Abstract
Thyroid hormones are important regulators of fetal neurodevelopment. Among preterm infants, TSH is highly variable. Understanding this variability will further improvements in screening for thyroid disorders in preterm infants. We examined 61 maternal and infant clinical and demographic factors for associations with neonatal TSH levels in 698 preterm neonates. TSH was measured as part of routine State-mandated newborn screening in Iowa. Of the maternal characteristics, nulliparous women (P=8x10−4), women with preeclampsia (P=2x10−3), and those with induced labor (P=3x10−3) had infants with higher TSH levels. TSH levels at the time of newborn screening were associated with respiratory distress syndrome (RDS) (P<0.0001) and sepsis (P=3x10−3). We replicated findings between parity and preeclampsia previously observed in primarily term infants. We also observed strong relationships between neonatal TSH and complications of prematurity including RDS and sepsis, which has implications for future studies examining this relationship both prenatally and longitudinally after birth.
Keywords: thyroid stimulating hormone, preterm birth, pregnancy complications, respiratory distress and sepsis
Introduction
Thyroid hormone is essential for fetal development, regulates neurodevelopment and is critical for brain maturation. Maternal and neonatal thyroid levels are tightly correlated and hypothyroidism in pregnancy is associated with pregnancy complications such as preterm birth and preeclampsia, as well as adverse neurodevelopmental outcomes in the offspring (1–3). Neonatal thyroid stimulating hormone (TSH), a hormone that stimulates the production of thyroid hormones, is highly variable among preterm infants and when measured 1–3 days after birth is significantly lower in preterm compared to term neonates (4). Associations between maternal and infant factors and TSH levels have generally been explored in the context of newborn screening programs but understanding the TSH variability in preterm infants also has implications for thyroid complications later-in-life as TSH, measured at birth is a highly heritable trait (5).
Several maternal and infant factors have been shown to contribute to the variability in TSH levels in term and preterm infants. Gestational age, infant gender, age at time of testing, month of birth, mode of delivery, and severity of illness in the neonate, have all been shown to affect the variability in TSH at birth (4, 6). Maternal characteristics such as parity, maternal race, gestational diabetes, and hypertension have all been associated with neonatal thyroid hormone levels; however, many of these studies have been performed with small sample sizes and results have been inconsistent (7–13).
Experimental and animal models demonstrate that thyroid hormones regulate surfactant metabolism (14–16). Surfactant insufficiency is associated with neonatal respiratory distress syndrome (RDS) which can occur in both preterm and term infants. Several studies have examined the association between TSH at birth and RDS in both term and preterm newborns; however, the results are inconsistent (6, 17–20). Less studied and known is the relationship between neonatal TSH and other complications of prematurity such as patent ductus arteriosus (PDA), interventricular hemorrhage (IVH), and sepsis.
Identifying factors that influence TSH variability in preterm infants may be useful, not only in the context of improving newborn screening, but also in understanding particular contributors to abnormal thyroid levels at birth that may carry forward into future thyroid problem in early childhood or adulthood. We examined the association between neonatal TSH levels and an extensive list of maternal, environmental, and neonatal factors, many of which have been examined in relation to thyroid hormone levels in primarily term infants (7), in 698 preterm (<37 weeks gestation) newborns.
Materials and Methods
This is a retrospective analysis of data collected as part of a prospective cohort for studying the epidemiology and genetics of preterm birth (21, 22). Study samples were collected at the University of Iowa Hospitals and Clinics in Iowa City, IA, after approval by the University of Iowa Institutional Review Board (IRB200506792). Signed informed consent was obtained from all families at enrollment. Existing data collected by an interview with the mother, medical chart review, or both, were mined for 61 maternal and infant clinical and demographic factors. Data extracted included infant demographic and newborn screening collection procedures including any changes to the TSH assay during the time of collection (Major Assay Change). Maternal demographics, exposures and medical history included questions women were asked about any smoking, alcohol, caffeine or drug use during pregnancy and any vitamin use (prenatal or otherwise) 3 or more times a week during pregnancy and 3 months prior to pregnancy. Maternal pregnancy complications included data on diagnosed diabetes, hypertension and infection (chorioamnionitis) during pregnancy as well as the type of delivery (vaginal or C-section) and type of labor (no labor or induced). Neonatal complications of preterm birth including bronchopulmonary dysplasia, respiratory distress syndrome, intraventricular hemorrhage, retinopathy of prematurity, sepsis and patent ductus arteriosus were extracted from the infant’s medical record. Completeness of the data varied across subjects, with a subset (N~300) of primarily earlier (<32 weeks) preterm birth, having complete and extensive data on all maternal and infant characteristics.
Data on TSH was obtained from the State of Iowa Hygienic Laboratory and linked to the clinical medical record data. TSH was measured by solid phase, time-resolved fluoroimmunoassay from dried newborn blood spots using PerkinElmer’s AutoDELFIA® platform (Waltham, MA, USA) as part of routine newborn screening. There were 698 preterm infants included for analysis. Infants were included if they were born preterm (delivery at 23–36 weeks gestation), had not received a blood transfusion at the time of sample collection, had their sample collected between 24 and 72 hours after birth, did not have a known congenital anomaly at birth, and had a normal (<2.2 mIU/L – 24.9 mIU/L) TSH level at initial screen. TSH levels that were under the limit of detection, i.e., <2.2 mIU/L, were assigned a value of 2.2.
Analysis of variance (ANOVA) was performed to test for association between the natural log transformed values of TSH and each demographic or clinic variable unadjusted (model 1) and adjusting for gestational age (model 2). Unadjusted one-way ANOVA models were assessed for homogeneity of variances using the Bartlett test of equal variances; deviations are noted in the tables. A Bonferroni significance threshold of P<1x10−3 was used to correct for multiple testing. Stata, version 12.0 (Stata Corporation, College Station, Tx) was used for all analyses.
