Qvist T, Gilljam M, Jönsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kötz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Høiby N, Katzenstein TL: Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 2014. ., [http://dx.doi.org/10.1016/j.jcf.2014.08.002] [DOI] [PMC free article] [PubMed]
- 3.Esther CR, Jr, Esserman DA, Gilligan P, Kerr A, Noone PG, Esther CR. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros. 2010;9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, Wallace RJ., Jr An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar P, Biswas D, Sindhwani G, Rawat J, Kotwal A, Kakati B. Application of lipoarabinomannan antigen in tuberculosis diagnostics: current evidence. Postgrad Med J. 2014;90(1061):155–163. doi: 10.1136/postgradmedj-2013-132053. [DOI] [PubMed] [Google Scholar]
- 6.Minion J, Leung E, Talbot E, Dheda K, Pai M, Menzies D. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur Respir J. 2011;38:1398–1405. doi: 10.1183/09031936.00025711. [DOI] [PubMed] [Google Scholar]
- 7.Lawn SD: Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis 2012, 12:103., [DOI] [PMC free article] [PubMed]
- 8.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dheda K, Davids V, Lenders L, Roberts T, Meldau R, Ling D, Brunet L, van Zyl Smit R, Peter J, Green C, Badri M, Sechi L, Sharma S, Hoelscher M, Dawson R, Whitelaw A, Blackburn J, Pai M, Zumla A: Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One 2010, 5:e9848., [DOI] [PMC free article] [PubMed]
- 10.Drain PK, Losina E, Coleman SM, Giddy J, Ross D, Katz JN, Walensky RP, Freedberg KA, Bassett IV: Diagnostic accuracy of a point-of-care urine test for tuberculosis screening among newly-diagnosed hiv-infected adults: a prospective, clinic-based study. BMC Infect Dis 2014, 14:110., [DOI] [PMC free article] [PubMed]
- 11.Gounder CR, Kufa T, Wada NI, Mngomezulu V, Charalambous S, Hanifa Y, Fielding K, Grant A, Dorman S, Chaisson RE, Churchyard GJ. Diagnostic accuracy of a urine lipoarabinomannan enzyme-linked immunosorbent assay for screening ambulatory HIV-infected persons for tuberculosis. J Acquir Immune Defic Syndr. 2011;58:219–223. doi: 10.1097/QAI.0b013e31822b75d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakiyingi L, Moodley VM, Manabe YC, Nicol MP, Holshouser M, Armstrong DT, Zemanay W, Sikhondze W, Mbabazi O, Nonyane BAS, Shah M, Joloba ML, Alland D, Ellner JJ, Dorman SE. Diagnostic accuracy of a rapid urine lipoarabinomannan test for tuberculosis in HIV-infected adults. J Acquir Immune Defic Syndr. 2014;66(3):270–279. doi: 10.1097/QAI.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudsen PK, Olesen HV, Høiby N, Johannesson M, Karpati F, Laerum BN, Meyer P, Pressler T, Lindblad A. Differences in prevalence and treatment of Pseudomonas aeruginosa in cystic fibrosis centres in Denmark, Norway and Sweden. J Cyst Fibros. 2009;8:135–142. doi: 10.1016/j.jcf.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD, Dheda K, Kerkhoff AD, Peter JG, Dorman S, Boehme CC, Nicol MP: Determine TB-LAM lateral flow urine antigen assay for HIV-associated tuberculosis: recommendations on the design and reporting of clinical studies. BMC Infect Dis 2013, 13:407., [DOI] [PMC free article] [PubMed]
- 15.Qvist T, Pressler T, Katzenstein TL, Hoiby N: Elevated levels of antibodies against Mycobacterium abscessus in cystic fibrosis patients is associated with active lung disease. PediatrPulmonol 2012, 47(Suppl 3(1099-0496)):335.,
- 16.Peter J, Green C, Hoelscher M, Mwaba P, Zumla A, Dheda K. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr Opin Pulm Med. 2010;16:262–270. doi: 10.1097/MCP.0b013e328337f23a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peter JG, Theron G, van Zyl-Smit R, Haripersad A, Mottay L, Kraus S, Binder A, Meldau R, Hardy A, Dheda K. Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. 2012;40:1211–1220. doi: 10.1183/09031936.00201711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 19.Boehme C, Molokova E, Minja F, Geis S, Loscher T, Maboko L, Koulchin V, Hoelscher M. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg. 2005;99:893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker L-G, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–1880. doi: 10.1097/QAD.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- 21.Reither K, Saathoff E, Jung J, Minja LT, Kroidl I, Saad E, Huggett JF, Ntinginya EN, Maganga L, Maboko L, Hoelscher M: Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC Infect Dis 2009, 9:141., [DOI] [PMC free article] [PubMed]
