Abstract
Objective:
To assess and compare the efficacy of 3% garlic extract, 0.2% tea tree oil, 0.2% chlorhexidine, 0.05% cetylpyridinium chloride, and ultravoilet (UV) toothbrush sanitizing device as toothbrush disinfectants against Streptococcus mutans.
Materials and Methods:
A double blind randomized controlled parallel study was done on 210 dental students. The subjects were divided into one control group using distilled water and five study groups representing 0.2% tea tree oil, 3% garlic extract, 0.2% chlorhexidine gluconate, 0.05% cetylpyridinium chloride and UV toothbrush sanitizing device. Participants were provided with new toothbrushes and toothpastes for both baseline and intervention phases. The toothbrushes were collected after two weeks for microbial analysis in both phases. The data were analysed and compared using appropriate statistical analysis.
Results:
On comparing pre– and post–intervention, S. mutans colony counts, a highly significant (P < 0.001) difference was observed in all the groups. Differences of 77.74 colony forming units (CFU) in tea tree oil group, 102.87 CFU in garlic group, 68.13 CFU in chlorhexidine group, 82.47 CFU in cetylpyridinium group and 42.67 CFU in UV toothbrush sanitizer group were observed. Garlic group showed the highest reduction (100%) whereas UV toothbrush sanitizer group showed the least reduction (47.4%) in S. mutans colonies.
Conclusions:
The antimicrobial agents used in this study effectively reduced the S. mutans counts and hence can be considered as toothbrush disinfectants to prevent dental caries. The 3% garlic was the most effective among the antimicrobial agents.
Keywords: Antimicrobial agents, chlorhexidine, garlic, S. mutans, toothbrush disinfection
INTRODUCTION
Dental caries continues to be a major oral health problem affecting people in developing and underdeveloped countries. The prevention of dental diseases is associated with a reduction in the number of microorganisms. The oral cavity is free of microorganisms at birth. There is a great variety of microbes in the oral cavity during the first day of life such as Streptococcus, Staphylococcus, Neisseria, Candida, Lactobacillus, and coliforms. Transmission of Streptococcus mutans was found to start even from the fourth month of a predentate child[1,2] either directly from the saliva of the mother or other family members or indirectly through fomites such as spoons, cups, toys, or contaminated toothbrushes.[3]
Tooth brushing is one of the important procedures in prevention of dental caries. Toothbrushes are manufactured free of microorganisms. After a single use, however, toothbrushes may become contaminated with a wide array of bacteria, viruses, yeasts, and fungi, present both in the oral cavity and in the external environment.[4,5] In toothbrushes used by children in a day care center heavy growth of enteric yeasts and molds was reported.[6]
Microorganisms can remain viable on toothbrush bristles for periods ranging from 24 hours to seven days.[7] The routine use of contaminated toothbrushes might contribute to disseminate microorganisms within the oral cavity of the same person or between different individuals. A method to reduce contamination of toothbrushes might be a helpful means to avoid re–infection and cross–infection risks. There are various methods for toothbrush decontamination, which include immersion in antimicrobial solutions like chlorhexidine,[8,9] triclosan,[10] cetylpyridinium chloride,[11,12] Listerine,[13] ultraviolet (UV) radiation,[14] spraying of disinfecting solutions on bristles,[7,15] use of microwave oven[11] etc.
With the rise in bacterial resistance to antibiotics, there is considerable interest in the development of natural antimicrobial agents for control of infection. Several studies have proved antibacterial effects of garlic[16,17] and tea tree oil extracts[17,18] on oral microorganisms.
Garlic (Allium sativum) is a flavor component used in a wide variety of dishes and has been used in native medicine since ancient times for the treatment of many diseases. There are a few reports on garlic antimicrobial activity against oral microorganisms in which 2.5[19] and 3%[20] concentrations showed good antimicrobial activity against S. mutans. The medicinal properties of the tea tree oil (TTO) were first reported by Penfold in the 1920s. The volatile essential oil is derived from the Australian native plant Melaleuca alternifolia. This oil is effective against a high number of gram–positive and gram–negative bacteria, viruses, protozoa, as well as fungi.[21] In some studies, maximum bactericidal concentrations of 2.5,[22] 2,[18] 0.25,[23] and 0.2%[19] TTO were reported against streptococcus species.
