Abstract
Recent studies have shown that the presence of a caring relational partner can attenuate neural responses to threat. Here we report reanalyzed data from Coan, Schaefer, and Davidson (2006), investigating the role of relational mutuality in the neural response to threat. Mutuality reflects the degree to which couple members show mutual interest in the sharing of internal feelings, thoughts, aspirations, and joys – a vital form of responsiveness in attachment relationships. We predicted that wives who were high (versus low) in perceived mutuality, and who attended the study session with their husbands, would show reduced neural threat reactivity in response to mild electric shocks. We also explored whether this effect would depend on physical contact (handholding). As predicted, we observed that higher mutuality scores corresponded with decreased neural threat responding in the right dorsolateral prefrontal cortex and supplementary motor cortex. These effects were independent of hand-holding condition. These findings suggest that higher perceived mutuality corresponds with decreased self-regulatory effort and attenuated preparatory motor activity in response to threat cues, even in the absence of direct physical contact with social resources.
Keywords: mutuality, threat, brain imaging, neuroscience, attachment, couples
Social support promotes physical health (Dekkers et al., 2001; Yarcheski & Mahon, 1999), decreases risk of mortality (Cobb, 1976; House, Landis & Umberson, 1988), buffers against risk for affective disorders (Jung-Soon & Kyung-Sook, 2001; Kessler & Essex, 1982), and increases health-promoting or maintaining behaviors (McNicholas, 2002). Touch is an important facilitator of this support. Soothing touch alleviates distress in children undergoing lumbar punctures (Vannorsdall, Dahlquist, Pendley, & Power, 2004), and HPA activity in women asked to give a socially stressful speech (Ditzen et al., 2007). The frequency of hugs in married couples is associated with lower blood pressure (Light, Grewen, & Amico, 2005), and the presence of a relational partner can decrease threat reactivity in the brain – an effect that is potentiated by high relationship quality (Coan et al., 2006; Frazier, Tix, & Barnett, 2003).
The health enhancing properties of social support are nowhere more evident and powerful than in attachment relationships (Coan, 2008; Mikulincer, Shaver, & Pereg, 2003). Infant–caregiver attachments are characterized by high levels of dependence on the part of the infant, necessitating one of the bedrock features of any attachment bond: a strong motivation to maintain close proximity to a responsive caregiver (Bowlby, 1973). Moreover, a caregiver’s ability to respond effectively to the infant’s needs plays a pivotal role in determining the quality of the attachment bond that develops (Ainsworth, Blehar, Waters, & Wall, 1978). A secure attachment bond is most likely to develop when a caregiver is sensitive to the child’s signals, and consistently responsive – both physically and emotionally – when needed. Adult attachment relationships are similarly rooted in partner responsiveness, especially during periods of stress (Hazan & Shaver, 1987; Mikulincer & Shaver, 2007). Adults are more likely to develop secure attachment relationships when their partners are sensitive and responsive to their needs (Collins & Feeney, 2000), and when they perceive that their partners are understanding, validating, and caring toward them (Reis, Clark, & Holmes, 2004).
One distinction between infant–caregiver versus adult attachment bonds is the notion of interdependence. Although infants are highly dependent upon caregivers for emotional and physiological support, caregivers are not similarly dependent upon infants. By contrast, adults in attachment relationships frequently require emotional support from one another. Within the attachment framework, a great emphasis upon perceived responsiveness in times of need or stress is common to both infant–caregiver and adult attachment literatures (Bowlby, 1973; Coan et al., 2006; Mikulincer & Shaver, 2007). However, the complexity of interdependence that characterizes adult attachment relationships suggests important differences in how adults manage attachment relationships.
In tacit recognition of this, researchers of adult attachment relationships have begun to study attachment processes from more complex dyadic perspectives (e.g., Bodenmann, Pihet, & Kayser, 2006; Kane et al., 2007). Moreover, researchers of adult attachment recognize great flexibility in attachment theory as a framework for understanding adult interpersonal relationships (Coan, 2010; Davidovitz, Mikulincer, Shaver, Ijzak, & Popper, 2007; Granqvist, Mikulincer, & Shaver, 2010). For example, adult attachment researchers have shown that when individuals can rely on their romantic partners to provide a secure base for goal pursuit and personal growth, they experience increases in self-esteem, positive mood, and confidence in achieving future goals (Feeney, 2004). New evidence also suggests that couples who are better at talking about and capitalizing on each others’ positive experiences – a manifestation of responsiveness rooted in the potentiation of positive affect – report higher levels of intimacy and relationship satisfaction (Gable, Reis, Impett, & Asher, 2004). Moreover, affective behavior observed during positive “love” interactions may predict variance in relationship satisfaction and divorce risk long neglected by overreliance on observations of interpersonal conflict (Graber, Laurenceau, Miga, Chango, & Coan, 2011).