Results
Associations between TSH and Infant Demographics
Neonatal TSH levels significantly decreased with decreasing gestational age (Figure 1a) and birth weight (Figure 1b) (Table 1). However, when including both variables in the same model only gestational age was significantly associated with TSH levels (P<0.0001) and explains 5.7% of the variance in TSH levels. Gestational age was the only infant demographic factor that remained significant after correction for multiple testing. Early and late age at sample collection (P=0.03), longer length at birth (P=0.03), winter month of birth (P=0.02), and male gender (P=0.02) were all marginally associated with higher neonatal TSH levels after adjustment for gestational age; however, these variables only contributed a small amount (≤1%) to the variability in TSH levels (Table 1).
Figure 1.
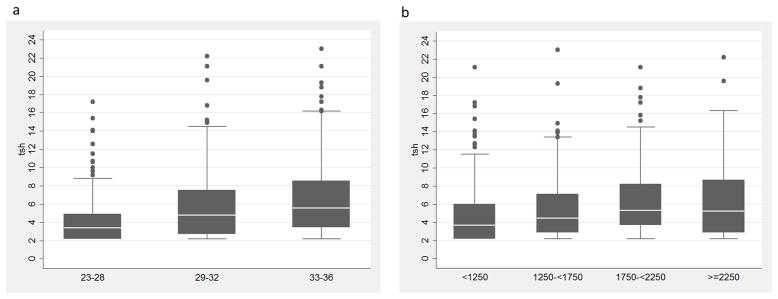
Distribution of TSH levels by gestational age in weeks (a) and birth weight in grams (b).
Table 1.
The Influence of Infant Demographics and Collection Procedures on Neonatal TSH
| Mean TSH (mIU/L)±SD | r2 | 1 P-value | r2 | 2 P-value | |
|---|---|---|---|---|---|
| Gestational age N=698 | 0.057 | <0.0001 | 0.060 | <0.0001* | |
| 23–28 wk (N=156) | 4.4±2.9 | ||||
| 29–32 wk (N=246) | 5.8±3.8 | ||||
| 33–36 wk (N=296) | 6.5±3.8 | ||||
| Birth weight N=698 | 0.034 | <0.0001 | 0.060 | 0.67 | |
| <1,250 g (N=185) | 5.0±3.6 | ||||
| 1,250–1749.9 g (N=179) | 5.6±3.5 | ||||
| 1,750–2,249.9 g (N=174) | 6.2±3.6 | ||||
| ≥2,250 g (N=160) | 6.4±4.0 | ||||
| Age at sample collection N=698 | 0.003 | 0.52 | 0.069 | 0.03 | |
| 24–36 hrs (N=588) | 5.8±3.6 | ||||
| 36–48 hrs (N=88) | 5.7±4.1 | ||||
| 48–60 hrs (N=18) | 4.8±.3.0 | ||||
| 60–72 hrs (N=4) | 6.5±6.6 | ||||
| Length at birth N=494 | 0.013 | 0.10 | 0.075 | 0.03 | |
| <25th (27–38.5 inches) (N=125) | 5.6±3.9 | ||||
| 25–50th (39–42.5 inches) (N=127) | 5.6±3.5 | ||||
| 50–75th (43–46 inches) (N=149) | 5.9±3.4 | ||||
| >75th (46.5–53.5 inches) (N=93) | 6.6±4.1 | ||||
| Head circumference at birth N=507 | 0.025 | 6x10−3 | 0.068 | 0.13 | |
| <25th (21–27.5 inches) (N=139) | 5.3±3.9 | ||||
| 25–50th (28–30 inches) (N=136) | 5.8±3.7 | ||||
| 50–75th (30.5–32 inches) (N=134) | 6.5±3.7 | ||||
| >75th (32.5–45.5 inches) (N=98) | 6.1±3.8 | ||||
| Season of Birth N=698 | 0.013 | 0.03 | 0.071 | 0.02 | |
| Winter (Dec-Feb) (N=182) | 6.1±3.7 | ||||
| Spring (Mar-May) (N=153) | 5.9±3.6 | ||||
| Summer (Jun-Aug) (N=182) | 5.2±3.5 | ||||
| Fall (Sep-Nov) (N=181) | 5.9±3.9 | ||||
| Year N=698 | 0.004 | 0.10 | 0.063 | 0.05 | |
| 2000–2004 (N=310) | 5.5±3.5 | ||||
| 2005–2009 (N=388) | 6.0±3.9 | ||||
| Major Assay Change N=698 | 0.002 | 0.54 | 0.060 | 0.42 | |
| Change #1 (N=3) | 5.6±3.7 | ||||
| Change #2 (N=433) | 5.7±3.7 | ||||
| Change #3 (N=262) | 5.9±3.7 | ||||
| Infant Gender N=698 | 0.006 | 0.05 | 0.065 | 0.02 | |
| Male (N=391) | 6.1±4.0 | ||||
| Female (N=307) | 5.4±3.3 | ||||
| Infant Race N=698 | <0.001 | 0.89 | 0.058 | 0.58 | |
| White (N=603) | 5.8±3.7 | ||||
| Non-White/Unknown (N=95) | 5.8±4.0 | ||||
| Multiple Birth N=698 | <0.001 | 0.76 | 0.058 | 0.80 | |
| Singleton (N=557) | 5.7±3.7 | ||||
| Twin/Multiple (N=141) | 5.8±3.9 | ||||
| Feeding method at birth N=538 | 0.020 | 0.01 | 0.066 | 0.23 | |
| Bottle (N=47) | 6.3±3.8 | ||||
| Breast (N=238) | 6.1±3.9 | ||||
| Both (N=12) | 6.3±3.7 | ||||
| NPO (N=241) | 5.3±3.6 | ||||
| Total parenteral nutrition at birth N=698 | <0.001 | 0.52 | 0.059 | 0.30 | |
| No (N=631) | 5.8±3.7 | ||||
| Yes (N=67) | 5.7±4.2 |
P-value: single variable one-way ANOVA.