Chlorhexidine gluconate (CHX), which has been studied extensively for many years, is currently one of the safest and most efficient chemotherapeutic agents against S. mutans and dental caries. Different concentrations of 0.2%,[24] 0.12%,[25,26,27] 0.06%[25] CHX have been shown to act against S. mutans. CHX used as a mouth rinse or in dental gels applied by toothbrush has been reported to result in a low-to-moderate reduction in S. mutans counts in plaque and saliva, and in only a trend toward less caries.[28]
Cetylpyridinium chloride (CPC) is a quarternary ammonium compound used in a wide variety of antiseptic mouth rinse products. CPC 0.07%[29] and 0.05%[11] have shown antibacterial activities.
Various UV toothbrush sanitizing devices are available in the market. Many of these new gadgets allow for multiple toothbrushes to be cleaned at the same time, and a number of them accommodate different sizes of brushes, like ones that are electric or kid-sized. UV radiation is effective against a number of bacteria, yeasts and viruses.[14,23]
There is limited information available comparing the potency of garlic, tea tree oil, CPC, CHX, and UV light as toothbrush disinfectants.
This study commenced with a null hypothesis: There is no difference in the antibacterial effect of garlic, tea tree oil, CPC, CHX and UV light against S.mutans as toothbrush disinfectants.
Hence, the present study was conducted with the following objectives:
To determine the efficacy of 3% garlic, 0.2% tea tree oil, 0.05% cetylpyridinium chloride, 0.2% chlorhexidine, and UV sanitizing device in reducing the S. mutans count in the contaminated toothbrush
To compare the potency of these antibacterial agents in reducing S. mutans count on the used toothbrushes.
MATERIALS AND METHODS
This study was a comparative experimental trial done on dental students aged 18 to 25 years residing in the hostel of a dental college. Ethical clearance was obtained from the Ethical Review Board of the dental college.
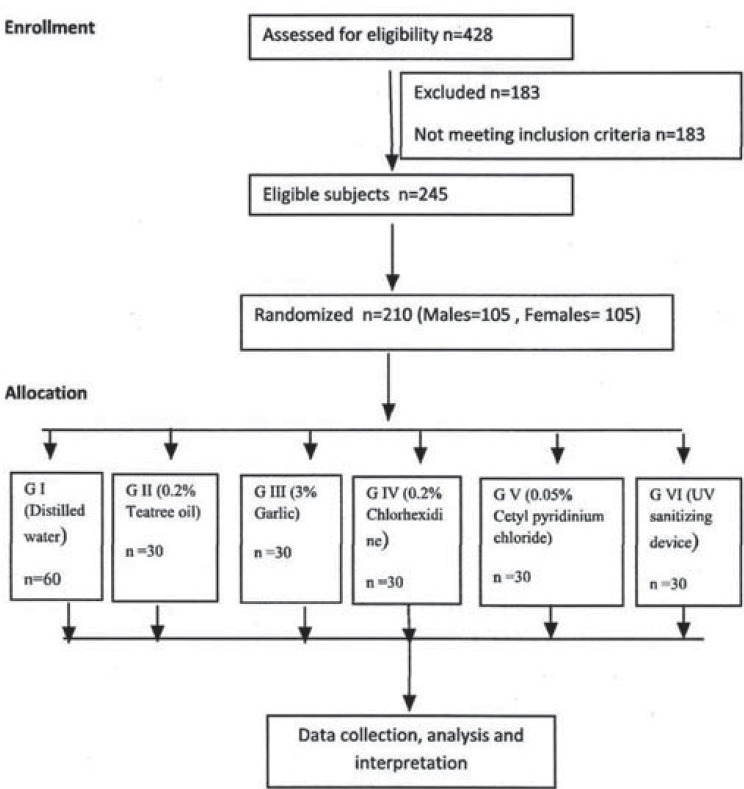
Out of the 428 dental students screened, 245 students fulfilled the inclusion criteria (dental students aged between 18 and 25 years who reside in the hostel inside the institution campus and having decayed, missing, and filled teeth index (DMFT) score not more than 3 and exclusion criteria (those undergoing orthodontic treatment, those with extensive intraoral prosthesis and using antibiotics or antiseptic mouthwashes for at least three months prior to the study or currently).
From the eligible 245 study participants, final required 210 subjects were randomly selected [Figure 1]. Written informed consent was obtained from all the subjects. A specially designed proforma was used to record information on each participant's demographic details, medical and dental history, and other observed findings. The caries experience was calculated using DMFT index by Klein, Palmer and Knutson (WHO modified 1997). Prior to the study, the investigator (1) was trained and calibrated. A pilot study on 24 subjects who were not included in the final study was carried out by the investigator.