Mutuality and responsiveness in attachment relationships
One perspective on positive dyadic interactions that may influence the regulatory functioning of attachment relationships is the notion of mutual psychological development, or mutuality. Mutuality implies a “shared sense of relationship” (Genero, Baker, Surrey, & Baldwin, 1992, p. 37) – a reciprocal sharing of thoughts and feelings in close relationships characterized by a genuine interest in the subjective reality of each member that emphasizes responsiveness during the routine sharing of internal feelings, thoughts, aspirations, and joys (Genero et al., 1992). Mutuality manifests as a genuine interest in fostering growth in one’s relational partner, an interest that is perceived to be reciprocal (Miller & Stiver, 1997). The key to this construct is its emphasis on authentic mutual interest coupled with perceived success in mutually felt understanding (cf., Oishi, Krochik, & Akimoto, 2010). Originally developed as part of “Self-in-Relation Theory” (Surrey, 1985), mutuality empowers relationships by bringing clarity, directness, and predictability to the thoughts, feelings, and intentions of the individuals who inhabit them.
The impact of mutuality on the regulatory functions associated with attachment relationships is evident in a variety of ways. For example, mutuality is associated with greater emotional resiliency (Gottlieb, 1992), more effective relational coping (Coyne & Bolger, 1990), faster recovery from eating disorders (Tantillo & Sanfter, 2003), lower levels of depressive symptoms (Genero et al., 1992), lower levels of daily anger (Sperberg & Stabb, 1998), and higher self-esteem (Lippes, 1998). Higher levels of mutuality are associated with higher quality of life and greater self-care agency among breast cancer patients (Kayser, Sormanti, & Strainchamps, 1999), as well as less depression, lower anxiety, lower disease impact, and even lower levels of physical disability among individuals suffering from rheumatoid arthritis (Kasle, Wilhelm, & Zautra, 2008). Similarly, higher levels of mutuality reported by women with rheumatoid arthritis prospectively predicted lower levels of inflammation for up to a year (Kasle, Wilhelm, McKnight, Sheikh, & Zautra, 2010). Ultimately, a key process in attachment bonding is perceived partner responsiveness, and mutuality contributes to an expanded view of how responsiveness manifests in attachment relationships.
The social regulation of neural threat responding
Outside of basic work in social cognition (Amodio & Frith, 2006; Pelphrey & Morris, 2006), little is known about the neurobiology of human social relationships, and still less is known about how the human brain transforms strong social relationships into decreased negative affect and improved physical health (Coan, 2008). Recently, Coan (2010) proposed the Social Baseline Theory (SBT), which states among other things that the neural substrates of socially mediated forms of emotion regulation are unlikely to involve neural systems supporting the self-regulation of emotion. This perspective derives from empirical observations that the successful provision of support does not appear to cause activations in self-regulatory circuits such as the dorsolateral prefrontal cortex (dlPFC). On the contrary, neural systems supporting self-regulatory efforts are typically less active when social support is provided, as are the many emotion-generative circuits those self-regulatory efforts are intended to inhibit (Coan et al., 2006; Connor et al., 2012; Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007). SBT proposes that this decrease in self-regulatory effort confers energy-saving advantages to the socially mediated forms of emotion regulation. It is in this sense that socially mediated forms of emotion regulation likely constitute a “baseline” or default emotion-regulation strategy for most people, most of the time (Beckes & Coan, 2011). Indeed, a large body of research suggests that self-regulation, including the self-regulation of emotion, is depleting if engaged for long periods of time, resulting in subjective feelings of exhaustion and steady decreases in self-regulatory capabilities (Galliot & Baumeister, 2007). By contrast, evidence suggests that people work less hard to regulate themselves when social resources are available (Fitzsimons & Finkel, 2011).
One of the important observations in the literature on socially mediated forms of emotion regulation is the moderating impact of relationship quality (Kiecolt-Glaser & Newton, 2001; Robles & Kiecolt-Glaser, 2003). For example, in Coan et al. (2006), when placed under threat of mild electric shock, relationship quality moderated the regulatory impact of supportive hand-holding in the superior frontal gyrus, right anterior insula, and hypothalamus (Coan et al., 2006). Specifically, although no association obtained between relationship quality and brain activity in either the alone or stranger conditions, brain activity in all three regions was lower during spouse hand-holding if relationship quality was higher. Thus, relationship quality impacted neural threat responding specifically in the physical presence of the spouse.
For this study, we have reanalyzed data reported in Coan et al. (2006) to investigate the role of mutuality on the neural response to threat. As reviewed above, mutuality measures a vital form of responsiveness within attachment relationships – one that emphasizes mutual interest in sharing internal feelings, thoughts, aspirations, and joys (Genero et al., 1992). We suspect that mutuality, by virtue of its broad focus on positive aspects of the relationship, is likely to have a similarly broad impact on neural threat responding. That is, mutuality reflects a form of responsiveness that is not contingent upon the presence of a potential threat. Thus, it may manifest as a moderating influence that reduces threat reactivity generally (i.e., not only during partner hand-holding; cf., Eisenberger et al., Lieberman). In this way, mutuality would distinguish itself from relationship quality per se. Importantly, all analyses reported below are first statistically adjusted for relationship quality in order to determine the degree to which mutuality impacts neural threat responding independently.