P-value: ANOVA adjusted for gestational age.
This model includes adjustment for birth weight. NPO = nil per os - nothing by mouth, Major Assay Change is defined as a manufacture’s kit change which could alter the assay variability in TSH levels.
Associations between TSH and Maternal Demographics and Pregnancy Complications
Maternal smoking, drug use during pregnancy, and prenatal steroids were significantly associated with lower neonatal TSH levels; however, none remained significant after adjustment for gestational age (Table 2). Multiparous women had infants with lower TSH levels (P=8x10−4) compared to women who were nulliparous, a result that remained significant after correction for multiple testing and contributes 1.5% to the variability in TSH levels (Table 2). Preeclampsia (P=2x10−3) and induced labor (P=3x10−3) were associated with higher neonatal TSH levels after adjustment for gestational age, and were close to the threshold for correction for multiple testing and contributed 1.1% and 2.4% to the variability in TSH levels (Table 3).
Table 2.
The Influence of Maternal Demographics, Exposures, and Medical History on Neonatal TSH
| Mean TSH (mIU/L)±SD | r2 | 1 P-value | r2 | 2 P-value | |
|---|---|---|---|---|---|
| Maternal age N=681 | 0.005 | 0.36 | 0.060 | 0.49 | |
| <18 years (N=16) | 5.0±3.9 | ||||
| 18–25 years (N=272) | 5.7±3.8 | ||||
| 26–35 years (N=315) | 5.8±3.7 | ||||
| >35 years (N=78) | 6.0±3.1 | ||||
| Maternal pre-pregnancy BMI N=262 | 0.003 | 0.72 | 0.079 | 0.50 | |
| <=25 (N=143) | 5.9±3.3 | ||||
| 25-<30 (N=48) | 6.2±3.7 | ||||
| >=30 (N=71) | 6.5±4.2 | ||||
| Smoking during pregnancy N=599 | 0.007 | 0.04 | 0.067 | 0.05 | |
| No (N=448) | 5.8±3.6 | ||||
| Yes (N=151) | 5.3±3.9 | ||||
| Alcohol use during pregnancy N=577 | <0.001 | 0.52 | 0.061 | 0.48 | |
| No (N=532) | 5.7±3.6 | ||||
| Yes (N=45) | 5.8±3.2 | ||||
| Drug use during pregnancy N=564 | 0.007 | 0.04 | 0.070 | 0.05 | |
| No (N=539) | 5.7±3.6 | ||||
| Yes (N=25) | 4.5±3.7 | ||||
| Caffeine use during pregnancy N=245 | 0.003 | 0.39 | 0.094 | 0.57 | |
| No (N=89) | 6.0±3.2 | ||||
| Yes (N=156) | 5.8±3.7 | ||||
| Vitamin use during pregnancy N=283 | <0.001 | 0.85 | 0.062 | 0.64 | |
| No (N=8) | 6.0±3.5 | ||||
| Yes (N=275 | 5.8±3.5 | ||||
| Vitamin use 3 months prior to pregnancy N=162 | <0.001 | 0.83 | 0.138 | 0.71 | |
| No (N=60) | 6.0±3.9 | ||||
| Yes (N=102) | 5.9±3.3 | ||||
| Parity N=629 | 0.015 | 2x10−3 | 0.072 | 8x10−4 | |
| Nulliparous (N=347) | 6.1±3.9 | ||||
| Multiparous (N=282) | 5.2±3.3 | ||||
| Prenatal Steroids N=647 | 0.018 | 6x10−4 | 0.057 | 0.24 | |
| No (N=178) | 6.5±4.1 | ||||
| Yes (N=469) | 5.4±3.5 | ||||
| Antibiotics during pregnancy or delivery N=231 | 0.003 | 0.43 | 0.064 | 0.44 | |
| No (N=32) | 6.3±3.7 | ||||
| Yes (N=199) | 5.8±3.7 | ||||
| Maternal thyroid disease N=277 | <0.001 | 0.66 | 0.101 | 0.51 | |
| No (N=256) | 5.7±3.4 | ||||
| Yes (N=21) | 6.0±3.5 | ||||
| Maternal urinary tract infection N=450 | 0.002 | 0.42 | 0.057 | 0.32 | |
| No (N=362) | 5.7±3.5 | ||||
| Yes (N=88) | 6.1±4.2 | ||||
| Sexually transmitted disease when pregnant N=238 | 0.012 | 0.09 | 0.075 | 0.16 | |
| No (N=216) | 5.7±3.6 | ||||
| Yes (N=22) | 4.3±2.5 |
P-value: single variable one-way ANOVA.
P-value: ANOVA adjusted for gestational age.
Table 3.