Figure 1.
Schematic representation of methodology
The present study followed a double blind randomized controlled parallel group design. The study subjects and the microbiologist remained blinded throughout the study. The duration of the study was three months. The selected 210 subjects were divided into one control group [Group I (G I) - distilled water] and 5 study groups [Group II (G II) - 0.2% tea tree oil, Group III (G III) - 3% garlic extract, Group IV (G IV) - 0.2% chlorhexidine gluconate, Group V (G V) - 0.05% cetylpyridinium chloride, and Group VI (G VI) - UV toothbrush sanitizing device). Each group consisted of 30 subjects (15 males and 15 females). The study procedure was divided into two phases for each group: Baseline phase and intervention phase.
For the study purpose, commercially available soft-bristled toothbrushes (Oral B shiny clean) and fluoridated toothpastes (Pepsodent) were provided to the participants. The students were provided precoded toothbrushes and toothpastes before each phase of the study. The participants were instructed to brush their teeth twice daily— in the morning before breakfast and in the night after dinner — and to rinse the toothbrushes under running tap water for 30 seconds after brushing. The subjects were instructed to keep their toothbrushes in disposable glasses provided to them and leave them for drying. Subjects were periodically reminded to follow the instructions through personal contact and over the mobile phone.
Five new toothbrushes, which were freshly opened from the packets, were subjected to microbial analysis to ensure that the new toothbrushes were free from contamination before their use by the study subjects.
Baseline phase
After giving necessary instructions, participants were provided new precoded toothbrushes and dentifrices. The used precoded toothbrushes were collected back from the participants after two weeks. These toothbrushes were stored in separate disposable sterile sealed plastic pouches and taken to the microbiological lab (Wingene Biotech Research Labs, Bangalore) within two hours of collecting the toothbrushes for baseline microbial analysis.
Preparation of solutions
For preparing 3% garlic extract, 100 g of fresh garlic obtained from local market was peeled and blended in a mixer and the extract was filtered first through a muslin cloth and then into a jar using a Whatman's No. 1 filter paper. It was then sterilized by filtration using a membrane filter of 0.2 μm.[19,20] To make it 3%, 12 ml of garlic extract was mixed with distilled water. Garlic solution was prepared freshly on the day of the experiment.
For preparing 0.2% TTO, 0.6 ml of commercially available TTO (Mother Herbs Private Limited, New Delhi) was mixed with distilled water and 0.5% Tween 80. As TTO is sparingly soluble in water, an emulsifying agent Tween 80 (with no antimicrobial activity) was added to enhance the solubility.
Chlorhexidine 0.2% (Hexidine, ICPA Health Products Ltd, India) was used, which was readily available in the market.
Cetylpyridinium chloride powder 15 mg (CDH Laboratory, India) was mixed with distilled water to obtain 0.05% cetylpyridinium chloride solution.
Intervention phase
On the day of collecting the first set of toothbrushes, another set of new precoded toothbrushes and toothpastes were provided to each group. Subjects were once again reminded on the necessary instructions to be followed. After two weeks the brushes were collected and stored in separate disposable sterile plastic pouches and sent to the laboratory immediately. The collected toothbrushes were aseptically introduced into separate test tubes containing the test solutions — distilled water (control), 0.2% tea tree oil, 3% garlic extract, 0.2% chlorhexidine gluconate and 0.05% cetylpyridinium chloride. The head of toothbrushes were kept immersed in the solutions for 12 hours. The UV toothbrush sanitizing device (VIOLight Toothbrush Sanitizer, Violight Inc., New York) was used as instructed by the manufacturer for G VI toothbrushes. At a time four toothbrushes were kept upside down inside the device with their bristles facing the UV light. Once the device completed its 7–minute cycle, it turns itself off.
After the prescribed time, the toothbrushes were withdrawn and subjected to microbial analysis for evaluation of post intervention S. mutans counts on the toothbrushes.
Laboratory method
On the open end of the test tube, the handle of the brush was covered and well plugged with sterile cotton pellet. The sample was subjected to a cyclomixer. Mitis salivarius agar medium was used. The plates were incubated for 24-48 hours at 37°C in an incubator. The plates were opened after 48 hours and the colonies were counted and were expressed as colony forming units (CFU)/ml. The laboratory procedure was similar for both the phases.
Descriptive statistical analysis was carried out in the present study. Significance was assessed at 5% level of significance. The F test (one way analysis of variance (ANOVA)) and Tukey's post-hoc test were used. The Statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0, and R environment ver. 2.11.1, were used for the analysis of the data.