Method
Participants
Participants included 16 married couples preselected to score “highly satisfied” on the Dyadic Adjustment Scale (DAS; Spanier, 1976), operationalized as scores above a 40 on the DAS’s Satisfaction subscale. For the original study, highly satisfied couples were selected in order to capture, as clearly as possible, the normative regulatory impact of supportive hand-holding on threat responding among high functioning attachment relationships. Mean ages of the husbands and wives were 33 (SD = 10) and 31 (SD = 6), respectively. Couples self-identified their ethnicities as Caucasian (N = 15) and Asian (N = 1). Couples were recruited by way of print advertisements in the Madison, WI, area. Exclusion criteria included pregnancy, psychopathology in the past or present, or other characteristics that would be risk factors in close proximity to the fMRI scanner. Later, total DAS scores were recorded from both husbands and wives. The total DAS score has a theoretical range extending from 0 to 151, with scores lower than 100 thought to indicate distressed marriages. Mean total DAS scores were 126 (SD = 6) and 127 (SD = 10) for husbands and wives, respectively, indicating a generally high level of marital quality among the couples in this sample. The Pearson correlation between husbands’ and wives’ DAS scores was 0.20, n.s. Total DAS scores were used for the analyses reported below.
Mutuality
Husbands and wives completed the Mutual Psychological Development Questionnaire (MPDQ; Genero et al., 1992), a robust instrument used to gauge bidirectional exchange of thoughts, openness to feelings, and other aspects of reciprocity in interest and conversation. The instrument is made up of two subscales designed to measure one’s own responsiveness to a partner (self-mutuality) and the partner’s responsiveness to the self (other-mutuality) respectively. Each subscale comprises a stem statement (“When we talk about things that matter to me, my spouse/partner is likely to …” and “When we talk about things that matter to me, I am likely to …”) and 11 items (such as “express an opinion clearly” and “have difficulty listening”). Each item receives a rating from 1 (low) to 6 (high). After reverse-scoring indicated items, average scores ranging from 1 to 6 were obtained separately for both husband and wife, as well as for self and other, yielding four mutuality scores total: wife–self (wife’s perceptions of her own responsiveness), wife–partner (wife’s perceptions of her partners’ responsiveness), husband– self, and husband–partner.
Procedure
Only the wives underwent fMRI scanning. Husbands completed questionnaires and provided hand-holding. All participants gave written informed consent in accord with the Human Subjects Committee of the University of Wisconsin medical school and received monetary compensation for participation.
Telephone screenings determined eligible participants, who were told they would be participating in a study on hand-holding. Two visits to the laboratory were scheduled for each couple. During the first visit, couples completed the MPDQ, the complete DAS, and other measures of relationship quality before experiencing a trial run in the laboratory’s mock fMRI scanner. Approximately one week later, participants returned for second visit, during which the experiment’s brain-imaging procedure was conducted. Couples completed an fMRI safety assessment in a waiting room while the wife’s left or right ankle, counterbalanced across participants, was attached to two Ag-AgCl shock electrodes. The wife then entered the MRI chamber and high-resolution anatomical scans were collected before the functional imaging.
During functional scanning for the experiment, the wife was presented with 12 threat cues (a red “X” on a dark background) and 12 safety cues (a blue “O” on a dark background). Cues were in random order, within each of three counterbalanced blocks of 8 cues per block, totaling 24 cue trials (see Coan et al., 2006). Trials were randomized within subjects, and block order was counterbalanced between subjects. During each of the three blocks, the wife held either her husband’s hand, the hand of an unseen, anonymous male experimenter, or no hand. All hand-holding involved participants’ right hands; left hands provided responses on a button box. All but three participants held the hand of the same male experimenter; two other male volunteers served as hand-holders when the experimenter was not available. Threat cues indicated a 20% chance of receiving an electric shock, while safety cues indicated no chance of shock. Electric shocks were delivered using an isolated physiological stimulator (Coulbourn Instruments, Allentown, PA) with 20 ms duration at 4 mA. Two shocks were delivered per block to each subject.
Each trial comprised a threat or safety cue with a duration of 1 s and then an anticipation period whose duration varied between 4 and 10 s. During the anticipation period, wives focused on a fixation cross. Shocks were delivered only at the end of the anticipation period. The end of each trial was indicated by the presentation of a small circle; between trials, a black screen was presented and subjects were told to rest until the start of the next trial. The resting period was also of varying duration, between 4 and 10 s.