The Influence of Maternal Pregnancy Complications and Delivery on Neonatal TSH
| Mean TSH (mIU/L)±SD | r2 | 1 P-value | r2 | 2 P-value | |
|---|---|---|---|---|---|
| Gestational diabetes N=660 | 0.003 | 0.14 | 0.057 | 0.33 | |
| No (N=618) | 5.6±3.6 | ||||
| Yes (N=42) | 6.5±4.2 | ||||
| Chronic hypertension N=429 | <0.001 | 0.74 | 0.068 | 0.42 | |
| No (N=389) | 5.6±3.6 | ||||
| Yes (N=40) | 5.5±2.9 | ||||
| Gestational hypertension N=591 | 0.004 | 0.15 | 0.057 | 0.28 | |
| No (N=553) | 5.7±3.6 | ||||
| Yes (N=38) | 6.8±4.6 | ||||
| Preeclampsia N=662 | 0.011 | 7x10−3 | 0.071 | 2x10−3 | |
| No (N=489) | 5.4±3.4 | ||||
| Yes (N=173) | 6.4±4.3 | ||||
| HELLP syndrome N=391 | <0.001 | 0.61 | 0.058 | 0.35 | |
| No (N=356) | 5.9±3.8 | ||||
| Yes (N=35) | 6.1±3.6 | ||||
| Oligohydraminos N=309 | 0.002 | 0.40 | 0.069 | 0.10 | |
| No (N=276) | 5.8±3.7 | ||||
| Yes (N=33) | 6.3±3.5 | ||||
| Polyhydraminos N=298 | 0.013 | 0.05 | 0.082 | 0.03 | |
| No (N=288) | 5.9±3.7 | ||||
| Yes (N=10) | 4.1±3.3 | ||||
| Type of Delivery N=695 | <0.001 | 0.92 | 0.059 | 0.31 | |
| Vaginal (N=315) | 5.8±3.8 | ||||
| C-section (N=380) | 5.7±3.6 | ||||
| Type of Labor N=671 | 0.024 | 3x10−4* | 0.070 | 3x10−3 | |
| No labor (N=159) | 6.1±3.8 | ||||
| Induced (N=98) | 7.1±4.8 | ||||
| Spontaneous (N=414) | 5.2±3.2 | ||||
| Rupture of membranes N=603 | 0.001 | 0.35 | 0.058 | 0.44 | |
| Spontaneous (N=290) | 5.4±3.2 | ||||
| Artificial (N=313) | 5.9±3.9 | ||||
| Placental abruption N=502 | 0.003 | 0.23 | 0.049 | 0.62 | |
| No (N=459) | 5.7±3.6 | ||||
| Yes (N=43) | 5.1±3.0 | ||||
| Chorioamnionitis N=574 | 0.009 | 0.03 | 0.062 | 0.16 | |
| No (N=529) | 5.9±3.7 | ||||
| Yes (N=45) | 4.8±3.0 | ||||
| Group B streptococcus N=410 | <0.001 | 0.74 | 0.062 | 0.61 | |
| No (N=316) | 5.9±3.9 | ||||
| Yes (N=94) | 5.8±3.5 |
P-value: single variable one-way ANOVA.
P-value: ANOVA adjusted for gestational age.
Bartlett’s test for equal variances was p=0.02.
Associations between TSH and Complications of Prematurity
Several complications of premature birth were associated with lower neonatal TSH levels including bronchopulmonary dysplasia (BPD), PDA, RDS, retinopathy of prematurity (ROP) and sepsis; however after adjusting for gestational age only associations with RDS (P<0.0001) and sepsis (P=3x10−3) remained significant(Table 4). RDS explained 8.4% of the variability of TSH measurements and when including gestational age in the model the variability explained was 10.3%. Sepsis explained 5.7% of the variability in TSH levels and when including gestational age in the model the variability explained was 8.0%. There were no associations between APGAR scores, bilirubin levels, or hemoglobin levels at birth and neonatal TSH after adjustment for gestational age. As the prevalence of most complications of prematurity increase with decreasing gestational age, we also examined the association between neonatal TSH and each complication in neonates born <32 weeks gestation (Table 5). Significantly lower neonatal TSH was observed in neonates <32 weeks gestation with RDS (P=2x10−4); a result significant after correction for multiple testing (Table 5).
Table 4.
The Association between Complications of Prematurity and Neonatal TSH
| Mean TSH (mIU/L)±SD | r2 | 1 P-value | r2 | 2 P-value | |
|---|---|---|---|---|---|
| Bronchopulmonary dysplasia N=500 | 0.035 | <0.0001 | 0.059 | 0.29 | |
| No (N=355) | 5.9±3.9 | ||||
| Yes (N=145) | 4.6±3.3 | ||||
| Intraventricular hemorrhage N=450 | 0.003 | 0.26 | 0.052 | 0.52 | |
| No (N=396) | 5.4±3.6 | ||||
| Yes (N=54) | 4.6±2.4 | ||||
| Necrotizing enterocolitis N=618 | 0.001 | 0.43 | 0.054 | 0.77 | |
| No (N=606) | 5.7±3.7 | ||||
| Yes (N=12) | 5.0±3.4 | ||||
| Patent ductus arteriosus N=678 | 0.027 | <0.0001 | 0.062 | 0.08 | |
| No (N=557) | 6.0±3.7 | ||||
| Yes (N=121) | 4.6±3.3 | ||||
| Periventricular leukomalacia N=556 | 0.005 | 0.08 | 0.056 | 0.17 | |
| No (N=547) | 5.6±3.7 | ||||