RESULTS
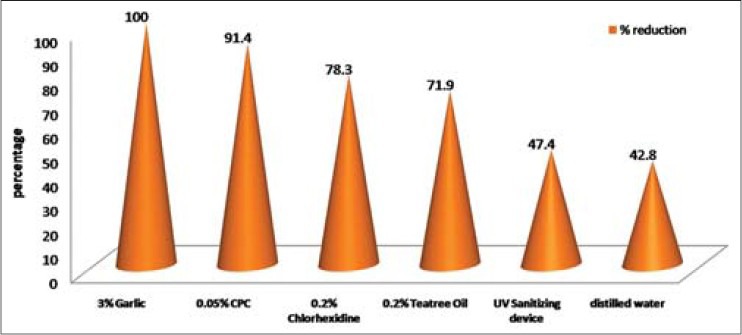
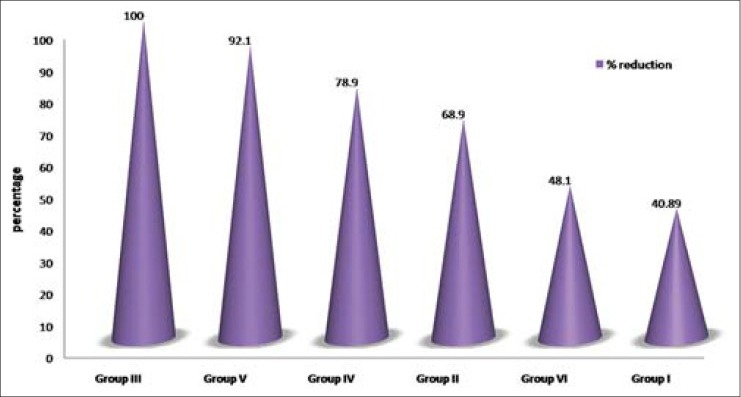
The data in Table 1 shows the comparison of mean CFU of S. mutans in control and experimental groups at baseline and intervention phases. In all the six groups, there were significant differences (P < 0.001) in the reduction of S. mutans counts from the baselines. The mean percentage reductions in S. mutans CFU are shown in Figure 2. The highest reduction in CFU was seen in the garlic group and the least reduction among antimicrobial agents was for UV sanitizing device. Among all the groups, distilled water showed the least reduction in S. mutans counts. Figures 3 and 4 show that in males and females garlic showed maximum reduction and distilled water showed least reduction in the bacterial counts, compared to all other agents.
Table 1.
Comparison of mean colony forming units of Streptococcus mutans in control and experimental groups at baseline and post-intervention
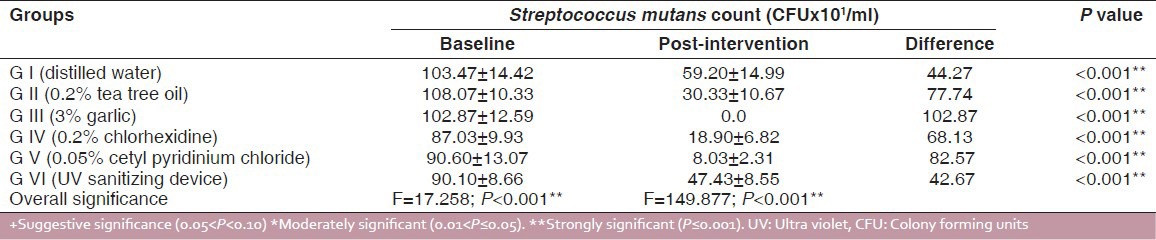
Figure 2.
Mean percentage reduction of Streptococcus mutans colony forming units in six study groups from baseline
Figure 3.
Comparison of percentage reduction of Streptococcus mutans colony forming units in six study groups from baseline among males
Figure 4.