Image acquisition and data analysis
Functional magnetic images were acquired with a General Electric (Fairfield, CT) Signa 3.0-T high-speed magnetic imaging device, with a quadrature head coil. A total of 215 functional images were collected per block, in volumes of 30 × 4 mm sagittal echo-planar slices (1 mm slice gap) covering the whole brain. A repetition time of 2 s was used, with an echo time of 30 ms, a 601 flip, and a field of view of 240 × 240 mm, with a 64 × 64 matrix, resulting in a voxel size of 3.75 × 3.75 × 5 mm. A T1-weighted spoiled-gradient-recalled anatomical scan consisting of 124 × 1.2 mm slices was acquired before functional imaging to assist with localization of function.
Using Analysis of Functional Neural Images (AFNI) software (Version 2.52; Cox, 1996), raw data was reconstructed off-line with a 1-voxel in-plane full-width/half-maximum Fermi window, six-parameter rigid body-motion correction, high-pass filtering of 1/60 s (to remove signal unrelated to stimulus presentation), and removal of ghost and skull artifacts. Trials during which shocks were delivered were excluded from analysis to minimize movement artifacts. Time series were fit to an ideal hemodynamic response with a least squares general linear model; the motion parameters were entered as covariates. The beta weights that resulted were converted to percentage signal change, and the maps transformed to standard Talairach space (Talairach & Tournoux, 1988).
Functional regions of interest (ROIs)
As an intermediate data-reduction step, activation to threat cues and safety cues were contrasted to determine the normative threat response in the no-hand-holding condition. Voxel-wise t-tests that indicated greater activation in threat-cue than safety-cue trials identified multisubject ROIs (p < .005 corrected, with corrections estimated from Monte Carlo simulations). This statistical procedure allowed us to empirically identify clusters of neural activity normatively associated with the presence of a threat – clusters that are available for subsequent analyses involving hand-holding condition and mutuality scores. This analysis identified activation in a network of regions consistently shown to be associated with responses to threat, negative affect, or anticipation of pain, including the ventral anterior cingulate cortex (vACC), right dorsolateral prefrontal cortex (right dlPFC), right inferior frontal gyrus, left superior frontal gyrus, right anterior insula, supplementary motor cortex (SMC), caudate–nucleus accumbens (NAcc), putamen, hypothalamus, right postcentral gyrus, superior colliculus, posterior cingulate, and left supramarginal gyrus (Davidson & Irwin, 1999; Ploghaus et al., 1999; Salomons, Johnstone, Backonja, & Davidson, 2004; Wager et al., 2004). It was thus possible to restrict statistical tests described below to only these regions.
Results
Linear Mixed Models were conducted using SPSS’s Linear Mixed Model Module. For these models, brain activation was predicted by Hand Holding Condition (Alone, Spouse, Stranger), Wife DAS, both husband and wife mutuality scores (Husband Other Mutuality, Husband Self Mutuality, Wife Other Mutuality, and Wife Self Mutuality), and all interactions between Hand Holding Condition and husband and wife mutuality scores. This statistical model utilized a type 1 sum of squares procedure that allowed us to remove variance in brain activity attributable to wife DAS scores first, before analyzing all other effects. The type 1 sum of squares also minimized the impact of multicolinearity among the various mutuality scores, all of which were at least moderately correlated, all rs ≥ 0.32, all ps ≤ .03. Thus, all effects of mutuality reported below are statistically independent of wife DAS score effects reported in Coan et al. (2006). Husband mutuality scores were included as well, in order to consider the possibility that husband mutuality scores are associated with wife threat-responding.
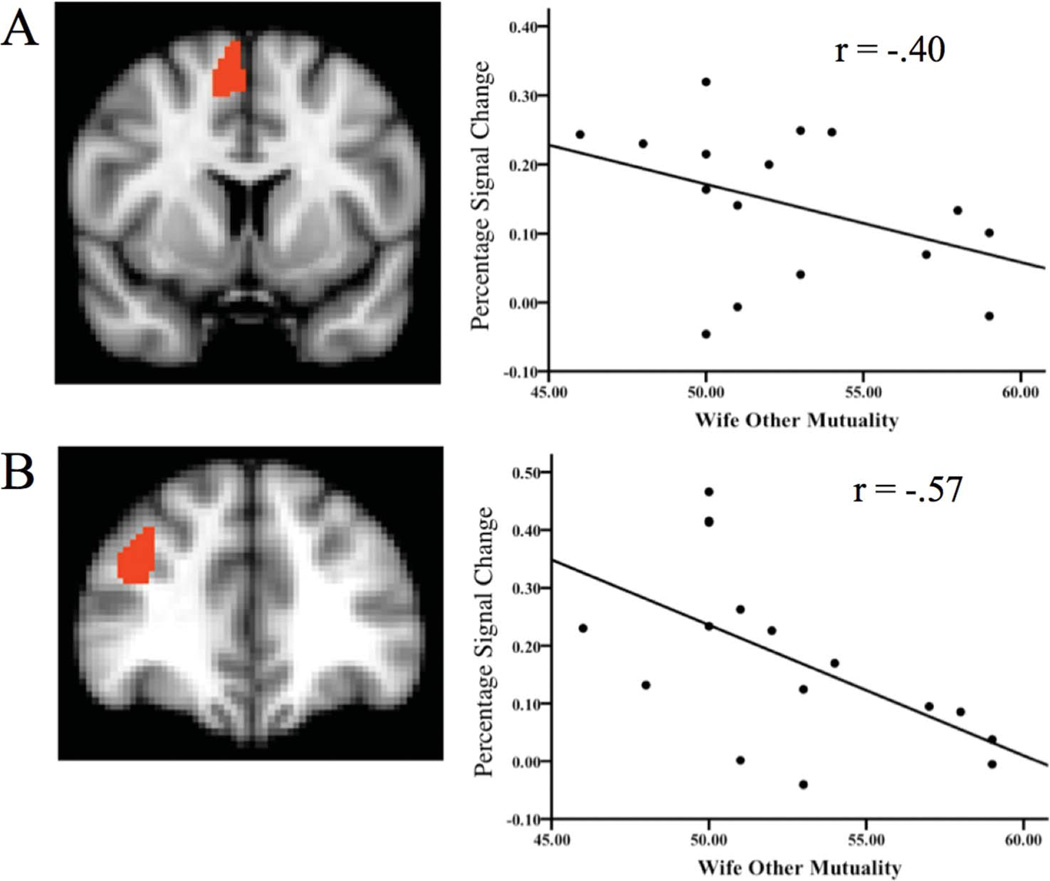