| Yes (N=9) | 3.6±1.9 | ||||
| Respiratory distress syndrome N=607 | 0.084 | <0.0001 | 0.103 | <0.0001 | |
| No (N=247) | 6.7±3.9 | ||||
| Yes (N=360) | 4.8±3.2 | ||||
| Retinopathy of prematurity N=504 | 0.017 | 3x10−3* | 0.060 | 0.43 | |
| No (N=431) | 5.7±3.8 | ||||
| Yes (N=73) | 4.2±2.3 | ||||
| Sepsis N=583 | 0.057 | <0.0001 | 0.080 | 3x10−3 | |
| No (N=190) | 6.7±3.8 | ||||
| Suspected (N=314) | 5.4±3.7 | ||||
| Yes (N=79) | 4.5±3.0 | ||||
| APGAR score at 1 minute N=688 | 0.003 | 0.13 | 0.058 | 0.54 | |
| <5 (N=138) | 5.4±3.7 | ||||
| ≥5 (N=550) | 5.9±3.7 | ||||
| APGAR score at 5 minutes N=689 | 0.001 | 0.32 | 0.059 | 0.79 | |
| <5 (N=21) | 5.2±3.5 | ||||
| ≥5 (N=668) | 5.8±3.7 | ||||
| Highest direct bilirubin at birth N=186 | 0.069 | 4x10−3 | 0.110 | 0.06 | |
| <25th (0–0.2) (N=21) | 5.8±4.8 | ||||
| 25–50th (0.3) (N=56) | 6.9±4.0 | ||||
| 50–75th (0.4–0.7) (N=61) | 5.4±2.9 | ||||
| >75th (0.8–18.6) (N=48) | 4.4±2.6 | ||||
| Highest total bilirubin at birth N=654 | 0.007 | 0.20 | 0.062 | 0.35 | |
| <25th (0.8–7.3) (N=157) | 5.4±3.8 | ||||
| 25–50th (7.4–9.2) (N=161) | 5.4±3.4 | ||||
| 50–75th (9.3–11.4) (N=174) | 6.1±4.1 | ||||
| >75th (11.5–27.1) (N=162) | 5.8±3.4 | ||||
| Hemoglobin at birth* N=306 | 0.018 | 0.14 | 0.075 | 0.63 | |
| <25th (10.2–15.5) (N=75) | 5.2±3.4 | ||||
| 25–50th (15.6–17) (N=82) | 5.3±3.3 | ||||
| 50–75th (17.1–18.4) (N=72) | 5.9±3.4 | ||||
| >75th (18.6–25.5) (N=77) | 6.3±4.1 |
P-value: single variable one-way ANOVA.
P-value: ANOVA adjusted for gestational age.
Bartlett’s test for equal variances was p=0.002.
Table 5.
The Association between Complications of Prematurity and Neonatal TSH in Infants Born <32 weeks
| Mean TSH (mIU/L)±SD | r2 | 1 P-value | r2 | 2 P-value | |
|---|---|---|---|---|---|
| Bronchopulmonary dysplasia N=288 | 0.011 | 0.08 | 0.016 | 0.38 | |
| No (N=148) | 5.0±3.2 | ||||
| Yes (N=140) | 4.5±3.2 | ||||
| Intraventricular hemorrhage N=320 | 0.001 | 0.54 | 0.017 | 0.59 | |
| No (N=274) | 4.9±3.4 | ||||
| Yes (N=46) | 4.4±2.3 | ||||
| Necrotizing enterocolitis N=321 | <0.001 | 0.84 | 0.021 | 0.75 | |
| No (N=311) | 4.9±3.3 | ||||
| Yes (N=10) | 5.3±3.7 | ||||
| Patent ductus arteriosus N=333 | 0.016 | 0.02 | 0.025 | 0.11 | |
| No (N=227) | 5.1±3.3 | ||||
| Yes (N=106) | 4.4±3.3 | ||||
| Periventricular leukomalacia N=331 | 0.004 | 0.24 | 0.022 | 0.22 | |
| No (N=322) | 4.9±3.3 | ||||
| Yes (N=9) | 3.6±1.9 | ||||
| Respiratory distress syndrome N=318 | 0.054 | <0.0001 | 0.061 | 2x10−4 | |
| No (N=65) | 6.2±3.7 | ||||
| Yes (N=253) | 4.5±3.1 | ||||
| Retinopathy of prematurity N=310 | 0.005 | 0.20* | 0.014 | 0.51 | |
| No (N=237) | 5.0±3.4 | ||||
| Yes (N=73) | 4.2±2.3 | ||||
| Sepsis N=302 | 0.017 | 0.07 | 0.022 | 0.21 | |
| No (N=38) | 5.4±3.0 | ||||
| Suspected (N=198) | 4.9±3.2 | ||||
| Yes (N=66) | 4.3±3.0 |
P-value: single variable one-way ANOVA.
P-value: ANOVA adjusted for gestational age.
Bartlett’s test for equal variances was p=0.006.
Discussion
We extensively examined associations between neonatal TSH levels and demographic factors, maternal exposures and infant outcomes. We replicated, in preterm infants, findings between maternal and infant demographics and TSH which, in general, were previously examined in term infants (7). We observe that within preterm infants there is a distinct and graded decrease in TSH levels between preterm (33–36 weeks), very preterm (29–32 weeks) and extremely preterm (23–28 weeks) neonates. There have been some reports examining neonatal screening measures of TSH that have found the opposite in that both decreasing gestational age and decreasing birth weight was associated with increasing TSH levels (23, 24). However, these studies measured TSH levels around the 6th day of life, whereas our study measured infants between day of life 1 and 3, but primarily on day 1 of life. Studies examining cord blood levels or levels obtained soon after delivery have observed similar results to ours (5, 6, 25).