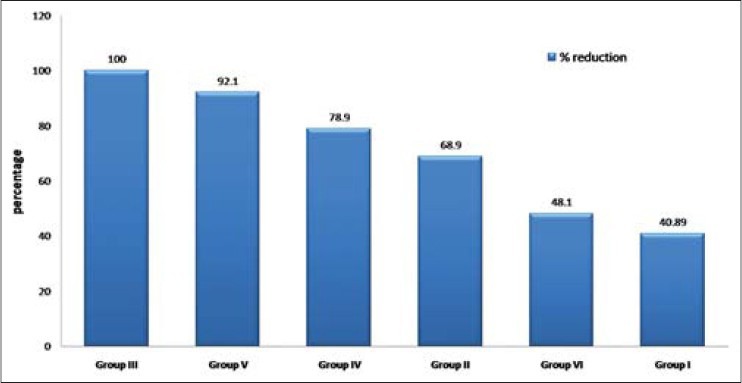
Comparison of mean percentage reduction of Streptococcus mutans colony forming units in six study groups from baseline among females
DISCUSSION
The most common device used for oral hygiene maintenance is a toothbrush. As most people live in communities, there is a further risk of cross-infection — microbes on one brush being transferred to others kept in close proximity or even in sharing of toothbrushes.[30]
American Dental Association (ADA) recommends routine change of toothbrushes every three months. Many patients, however, reported psychological, economical, and environmental barriers to changing their toothbrushes frequently.[31,32] Sterilizing toothbrushes in between uses has its own drawbacks.[30,33] Hence, establishing an easy and effective method for disinfecting a toothbrush would be an important and economical way to prevent reinfection with oral diseases.
In the present study involving 210 dental students, an attempt has been made to evaluate and compare the efficacy of different antimicrobial substances as toothbrush disinfectants against S. mutans. All the study subjects had a DMFT less than 3. The WHO classifies less than 3 DMFT to be ideal for 18-year-old individuals.[34] The same type of commercially available toothbrushes and toothpastes were supplied to all the participants. The participants were provided oral hygiene instructions for standardization of the study procedure.
Storage conditions of toothbrushes are an important factor for bacterial survival. The number of bacteria on the toothbrushes kept in aerated conditions after use decreases more quickly than on brushes stored in plastic containers.[31,35] Toothbrushes should be stored in the bedroom rather than in the bathroom, which is the most contaminated room in the house.[32] To avoid any such factors, participants were instructed to use their own toothbrushes and toothpastes provided to them and to keep their toothbrushes in disposable glasses in an open room on their individual tables.
The toothbrushes from the participants were collected after two weeks (14 days), which was similar to an earlier study.[23] However, different study intervals like five days,[10,31] seven days,[11,13,15] 48 hours,[36] and 24 hours,[36] have been used in different studies.
The toothbrushes were immersed in disinfecting solutions for 12 hours, which was similar to other studies.[10,13] Twice-a-day tooth brushing was used in this study. Hence, a soaking period of 12 hours in disinfectant solutions was employed. However, other studies have used different soaking times of 20 hours,[31] 24 hours,[8] or 20 minutes[36] and all had employed once-a-day brushing.
Toothbrushes were kept in UV toothbrush sanitizer for seven minutes as per the instructions given by the manufacturer. This was concurrent with other studies where the same UV sanitizer was used,[23,37] whereas in another study in which a different sanitizer[38] was employed, one hour was used.
The range of S. mutans colony counts in the present study at the baseline phase was 69-142 × 101 CFU. In a study,[31] 21-210 S. mutans CFU were reported where 5-12 year old children were recruited for five days. There was variance in the S. mutans colony counts, showing a range of 3-5.8 × 103 CFU after five days,[10] 105- 106 CFU after 24 hours,[39] 104-106 CFU up to 48 hours, and[40] 50-100 CFU up to eight hours,[3] in different studies. The discrepancy in CFU may be due to the different age groups and various time intervals for toothbrush collection employed in these studies.
In most households, the daily procedure for toothbrush care is rinsing and drying them. The results of our study revealed that S. mutans colonies remained on the bristles after usage and drying by the usual method. Hence, disinfecting toothbrushes after their use is of great importance to prevent reinfection of a person with pathogenic bacteria and the toothbrush becoming a reservoir of environmental microorganisms.[11]
Five toothbrushes, which were freshly removed from their packets were checked for S. mutans colonies on their bristles. All the toothbrushes were free of S. mutans colonies suggesting that the origin for these microbes is the oral cavity.
In the present study, toothbrushes soaked in 0.2% TTO solution for 12 hours showed a significant reduction (71.9%) of S. mutans colonies from the baseline. A study[19] with the same concentration of TTO showed more reduction in salivary S. mutans counts than the 0.12% chlorhexidine mouthwash.