No main effects of Hand Holding Condition or DAS were observed that have not been reported elsewhere from these data, nor were any Mutuality by Hand Holding Interaction effects observed. We did, however, observe main effects of Wife Other Mutuality in predicting threat related neural activation independent of Hand Holding Condition in both the SMC, F(1, 30) = 4.80, p = .04, and right dlPFC, F(1, 31) = 4.24, p = .05. Subsequent regressions (depicted in Figure 1) revealed that greater Wife Other Mutuality corresponded with reduced threat-related activation in both regions, although the specific correlation was only statistically significant in the right dlPFC, r = −0.57 (p = .02), with SMC r = −0.40 (p = .12).
Figure 1.
Main effects of Wife Other Mutuality score on threat related brain activity averaged across hand-holding conditions. Percentage signal change is graphed as a function of Wife Other Mutuality score, with correlation coefficients included. Section A depicts this association in the supplementary motor cortex (x = 4, y = 6, z = 46; t-score = 3.63; size = 4043 mm3). Section B depicts this association in the dorsolateral prefrontal cortex (x = 3, y = 44, z = 2; t-score = 3.81; size = 350 mm3).
Discussion
Higher mutuality scores corresponded with decreased activations in a limited set of threat-responsive regions, but across all experimental conditions. Specifically, the down-regulatory impact of mutuality was (a) independent of hand-holding condition, and (b) particularly pronounced in the right dlPFC, a region powerfully implicated in the effortful self-regulation of emotion. Importantly, higher mutuality scores also corresponded with decreased threat reactivity in the SMC, a structure thought to utilize emotional information to modify and prepare behavioral responses in the primary motor cortex (Hajcak et al., 2007). As predicted, higher levels of mutuality were nowhere under any circumstances associated with increased threat responding. In sum, it appears that higher perceived mutuality corresponds with decreased self-regulatory effort and attenuated preparatory motor activity in response to threat cues, even in the absence of direct physical access to social resources.
The MPDQ is designed to measure mutual felt understanding and genuine interest – generalized forms of responsiveness to and by relational partners. We know already that relationship quality is associated with decreased threat-related neural activity when holding the hand of one’s relational partner. Perhaps the reason there was no interaction between mutuality and hand-holding condition is because mutuality confers a global, trait-like sense of relative attachment security, expressed here at the neural level. That is, it is possible that relationship experiences that lead to high mutuality (e.g., mutual intimate disclosure) lead to the development of stable neural orientations (such as increased basal levels of opioid activity – see below) that result, in turn, in general decreases in the need for self-regulatory effort. By contrast, it is possible that the DAS captures aspects of the relationship (consensus, satisfaction, cohesion) that are more state-like, thus placing greater regulatory dependence upon the physical presence (hand-holding) of the relational partner. In the current study, it is also worth noting that partners were present in the laboratory setting throughout the experiment. Thus, wives high in perceived mutuality may have derived a sense of increased security by the mere presence of their husband in the laboratory setting (Kane, McCall, Collins, & Blascovich, 2012), even in the absence of direct physical contact.
Possible mechanisms linking mutuality to decreased threat responding
SBT argues that structures implicated in the self-regulation of emotion are themselves regulated by social proximity and interaction. In this sense, social affect regulation is not so much “down-regulatory” in the sense that effortful emotion regulation is typically characterized (e.g., via cognitive reappraisal or mindful awareness). Rather, perceived social resources obviate the need for the activation of emotion, returning the individual to a baseline state of relative calm by other, likely perceptual, means.