Several studies have examined the impact of maternal demographics (e.g., parity), maternal exposures (e.g., smoking), pregnancy complications (e.g., hypertension), and delivery outcomes (e.g., delivery method) on neonatal thyroid hormone levels. One such study (Herbstman et al., 2008) examined an extensive list of maternal and infant demographics and delivery outcomes on cord blood levels of total T4, free T4 and TSH (7). The authors studied 300 neonates from Baltimore, the majority (87%) of which were term infants, and found that infants of mothers delivering their first child had higher TSH levels than multiparous mothers. We observed a similar association between higher neonatal TSH and first-born children. Another study by Sullivan et al., also found this association; however the mechanism for this relationship is unclear (10). A likely explanation would be that altered levels of maternal TSH in subsequent pregnancies affect neonatal levels at birth. However, while there are contradictory data on whether maternal TSH stimulation influences fetal TSH levels, one study found no such evidence (26). To our knowledge, maternal levels of TSH throughout pregnancy have not been directly examined by parity.
Herbstman et al., also found that neonates delivered by C-section or unassisted vaginal delivery had lower TSH levels compared to vaginal or assisted vaginal delivery, respectively (7). We did not observe this same trend, but found infants that were delivered spontaneously had lower TSH levels. The differences observed between our two studies are likely due to study design. Their population was comprised of mainly term infants whereas we studied preterm infants who already have lower TSH levels. Reasons for C-section between these two groups would also be very different, and the severity of illness, which affects neonatal TSH, would also differ, likely contributing to these differences. We also observed an association between higher neonatal TSH levels in infants born to mothers with preeclampsia. Two studies found higher cord blood TSH in preterm neonates born to mothers with preeclampsia; however, other studies, some of which evaluated term infants, have not observed this finding (7, 12, 27, 28). Our findings, in combination with the other studies that found higher neonatal TSH levels in preterm infants born to mothers with preeclampsia, suggest that there may be placental insufficiency and/or fetal hypoxia which in turn triggers higher TSH levels in the neonate.
Critical illness in both children and adults has been shown to suppress thyroid hormone secretion (29–32). This is defined as non-thyroidal illness syndrome (NTI). Our results support this concept in that we observed significant associations in preterm infants between TSH levels and RDS and sepsis. The effect of thyroid hormones on RDS has been extensively studied as thyroid hormones are regulators of surfactant metabolism, and surfactant insufficiency results in RDS (14–16). Several studies have shown an association between low TSH and RDS; however, the results have been inconsistent (6, 17–20). Additionally, a randomized, multicenter clinical trial found no difference in the frequency of RDS in the infants of women that were given antenatal thyrotropin-releasing hormone at <33 weeks gestation at the time of corticosteroid treatment compared to the group that received the placebo (33). We observed that steroid administration during pregnancy was associated with lower TSH levels at birth but this was not significant when adjusted by gestational age. Additionally, the strong association between low TSH levels and RDS (P<0.001) remained when adjusting for gestational age and prenatal steroids. Infants born preterm and those (term or preterm) that had RDS are at an increased risk for hypothyroidism, both in infancy and young adulthood (34, 35).
Several studies have suggested that thyroid hormones, specifically low total T3 and T4 levels are predictors of bacterial sepsis in children and neonates (36–39). We observed lower levels of TSH in infants with both suspected and confirmed sepsis. Sepsis was confirmed by a positive blood, urine or cerebral spinal fluid culture and included both early and late diagnosed sepsis. This may be a result of transient hypothyroxinemia which is associated with “normal” or low levels of TSH (40). Unfortunately, we were unable to assess transient hypothroxinemia as we did not have T4 measurements. More investigation is needed not only in relation to TSH and the timing of the development of sepsis but also the effect and timing of medications to treat sepsis that may also affect thyroid hormone levels.
Our study examined an extensive list of maternal, fetal, and delivery characteristics for associations with neonatal TSH levels. We replicated many previous findings in our preterm population from studies that have mostly focused on term infants. Our study was limited to examining only TSH and not the other thyroid hormones, such as T4, as Iowa’s newborn screening program only captures TSH. Additionally, future studies focused on longitudinal thyroid measurements in preterm infants, and analyzing medications administered during the NICU stay, would be ideal for identifying changes in levels in relation to the complications of preterm birth such as sepsis and RDS. We had limited information on maternal thyroid conditions and no information on iodine deficiency or subclinical hypothyroidism in pregnancy, and the presence of these conditions could impact our findings. The strength in our study was the ability to examine many demographic and clinical factors in a large sample of preterm infants.
Our study suggests that lower TSH levels are present at birth in infants with complications of prematurity including RDS and sepsis and this may warrant additional screening in these infants. Identifying factors that influence TSH variability in preterm infants will identify groups of infants that may be at increased risk for thyroid conditions later in childhood and adulthood.
Acknowledgments
We would like to express our thanks to all the participating families in our study. We would also like to express our gratitude to the coordinating medical and research staff at the University of Iowa Hospitals and Clinics in Iowa City, IA; including a special thanks to research coordinators Laura Knosp and Susan Berends. We would also like to thank Franklin Delin, Dariush Shirazi, and Stanton Berberich from the State Hygienic Laboratory for their assistance in the acquisition of the newborn screening data.
Financial Statement: This work was supported by the March of Dimes (1-FY05-126 and 6-FY08-260), National Institute of Health Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD-52953, R01 HD-57192, and R00 HD-065786).