- 22.Etard J-F, Ndiaye I, Thierry-Mieg M, Guèye NFN, Guèye PM, Lanièce I, Dieng AB, Diouf A, Laurent C, Mboup S, Sow PS, Delaporte E. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 23.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 24.Wong EB, Omar T, Setlhako GJ, Osih R, Feldman C, Murdoch DM, Martinson NA, Bangsberg DR, Venter WDF: Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS One 2012, 7:e47542., [DOI] [PMC free article] [PubMed]
- 25.Cox JA, Lukande RL, Lucas S, Nelson AM, Van Marck E, Colebunders R. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev. 2010;12(4):183–194. [PubMed] [Google Scholar]
- 26.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002;23:553–567. doi: 10.1016/S0272-5231(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 27.Bonard D, Messou E, Seyler C, Vincent V, Gabillard D, Anglaret X. High incidence of atypical mycobacteriosis in African HIV-infected adults with low CD4 cell counts: a 6-year cohort study in Côte d’Ivoire. AIDS. 2004;18:1961–1964. doi: 10.1097/00002030-200409240-00015. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy KD, Cain KP, Winthrop KL, Udomsantisuk N, Lan NTN, Sar B, Kimerling ME, Kanara N, Lynen L, Monkongdee P, Tasaneeyapan T, Varma JK. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. Am J Respir Crit Care Med. 2012;185:981–988. doi: 10.1164/rccm.201107-1327OC. [DOI] [PubMed] [Google Scholar]
- 29.Buijtels PCAM, van der Sande MAB, Parkinson S, Verbrugh HA, Petit PLC, van Soolingen D. Isolation of non-tuberculous mycobacteria at three rural settings in Zambia; a pilot study. Clin Microbiol Infect. 2010;16:1142–1148. doi: 10.1111/j.1469-0691.2009.03072.x. [DOI] [PubMed] [Google Scholar]
- 30.Crump JA, van Ingen J, Morrissey AB, Boeree MJ, Mavura DR, Swai B, Thielman NM, Bartlett JA, Grossman H, Maro VP, van Soolingen D. Invasive disease caused by nontuberculous mycobacteria, Tanzania. Emerg Infect Dis. 2009;15:53–55. doi: 10.3201/eid1501.081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, Blattner W: Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One 2013, 8:e63170., [DOI] [PMC free article] [PubMed]
- 32.Kulich M, Rosenfeld M, Goss CH, Wilmott R. Improved survival among young patients with cystic fibrosis. J Pediatr. 2003;142:631–636. doi: 10.1067/mpd.2003.197. [DOI] [PubMed] [Google Scholar]
- 33.Chalmers JD, Fleming GB, Hill AT, Kilpatrick DC. Impact of mannose-binding lectin insufficiency on the course of cystic fibrosis: a review and meta-analysis. Glycobiology. 2011;21(1460-2423 (Electronic)):271–282. doi: 10.1093/glycob/cwq161. [DOI] [PubMed] [Google Scholar]
- 34.Olesen HV, Jensenius JC, Steffensen R, Thiel S, Schiøtz PO. The mannan-binding lectin pathway and lung disease in cystic fibrosis–disfunction of mannan-binding lectin-associated serine protease 2 (MASP-2) may be a major modifier. Clin Immunol. 2006;121:324–331. doi: 10.1016/j.clim.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Høiby N, Schwartz M, Koch C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–437. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvaraj P, Narayanan PR, Reetha AM. Association of functional mutant homozygotes of the mannose binding protein gene with susceptibility to pulmonary tuberculosis in India. Tuber Lung Dis. 1999;79:221–227. doi: 10.1054/tuld.1999.0204. [DOI] [PubMed] [Google Scholar]
- 37.Søborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777–782. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- 38.Garred P, Richter C, Andersen AB, Madsen HO, Mtoni I, Svejgaard A, Shao J. Mannan-binding lectin in the sub-Saharan HIV and tuberculosis epidemics. Scand J Immunol. 1997;46:204–208. doi: 10.1046/j.1365-3083.1997.d01-111.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoal-Van Helden EG, Epstein J, Victor TC, Hon D, Lewis LA, Beyers N, Zurakowski D, Ezekowitz AB, Van Helden PD. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr Res. 1999;45(4 Pt 1):459–464. doi: 10.1203/00006450-199904010-00002. [DOI] [PubMed] [Google Scholar]
- 40.Wood R, Racow K, Bekker L-G, Middelkoop K, Vogt M, Kreiswirth BN, Lawn SD: Lipoarabinomannan in urine during tuberculosis treatment: association with host and pathogen factors and mycobacteriuria. BMC Infect Dis 2012, 12:47., [DOI] [PMC free article] [PubMed]