Several candidate mechanisms, including endogenous opioids, oxytocin, and dopamine, have been proposed as links between social resources and decreased negative affect. For example, opioid tone, or basal levels of opioid activity, may play a role in limiting levels of stress reactivity in a variety of contexts (LaPrairie & Murphy, 2009; Slattery & Neumann, 2008), and opioid activity can increase in the presence of social resources (Panksepp, 1998). Indeed, Eisenberger et al. (2007) has suggested that the activity of endogenous opioids associated with social relationships may be particularly pronounced in regions such as the dorsal anterior cingulate cortex (dACC). The dACC has a very high density of opioid receptors (Rainville, Duncan, Price, Carrier, & Bushnell, 1997), and opioid activity in the dACC is known to play a powerful role in modulating sensitivity to both pain and stress (Eippert et al., 2009; Zubieta et al., 2001). Moreover, abundant evidence suggests endogenous opioid activity throughout the brain is highly sensitive to social contact (Nocjar & Panksepp, 2007). It is possible that higher levels of mutuality correspond with higher opioid tone throughout the brain, requiring higher levels of stress before the brain will activate stress responses via circuits like the dACC. If true, and if mutuality is indeed contributing to the modulation of opioid tone in the dACC and elsewhere, the notion that mutuality confers a stable, trait-like advantage in confronting various life stressors would seem particularly likely.
The neuropeptide oxytocin is also likely to play a role mediating the effects of mutuality on threat reactivity in the brain (Gainer & Wray, 1994). Oxytocin activity is also highly responsive to social proximity, increasing levels of trust and sensitivity to social cues (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005; Ross et al., 2009; Uvnaes-Moberg, 1998), and is associated with decreased threat reactivity (Windle, Shanks, Lightman, & Ingram, 1997), even in the amygdala (Kirsch et al., 2005). Still others have suggested that dopaminergic, reward-related processing in the presence of social resources may inhibit negative affect (Depue & Morrone-Strupinsky, 2005; Younger, Aron, Parke, Chatterjee, & Mackey, 2010). More research is needed to assess the likelihood that basal levels of these latter possible mechanisms can be modulated by experiences similar to those tapped by the MPDQ.
Conclusion
Higher levels of mutuality correspond with decreased threat respond in the brain, regardless of the “on-line” or direct physical contact with social resources. The associations between mutuality and neural threat responding are neither as great nor as pervasive as those observed during supportive hand-holding (cf., Coan et al., 2006). Nevertheless, mutuality’s impact on the regulation of emotion is not likely to be trivial. The region most strongly associated with mutuality was the dlPFC, a region widely known for its role in working memory and effortful emotion regulation. Also implicated was the SMC, a region that likely supports preparation for the activation of behavioral motor responses to affectively salient situations. These effects suggest that individuals who experience their relationships as characterized by higher levels of mutual understanding and genuine interest are not working as hard to regulate their negative affect, and are preparing fewer or less intense behavioral affective responses. According to SBT, this is because higher levels of mutuality signal the increased dependability of the relational partner, and a commensurably decreased need to deploy affective responses to uncertain threats. This could result in real savings in cognitive and metabolic effort (Beckes & Coan, 2011). Ultimately, our focus on mutuality has the potential to expand the conceptualization and measurement of partner responsiveness as applied to attachment relationships, much as recent work on the capitalization of positive affect has done (cf., Coan, 2011; Ditzen et al., 2007; Gable et al., 2004; Graber et al., 2011 Reis & Gable, 2003). Indeed, we view mutuality as an important marker of perceived partner responsiveness – a critical component of secure and well-functioning attachment bonds (see Kane et al., 2012). If true, our findings suggest that the regulatory impact of one’s closest relationship is yoked in part to the perception that one’s partner is responsive to a wide range of needs, including a need for felt understanding and genuine interest. And the impact of such responsiveness may even decrease the processing load imposed on circuits of the brain supporting emotion regulation and action planning during stressful events.
Acknowledgements
This work was supported by National Institute of Mental Health (NIMH) Grants P50-MH06931 and MH43454 to R.J.D. We thank David Sbarra for his thoughtful suggestions, and Josh Glazer, Josie Golembiewski, and Megan Roach for their assistance in data collection and reduction.