Footnotes
Authors Disclosure Statement
No competing financial interests exist.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
References
- 1.Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VV, Hofman A, Hooijkaas H, Steegers EA, Tiemeier H, Bongers-Schokking JJ, Visser TJ. Maternal early pregnancy and newborn thyroid hormone parameters: The generation R study. J Clin Endocrinol Metab. 2012;97(2):646–52. doi: 10.1210/jc.2011-2398. [DOI] [PubMed] [Google Scholar]
- 2.Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. 2012;119(2 Pt 1):315–20. doi: 10.1097/AOG.0b013e318240de6a. [DOI] [PubMed] [Google Scholar]
- 3.Schneuer FJ, Nassar N, Tasevski V, Morris JM, Roberts CL. Association and predictive accuracy of high TSH serum levels in first trimester and adverse pregnancy outcomes. J Clin Endocrinol Metab. 2012;97(9):3115–22. doi: 10.1210/jc.2012-1193. [DOI] [PubMed] [Google Scholar]
- 4.Ryckman KK, Berberich SL, Shchelochkov OA, Cook DE, Murray JC. Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin Biochem. 2013;46(1–2):133–8. doi: 10.1016/j.clinbiochem.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alul FY, Cook DE, Shchelochkov OA, Fleener LG, Berberich SL, Murray JC, Ryckman KK. The heritability of metabolic profiles in newborn twins. Heredity (Edinb) 2013;110(3):253–8. doi: 10.1038/hdy.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K, Shimizu T, Hosaka A, Tokita A, Shiga S, Yamashiro Y. Serum free T4 and thyroid stimulating hormone levels in preterm infants and relationship between these levels and respiratory distress syndrome. Pediatr Int. 2007;49(4):447–51. doi: 10.1111/j.1442-200X.2007.02390.x. [DOI] [PubMed] [Google Scholar]
- 7.Herbstman J, Apelberg BJ, Witter FR, Panny S, Goldman LR. Maternal, infant, and delivery factors associated with neonatal thyroid hormone status. Thyroid. 2008;18(1):67–76. doi: 10.1089/thy.2007.0180. [DOI] [PubMed] [Google Scholar]
- 8.Wilker RE, Fleischman AR, Saenger P, Pan C, Surks MI. Thyroid hormone levels in diabetic mothers and their neonates. Am J Perinatol. 1984;1(3):259–62. doi: 10.1055/s-2007-1000015. [DOI] [PubMed] [Google Scholar]
- 9.Ward LS, Kunii IS, de Barros Maciel RM. Thyroid stimulating hormone levels in cord blood are not influenced by non-thyroidal mothers’ diseases. Sao Paulo Med J. 2000;118(5):144–7. doi: 10.1590/S1516-31802000000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr. 1997;127(1):55–8. doi: 10.1093/jn/127.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Franklin R, O’Grady C, Carpenter L. Neonatal thyroid function: Comparison between breast-fed and bottle-fed infants. J Pediatr. 1985;106(1):124–6. doi: 10.1016/s0022-3476(85)80481-9. [DOI] [PubMed] [Google Scholar]
- 12.Chan LY, Chiu PY, Lau TK. Cord blood thyroid-stimulating hormone level in high-risk pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003;108(2):142–5. doi: 10.1016/s0301-2115(02)00418-9. [DOI] [PubMed] [Google Scholar]
- 13.Lao TT, Lee CP. Gestational diabetes mellitus and neonatal hyperthyrotropinemia. Gynecol Obstet Invest. 2002;53(3):135–9. doi: 10.1159/000058363. [DOI] [PubMed] [Google Scholar]
- 14.Hitchcock KR. Lung development and the pulmonary surfactant system: Hormonal ifluences. Anat Rec. 1980;198(1):13–34. doi: 10.1002/ar.1091980103. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock KR. Hormones and the lung. I. thyroid hormones and glucocorticoids in lung development. Anat Rec. 1979;194(1):15–39. doi: 10.1002/ar.1091940103. [DOI] [PubMed] [Google Scholar]
- 16.Wilson M, Hitchcock KR, Douglas WH, DeLellis RA. Hormones and the lung. II. immunohistochemical localization of thyroid hormone binding in type II pulmonary epithelial cells clonally-derived from adult rat lung. Anat Rec. 1979;195(4):611–9. doi: 10.1002/ar.1091950404. [DOI] [PubMed] [Google Scholar]
- 17.van Wassenaer AG, Kok JH, Dekker FW, de Vijlder JJ. Thyroid function in very preterm infants: Influences of gestational age and disease. Pediatr Res. 1997;42(5):604–9. doi: 10.1203/00006450-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Klein RZ, Carlton EL, Faix JD, Frank JE, Hermos RJ, Mullaney D, Nelson JC, Rojas DA, Mitchell ML. Thyroid function in very low birth weight infants. Clin Endocrinol (Oxf) 1997;47(4):411–7. doi: 10.1046/j.1365-2265.1997.2511064.x. [DOI] [PubMed] [Google Scholar]
- 19.Cuestas RA, Lindall A, Engel RR. Low thyroid hormones and respiratory-distress syndrome of the newborn. studies on cord blood. N Engl J Med. 1976;295(6):297–302. doi: 10.1056/NEJM197608052950601. [DOI] [PubMed] [Google Scholar]
- 20.Cuestas RA, Engel RR. Thyroid function in preterm infants with respiratory distress syndrome. J Pediatr. 1979;94(4):643–6. doi: 10.1016/s0022-3476(79)80042-6. [DOI] [PubMed] [Google Scholar]