Garlic extracts of 2.5[16,19] and 10%[41] were used as mouthwash in studies, which also showed significant reduction in salivary S. mutans counts. Garlic mouthwash, 3%, had reduced more salivary S. mutans counts than 0.2% chlorhexidine mouthwash,[20] which was similar to our study. In our study the 3% garlic extract has proved to be a highly effective antimicrobial solution with 100% reduction of S. mutans. CHX exhibits a broad spectrum of antimicrobial activity and is often used as a positive control for the assessment of the anticariogenic potential of other agents.[28] There was 100% reduction in S. mutans colonies from toothbrushes in some studies where 0.2% CHX was used with an immersion period of 12 hours[13] and 24 hours.[8] Our study results proved that 0.2% CHX is effective as a toothbrush disinfectant against S. mutans with a reduction of 78.3%.
Cetylpyridinium chloride showed a significant reduction of 91.4% S.mutans counts in our study. In a clinical trial conducted for one week using 0.05% CPC spray showed significant decrease in toothbrush contamination[11] whereas another study employed Cepacol (antiseptic containing CPC), which showed 100% reduction of all bacteria after 20 minutes exposure.[42]
A study conducted using the same UV device showed 86% reduction, which may be due to the short duration (one week) of brushing period employed.[23] An in vitro study[37] showed that the antibacterial effect of Violight was least compared to Listerine and 3% hydrogen peroxide. A reduction of 47.4% was observed in our study.
When the efficacy was compared among different antimicrobial agents used in the present study, 3% garlic had the highest efficacy followed by 0.05% CPC, 0.2% CHX, 0.2% TTO and the least efficacious was UV toothbrush sanitizing device.
A precise comparison of present study findings with others could not be done due to variations in age groups and methodology (brushing frequency, number of days of brushing), and differences in antimicrobial agents with varying concentrations, immersion timings, methods of application, and organisms analyzed. Microbiological analysis is dependent on methodological accuracy and crucial technical details, which makes comparing results difficult.
The present study considered only S. mutans for evaluation of the efficacy of disinfecting agents and did not take into account all other microorganisms present on toothbrushes. Only single immersion period (12 hours) was used. It also did not assess the acceptability of toothbrushes dipped in these solutions by the study subjects as antimicrobial agents are known to have bitter and altered taste especially with garlic, tea tree oil, and chlorhexidine. Therefore, further research is required to analyze various antimicrobial agents with different concentrations and different soaking time.
CONCLUSIONS
The study showed that all antimicrobial agents were effective in reducing S. mutans counts in toothbrushes. However 3% garlic had the highest efficacy followed by 0.05% CPC, 0.2% CHX, 0.2% TTO, and UV toothbrush sanitizing device. Further clinical research is required to broaden our understanding of various antimicrobial agents, particularly natural agents, in the prevention of dental caries.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: Evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Caufield PW. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 1995;74:681–5. doi: 10.1177/00220345950740020901. [DOI] [PubMed] [Google Scholar]
- 3.Saravia ME, Nelson-Filho P, da Silva RAB, Rossi MA, Ito IY. Viability of Streptococcus mutans toothbrush bristles. J Dent Child. 2008;75:29–32. [PubMed] [Google Scholar]
- 4.Glass RT, Jensen HG. More on the contaminated toothbrush: The viral Story. Quintessence Int. 1989;19:7–10. [PubMed] [Google Scholar]
- 5.Verran J, Gilmartin AL, Watson GK, Hammond K, Huntington E, Raven SJ. Microbial contamination of toothbrushes during an in home trial. J Dent Res. 1997;76:437. [Google Scholar]