References
- Ainsworth MD, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Lawrence Erlbaum; 1978. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Beckes L, Coan JA. Social baseline theory: The role of social proximity in emotion and economy of action. Social and Personality Psychology Compass. 2011;5:976–988. [Google Scholar]
- Bodenmann G, Pihet S, Kayser K. The relationship between dyadic coping and marital quality: A 2-year longitudinal study. Journal of Family Psychology. 2006;20:485–493. doi: 10.1037/0893-3200.20.3.485. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol. 2. Separation: Anxiety and anger. New York, NY: Basic Books; 1973. [Google Scholar]
- Coan JA. Toward a neuroscience of attachment. In: Shaver PR, editor. Handbook of attachment: Theory, research, and clinical applications, 2nd edition. New York, NY: Guilford Press; 2008. pp. 241–265. [Google Scholar]
- Coan JA. Adult attachment and the brain. Journal of Social and Personal Relationships. 2010;27:210–217. doi: 10.1177/0265407510368966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA. The social regulation of emotion. In: Decety J, Cacioppo JT, editors. Oxford Handbook of Social Neuroscience. New York: Oxford University Press; 2011. pp. 614–623. [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cobb S. Presidential Address-1976. Social support as a moderator of life stress. Psychosomatic Medicine. 1976;38(5):300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- Collins NL, Feeney BC. A safe haven: Support-seeking and caregiving processes in intimate relationships. Journal of Personality and Social Psychology. 2000;78:1053–1073. doi: 10.1037//0022-3514.78.6.1053. [DOI] [PubMed] [Google Scholar]
- Connor OL, Siegle GJ, McFarland AM, Silk JS, Ladouceur CD, Dahl RE, Coan JA, Ryan ND. Mom – it helps when you’re right here! Attenuation of neural stress markers in anxious adolescents whose caregivers are present during fMRI. PLoS One. 2012;7:e50680. doi: 10.1371/journal.pone.0050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Coyne JC, Bolger N. Doing without social support as an explanatory concept. Journal of Social and Clinical Psychology. 1990;9:148–158. [Google Scholar]
- Davidovitz R, Mikulincer M, Shaver PR, Ijzak R, Popper M. Leaders as attachment figures: Their attachment orientations predict leadership-related mental representations and followers’ performance and mental health. Journal of Personality and Social Psychology. 2007;93:632–650. doi: 10.1037/0022-3514.93.4.632. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Dekkers JC, Geenen R, Evers AWM, Kraaimaat FW, Bijlsma JWJ, Godaert GLR. Biopsychosocial mediators and moderators of stress-health relationships in patients with recently diagnosed rheumatoid arthritis. Arthritis Care & Research. 2001;45(4):307–316. doi: 10.1002/1529-0131(200108)45:4<307::AID-ART342>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–395. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, et al. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32(5):565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney BC. A secure base: Responsive support of goal strivings and exploration in adult intimate relationships. Journal of Personality and Social Psychology. 2004;87:631–648. doi: 10.1037/0022-3514.87.5.631. [DOI] [PubMed] [Google Scholar]
- Fitzsimons GM, Finkel EJ. Outsourcing self-regulation. Psychological Science. 2011;22:369–375. doi: 10.1177/0956797610397955. [DOI] [PubMed] [Google Scholar]
- Frazier PA, Tix AP, Barnett CL. The relational context of social support: Relationship satisfaction moderates the relations between enacted support and distress. Personality & Social Psychology Bulletin. 2003;29(9):133–1146. doi: 10.1177/0146167203254545. [DOI] [PubMed] [Google Scholar]
- Gable SL, Reis HT, Impett EA, Asher ER. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. Journal of Personality and Social Psychology. 2004;87:228–245. doi: 10.1037/0022-3514.87.2.228. [DOI] [PubMed] [Google Scholar]
- Gainer H, Wray S. Cellular and molecular biology of oxytocin and vasopressin. In: Knobil E, Neill J, editors. The physiology of reproduction. New York, NY: Raven Press; 1994. [Google Scholar]
- Galliot MT, Baumeister RF. The physiology of willpower: Linking blood glucose to self-control. Personality and Social Psychology Review. 2007;11:303–327. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- Genero NP, Baker MJ, Surrey J, Baldwin LM. Measuring perceived mutuality in close relationships: Validation of the Mutual Psychological Development Questionnaire. Journal of Family Psychology. 1992;6:36–48. [Google Scholar]
- Gottlieb BH. Quandries in translating support concepts to intervention. In: Veiel HOF, Bauman U, editors. The meaning and measurement of social support. Washington, DC: Hemisphere Press; 1992. pp. 293–309. [Google Scholar]
- Graber E, Laurenceau JP, Miga E, Chango J, Coan JA. Conflict versus love: Predicting newlywed outcomes from two interaction contexts. Journal of Family Psychology. 2011;25:541–550. doi: 10.1037/a0024507. [DOI] [PubMed] [Google Scholar]
- Granqvist P, Mikulincer M, Shaver PR. Religion as attachment: Normative processes and individual differences. Personality and Social Psychology Review. 2010;14:49–59. doi: 10.1177/1088868309348618. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, Nahas Z. Emotion facilitates action: A transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44(1):91–97. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver PR. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jung-Soon M, Kyung-Sook C. The effects of handholding on anxiety in cataract surgery patients under local anaesthesia. Journal of Advanced Nursing. 2001;35:407–415. doi: 10.1046/j.1365-2648.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Kane HS, Jaremka LM, Guichard AC, Ford MB, Collins NL, Feeney BC. Feeling supported and feeling satisfied: How one partner’s attachment style predicts the other partner’s relationship experiences. Journal of Social and Personal Relationships. 2007;24:535–555. [Google Scholar]