- 21.Steffen KM, Cooper ME, Shi M, Caprau D, Simhan HN, Dagle JM, Marazita ML, Murray JC. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007;27(11):672–80. doi: 10.1038/sj.jp.7211806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehn NL, Cooper ME, Orr K, Shi M, Johnson MK, Caprau D, Dagle J, Steffen K, Johnson K, Marazita ML, Merrill D, Murray JC. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr Res. 2007;62(5):630–5. doi: 10.1203/PDR.0b013e3181567bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson J, Williams FL, Delahunty C, van Toor H, Wu SY, Ogston SA, Visser TJ, Hume R Scottish Preterm Thyroid Group. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J Clin Endocrinol Metab. 2005;90(3):1271–9. doi: 10.1210/jc.2004-2091. [DOI] [PubMed] [Google Scholar]
- 24.Ng SM, Wong SC, Paize F, Chakkarapani E, Newland P, Isherwood D, Didi M. Multivariate analyses of factors that affect neonatal screening thyroid stimulating hormone. J Pediatr Endocrinol Metab. 2011;24(9–10):727–32. doi: 10.1515/jpem.2011.234. [DOI] [PubMed] [Google Scholar]
- 25.Korada M, Pearce MS, Avis E, Turner S, Cheetham T. TSH levels in relation to gestation, birth weight and sex. Horm Res. 2009;72(2):120–3. doi: 10.1159/000232165. [DOI] [PubMed] [Google Scholar]
- 26.Dokumov SI, Milanov SC, Trepetshov SP. Thyroid-stimulating hormone in serum of mother and newborn at birth. J Obstet Gynaecol Br Commonw. 1971;78(9):809–11. doi: 10.1111/j.1471-0528.1971.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 27.Belet N, Imdat H, Yanik F, Kucukoduk S. Thyroid function tests in preterm infants born to preeclamptic mothers with placental insufficiency. J Pediatr Endocrinol Metab. 2003;16(8):1131–5. doi: 10.1515/jpem.2003.16.8.1131. [DOI] [PubMed] [Google Scholar]
- 28.Narin N, Kurtoglu S, Basbug M, Caksen H, Kafali M, Durak AC, Genc E, Narin F. Thyroid function tests in the newborn infants of preeclamptic women. J Pediatr Endocrinol Metab. 1999;12(1):69–73. doi: 10.1515/jpem.1999.12.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Faber J, Kirkegaard C, Rasmussen B, Westh H, Busch-Sorensen M, Jensen IW. Pituitary-thyroid axis in critical illness. J Clin Endocrinol Metab. 1987;65(2):315–20. doi: 10.1210/jcem-65-2-315. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell IM, Pollock JC, Jamieson MP, Donaghey SF, Paton RD, Logan RW. The effects of cardiopulmonary bypass on thyroid function in infants weighing less than five kilograms. J Thorac Cardiovasc Surg. 1992;103(4):800–5. [PubMed] [Google Scholar]
- 31.Murzi B, Iervasi G, Masini S, Moschetti R, Vanini V, Zucchelli G, Biagini A. Thyroid hormones homeostasis in pediatric patients during and after cardiopulmonary bypass. Ann Thorac Surg. 1995;59(2):481–5. doi: 10.1016/0003-4975(94)00879-c. [DOI] [PubMed] [Google Scholar]
- 32.Van den Berghe G, de Zegher F, Lauwers P. Dopamine suppresses pituitary function in infants and children. Crit Care Med. 1994;22(11):1747–53. [PubMed] [Google Scholar]
- 33.Collaborative trial of prenatal thyrotropin-releasing hormone and corticosteroids for prevention of respiratory distress syndrome. collaborative santiago surfactant group. Am J Obstet Gynecol. 1998;178(1 Pt 1):33–9. doi: 10.1016/s0002-9378(98)70622-2. [DOI] [PubMed] [Google Scholar]
- 34.Fisch RO, Bilek MK, Miller LD, Engel RR. Physical and mental status at 4 years of age of survivors of the respiratory distress syndrome. follow-up report from the collaborative study. J Pediatr. 1975;86(4):497–503. doi: 10.1016/s0022-3476(75)80137-5. [DOI] [PubMed] [Google Scholar]
- 35.Crump C, Winkleby MA, Sundquist J, Sundquist K. Preterm birth and risk of medically treated hypothyroidism in young adulthood. Clin Endocrinol (Oxf) 2011;75(2):255–60. doi: 10.1111/j.1365-2265.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yildizdas D, Onenli-Mungan N, Yapicioglu H, Topaloglu AK, Sertdemir Y, Yuksel B. Thyroid hormone levels and their relationship to survival in children with bacterial sepsis and septic shock. J Pediatr Endocrinol Metab. 2004;17(10):1435–42. [PubMed] [Google Scholar]
- 37.den Brinker M, Joosten KF, Visser TJ, Hop WC, de Rijke YB, Hazelzet JA, Boonstra VH, Hokken-Koelega AC. Euthyroid sick syndrome in meningococcal sepsis: The impact of peripheral thyroid hormone metabolism and binding proteins. J Clin Endocrinol Metab. 2005;90(10):5613–20. doi: 10.1210/jc.2005-0888. [DOI] [PubMed] [Google Scholar]
- 38.den Brinker M, Dumas B, Visser TJ, Hop WC, Hazelzet JA, Festen DA, Hokken-Koelega AC, Joosten KF. Thyroid function and outcome in children who survived meningococcal septic shock. Intensive Care Med. 2005;31(7):970–6. doi: 10.1007/s00134-005-2671-8. [DOI] [PubMed] [Google Scholar]
- 39.Das BK, Agarwal P, Agarwal JK, Mishra OP. Serum cortisol and thyroid hormone levels in neonates with sepsis. Indian J Pediatr. 2002;69(8):663–5. doi: 10.1007/BF02722699. [DOI] [PubMed] [Google Scholar]
- 40.Williams F, Hume R. The measurement, definition, aetiology and clinical consequences of neonatal transient hypothyroxinaemia. Ann Clin Biochem. 2011;48(Pt 1):7–22. doi: 10.1258/acb.2010.010174. [DOI] [PubMed] [Google Scholar]