- 6.Malmberg E, Birkhed D, Gunnar N, Jorgen GN, Dahlen G. Microorganisms on toothbrushes at day-care centers. Acta Odontol Scand. 1994;52:93–8. doi: 10.3109/00016359409029061. [DOI] [PubMed] [Google Scholar]
- 7.Nelson-Filho P, Faria G, da Silva RAB, Rossi MA, Ito IY. Evaluation of the contamination and disinfection methods of toothbrushes used by 24- to 48-month-old children. J Dent Child. 2006;73:152–8. [PubMed] [Google Scholar]
- 8.Bhat SS, Hegde KS, George RM. Microbial contamination of toothbrushes and their decontamination. J Indian Soc Pedo Prev Dent. 2003;21:108–12. [PubMed] [Google Scholar]
- 9.Komiyama EY, Back-Brito GN, Balducci I, Koga-Ito CY. Evaluation of alternative methods for the disinfection of toothbrushes. Braz Oral Res. 2010;24:28–33. doi: 10.1590/s1806-83242010000100005. [DOI] [PubMed] [Google Scholar]
- 10.Balappanavar A, Nagesh L, Ankola A, Tangde P, Kakodkar P, Varun S. Antimicrobial efficacy of various disinfecting solutions in reducing the contamination of the toothbrush- a comparative study. Oral Health Prev Dent. 2009;7:139–45. [PubMed] [Google Scholar]
- 11.Sato S, Ito IY, Lara EH, Panzeri H, Albuquerque Junior RF, Pedrazzi V. Bacterial survival rate on toothbrushes and their decontamination with antimicrobial solutions. J Appl Oral Sci. 2004;12:99–103. doi: 10.1590/s1678-77572004000200003. [DOI] [PubMed] [Google Scholar]
- 12.Meier S, Collier C, Scaletta MG, Stephens J, Kimbrough R, Kettering JD. An in vitro investigation of the efficacy of CPC for use in toothbrush decontamination. J Dent Hyg. 1996;70:161–5. [PubMed] [Google Scholar]
- 13.Mehta A, Sequeira PS, Bhat G. Bacterial contamination and decontamination of toothbrushes after use. N Y State Dent J. 2007;73:20–2. [PubMed] [Google Scholar]
- 14.Glass RT, Jensen HG. The effectiveness of a UV toothbrush sanitizing device in reducing the number of bacteria, yeasts and viruses on toothbrushes. J Okla Dent Assoc. 1994;84:24–8. [PubMed] [Google Scholar]
- 15.Sato S, Pedrazzi V, Guimarães Lara EH, Panzeri H, Ferreira de Albuquerque R, Jr, Ito IY. Antimicrobial spray for toothbrush disinfection: An in vivo evaluation. Quintessence Int. 2005;36:812–6. [PubMed] [Google Scholar]
- 16.Prabhakar AR, Ahuja V, Bassappa N. Effect of curry leaves, garlic and tea tree oil on Streptococcus mutans and Lactobacilli in Children: A Clinical and Microbiological Study. Bras Res Ped Dent Int Clin. 2009;9:259–63. [Google Scholar]
- 17.Dalirsani Z, Aghazadeh M, Adibpour M, Amirchaghm M, Pakfetrat A, Mosannen MP, et al. In-vitro comparison of the antimicrobial activity of ten herbal extracts against Streptococcus mutans with chlorhexidine. J Appl Sci. 2011;11:878–82. [Google Scholar]
- 18.Hammer KA, Dry L, Jhonson M, Michalak EM, Carson CF, Riley TV. Susceptibility of oral bacteria to Malaleuca alternifolia (tea tree) oil invitro. Oral Microbiol Immunol. 2003;18:389–92. doi: 10.1046/j.0902-0055.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 19.Groppo FC, Ramacciato JC, Simoes RP, Florio FM, Sartoratto A. Antimicrobial activity of garlic, tea tree oil and chlorhexidine against oral microorganisms. Int Dent J. 2002;52:433–7. doi: 10.1111/j.1875-595x.2002.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 20.Devanand S, Nagesh L, Muralikrishna K. Comparative evaluation of garlic extract mouthwash and chlorhexidine mouthwash on salivary streptococcus mutans count- an invivo study. Oral Health Prev Dent. 2010;8:369–74. [PubMed] [Google Scholar]
- 21.Carson CF, Hammer KA, Riley TV. Malaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulik E, Lenkeit K, Meyer J. Antimicrobial effects of tea tree oil (Melaleuca alternifolia) on oral microorganisms. Schweiz Monatsschr Zahnmed. 2000;110:125–30. [PubMed] [Google Scholar]
- 23.Boylan R, Li Y, Simeonova L, Sherwin G, Kreismann J, Craig RG, et al. Reduction in bacterial contamination of toothbrushes using the violight ultraviolet light activated toothbrush sanitizer. Am J Dent. 2008;21:313–7. [PubMed] [Google Scholar]
- 24.Zanela NL, Bijella MF, da Silva Rosa OP. The influence of mouthrinses with antimicrobial solutions on the inhibition of dental plaque and on the levels of mutans streptococci on children. Bras Dent Res. 2002;16:101–6. doi: 10.1590/s1517-74912002000200002. [DOI] [PubMed] [Google Scholar]