- Kane HS, McCall C, Collins NL, Blascovich J. Mere presence is not enough: Responsive social support in a virtual world. Journal of Experimental Social Psychology. 2012;48:37–44. [Google Scholar]
- Kasle S, Wilhelm MS, McKnight PE, Sheikh SZ, Zautra AJ. Mutuality’s prospective beneficial effects on inflammation in female patients with rheumatoid arthritis. Arthritis Care and Research. 2010;61:92–100. doi: 10.1002/acr.20019. [DOI] [PubMed] [Google Scholar]
- Kasle S, Wilhelm MS, Zautra AJ. Rheumatoid arthritis patients’ perceptions of mutuality in conversations with spouses/partners and their links with psychological and physical health. Arthritis & Rheumatism. 2008;59:921–928. doi: 10.1002/art.23821. [DOI] [PubMed] [Google Scholar]
- Kayser K, Sormanti M, Strainchamps E. Women coping with cancer: The influence of relationship factors on psychosocial adjustment. Psychology of Women Quarterly. 1999;23(4):725–739. [Google Scholar]
- Kessler RC, Essex M. Marital status and depression: The importance of coping resources. Social Forces. 1982;61(2):484–507. [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- LaPrairie J, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Frontiers in Behavioral Neuroscience. 2009;3:1–11. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological Psychology. 2005;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Lippes TE. Unpublished Unpublished doctoral dissertation. Chicago, Illinois: Chicago School of Professional Psychology; 1998. Mutuality in marriage: The self-esteem connection. [Google Scholar]
- McNicholas SL. Social support and positive health practices. Western Journal of Nursing Research. 2002;24(7):772–787. doi: 10.1177/019394502237387. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in adulthood: Structure, dynamics, and change. New York, NY: Guilford Press; 2007. [Google Scholar]
- Mikulincer M, Shaver PR, Pereg D. Attachment theory and affect regulation: The dynamics, development, and cognitive consequences of attachment-related strategies. Motivation & Emotion. 2003;27:77–102. [Google Scholar]
- Miller JB, Stiver IP. The healing connection: How women form relationships in therapy and in life. Boston, MA: Beacon Press; 1997. [Google Scholar]
- Nocjar C, Panksepp J. Prior morphine experience induces long-term increases in social interest and in appetitive behavior for natural reward. Behavioural Brain Research. 2007;181:191–199. doi: 10.1016/j.bbr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Oishi S, Krochik M, Akimoto S. Felt understanding as a bridge between close relationships and subjective well-being: Antecedents and consequences across individuals and cultures. Social and Personality Psychology Compass. 2010;4:403–416. [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Pelphrey KA, Morris JP. A role for the superior temporal sulcus region in understanding the actions and intentions of others. Current Directions in Psychological Science. 2006;15:136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain fron its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Reis HT, Clark MS, Holmes JG. Perceived partner responsiveness as an organizing construct in the study of intimacy and closeness. In: Mashek DJ, Aron A, editors. Handbook of closeness and intimacy. Mahwah, NJ: Erlbaum; 2004. pp. 415–428. [Google Scholar]
- Reis HT, Gable SL. Toward a positive psychology of relationships. In: Haidt J, editor. Flourishing: Positive psychology and the life well-lived. Washington, DC: American Psychological Association; 2003. [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiology & Behavior. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. Journal of Neuroscience. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja M-M, Davidson RJ. Perceived controllability modulates the neural response to pain. Journal of Neuroscience. 2004;24(32):7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. The Journal of Physiology. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of a marriage and similar dyads. Journal of Marriage and the Family. 1976;38:15–28. [Google Scholar]
- Sperberg ED, Stabb SD. Depression in women as related to anger and mutuality in relationships. Psychology of Women Quarterly. 1998;22(2):223–238. [Google Scholar]
- Surrey J. The “self in relation”: A theory of women’s development. Wellesley, MA: Wellesley College; 1985. [Google Scholar]
- Talairach JT, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. New York, NY: Thieme; 1988. [Google Scholar]
- Tantillo M, Sanfter J. The relationship between perceived mutuality and bulimic symptoms, depression, and therapeutic change in group. Eating Behaviors. 2003;3:349–364. doi: 10.1016/s1471-0153(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Uvnaes-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Vannorsdall T, Dahlquist L, Pendley JS, Power T. The relation between nonessential touch and children’s distress during lumbar punctures. Children’s Health Care. 2004;33(4):299–315. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Yarcheski A, Mahon NE. The moderator-mediator role of social support in early adolescents. Western Journal of Nursing Research. 1999;21(5):685–698. doi: 10.1177/01939459922044126. [DOI] [PubMed] [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PloS One. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J-K, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]