- 25.Jayaprakash R, Sharma A, Moses J. Comparative evaluation of the efficacy of different concentrations of chlorhexidine mouthrinses in reducing the mutans streptococci in saliva: An in-vivo study. J Indian Soc Pedo Prev Dent. 2010;28:162–6. doi: 10.4103/0970-4388.73792. [DOI] [PubMed] [Google Scholar]
- 26.Himratul-Aznita WH, Fathila AR. The potential use of chlorhexidine and hexitidine containing mouthrinse in maintaining toothbrush sterility. J Med Sci. 2006;6:59–62. [Google Scholar]
- 27.De Albuquerque RF, Head TW, Mian H, Rodrigo A, Muller K, Sanches K, et al. Reduction of salivary S aureus and mutans group streptococci by a preprocedural chlorhexidine rinse and maximal inhibitory dilutions of chlorhexidine and cetylpyridinium. Quintessence Int. 2004;35:635–40. [PubMed] [Google Scholar]
- 28.Emilson CG. Potential efficacy of chlorhexidine against mutans streptococcus and human dental caries. J Dent Res. 1994;73:682–91. doi: 10.1177/00220345940730031401. [DOI] [PubMed] [Google Scholar]
- 29.Albert-Kiszely A, Pjetursson BE, Salvi GE, Witt J, Hamilton A, Persson GR, et al. Comparison of the effects of cetylpyridinium chloride with an essential oil mouthrinse on dental plaque and gingivitis- a six month randomized controlled trial. J Clin Periodontol. 2007;34:658–67. doi: 10.1111/j.1600-051X.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 30.Neal PR, Rippin JW. The efficacy of a toothbrush disinfectant spray- an in vitro study. J Dent. 2003;31:153–7. doi: 10.1016/s0300-5712(02)00081-7. [DOI] [PubMed] [Google Scholar]
- 31.Nelson-Filho P, Macari S, Faria G, Assed S, Ito IY. Microbial contamination of toothbrushes and their decontamination. Pediatr Dent. 2000;22:381–4. [PubMed] [Google Scholar]
- 32.Glass RT. Toothbrush Types and Retention of Microorganisms: How to choose a biologically sound toothbrush? J Okla Dent Assoc. 1992;82:26–8. [PubMed] [Google Scholar]
- 33.Glass RT. Toothbrush care. J Am Dent Assoc. 1998;129:1076. doi: 10.14219/jada.archive.1998.0363. [DOI] [PubMed] [Google Scholar]
- 34.WHO. Copenhagen: WHO Regional Office for Europe; 1982. A review of current recommendations for the organization and administration of community oral health services in Northern and Western Europe – Report of WHO Workshop. [Google Scholar]
- 35.Dayoub MB, Rusilko D, Gross A. Microbial contaminatin of toothbrushes. J Dent Res. 1977;56:706. doi: 10.1177/00220345770560063501. [DOI] [PubMed] [Google Scholar]
- 36.Sogi S, Subbareddy, Kiran S. Contamination of toothbrush at different time intervals and effectiveness of various disinfecting solutions in reducing the contamination of toothbrush. J Indian Soc Pedo Prev Dent. 2002;20:81–5. [PubMed] [Google Scholar]
- 37.Beneduce C, Baxter KA, Bowman J, Haines M, Andreana S. Germicidal activity of antimicrobials and violight personal travel toothbrush sanitizer: An in vitro study. J Dent. 2010;38:621–5. doi: 10.1016/j.jdent.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Purebrush. [Last accessed on 2011 Oct 21]. Available from: http://www.purebrush.com/clinical .
- 39.Quirynen M, De Soete M, Pauwels M, Gizani S, Meerbeek BV, Steenberghe DV. Can a toothpaste or a toothbrush with antibacterial tufts prevent toothbrush contamination? J Periodontol. 2003;74:312–22. doi: 10.1902/jop.2003.74.3.312. [DOI] [PubMed] [Google Scholar]
- 40.Napimoga MH, Kamiya RU, Rosa RT, Rosa EA, Hofling JF, Mattos-Graner RO, et al. Genotypic diversity and virulence traits of streptococcus mutans in caries free and caries active individuals. J Med Microbiol. 2004;53:697–703. doi: 10.1099/jmm.0.05512-0. [DOI] [PubMed] [Google Scholar]
- 41.Elnima EI, Ahmed SA, Mekkawi AG, Mossa JS. The antimicrobial activity of garlic and onion extracts. Pharmazie. 1983;38:747–8. [PubMed] [Google Scholar]
- 42.Caudry SD, Kiltorinos A, Chan EC. Contaminated toothbrushes and their disinfection. J Can Dent Assoc. 1995;61:511–6. [PubMed] [Google Scholar]


