Abstract
Repeated, extreme, or traumatic stressors can elicit pathological effects leading to many negative physical and psychological outcomes. Stressors can precipitate the onset of psychiatric diseases, or exacerbate pre-existing disorders including various anxiety and mood disorders. As stressors can negatively impact human psychiatric health, it is essential to identify neurochemicals that may confer protection from the negative sequelae of repeated or extreme stress exposure. Elucidating the neurobiological underpinnings of stress resilience will enhance our ability to promote resilience to, or recovery from, stress-related psychiatric disease. Herein, we will review the evidence for neuropeptide Y as an endogenous mediator of resilience and its potential relevance for the treatment of stress-related psychiatric diseases.
Keywords: Neuropeptide Y, Stress resilience, Stress-related psychiatric disorders, Rodent models, Emotionality
Highlights
-
•
Overview of neuropeptide Y and receptor subtypes in the central nervous system.
-
•
Alterations of neuropeptide Y in human stress-related psychiatric disorders.
-
•
Evidence for neuropeptide Y in resilience to stress-related emotionality in rodent behavioral models.
-
•
Pharmacotherapeutic implications for neuropeptide Y in the treatment of stress-related psychiatric disorders.
1. Introduction
Stressors elicit a cascade of neuronal, endocrine, and behavioral responses that promote homoeostatic adaptation to changing or threatening environments. Stressors maintained over prolonged periods of time or perceived as extreme can lead to maladaptive responses within stress-integrative circuitry. Pathological neurochemical and behavioral mechanisms can then manifest in the form of stress-related psychiatric diseases including anxiety disorders, post-traumatic stress disorder (PTSD), and depression. Neuropeptides have been shown to be influential neuromodulators of stress-related emotionality (Kormos and Gaszner, 2013). A growing body of evidence supports a role for neuropeptide Y (NPY) as a protective neurochemical that mediates stress resilience. NPY is a 36-amino acid peptide derived from preproNPY and belonging to a family that also includes pancreatic polypeptide (PP) and peptide YY (PYY) (Larhammar et al., 1993). NPY is highly conserved across mammalian species and is expressed throughout the central nervous system (CNS) (Larhammar and et al, 2001, Adrian and et al, 1983, Allen and et al, 1983, Lundberg and Hokfelt, 1986, Hirsch and Zukowska, 2012). In the periphery, NPY is expressed primarily in sympathetic ganglia, the adrenal medulla, and in platelets (Larhammar and et al, 2001, Adrian and et al, 1983, Allen and et al, 1983, Lundberg and Hokfelt, 1986, Hirsch and Zukowska, 2012). NPY is the most abundant and widely distributed neuropeptide in the human brain (Adrian et al., 1983), and has been shown to have a significant impact on brain activity. In the CNS, NPY and its receptors (Y1, Y2, Y4, Y5) play important roles in the control of food intake, energy homeostasis, pain, and many behavioral and physiological processes associated with stress and stress resilience (Hirsch and Zukowska, 2012, Brothers and Wahlestedt, 2010). In this review, we will discuss the role of NPY in stress-related behaviors and its relevance to select psychiatric disorders.
2. Neuropeptide Y (NPY)
2.1. NPY and NPY receptor subtypes in the brain
NPY immunopositive cell bodies and fibers are generally found in cortical, limbic, hypothalamic, and brainstem regions (Allen et al., 1983). Expression of NPY in the human and rodent brain is similar, with abundant NPY mRNA or immunoreactivity located in the neocortex, amygdala, hippocampus, basal ganglia, hypothalamus, periaqueductal grey, dorsal raphe nucleus, and the A1-3 and A6 noradrenergic cells groups in the brainstem (Adrian and et al, 1983, Allen and et al, 1983, Caberlotto et al., 2000, Wahlestedt et al., 1989, Yamazoe and et al, 1985, de Quidt and Emson, 1986a, de Quidt and Emson, 1986b). The effects of NPY are mediated by at least four subtypes of G-protein coupled receptors termed Y1, Y2, Y4, and Y5. Y6 receptors are expressed in the mouse brain, but this isoform is absent in the rat and nonfunctional in human and non-human primates (Larhammar and Salaneck, 2004). Autoradiographic and immunohistochemical examinations indicate that Y1 and Y2 receptors (Y1R and Y2R) exhibit the greatest expression in the brain, whereas lower levels of Y4 and Y5 receptors (Y4R and Y5R) are also present (Dumont and et al, 1993, Stanic and et al, 2006, Stanic and et al, 2011, Dumont and et al, 1998, Kask and et al, 2002, Wolak and et al, 2003). Significant differences in the distribution of NPY receptors are detectable between the rodent and human brain, warranting caution in the generalization of the role of NPY receptors from preclinical animal models to humans (Dumont et al., 1998). NPY receptors can couple to various effectors systems by associating with inhibitory Gi/o proteins (see review (Sah and Geracioti, 2013)). NPY receptors inhibit adenylyl cyclase and the accumulation of cAMP, mobilize calcium through phospholipase C and phosphatidylinositol 3-kinase activity, and have effects on multiple ion channels (Sah and Geracioti, 2013). Within stress responsive brain regions such as the cortex, amygdala, hypothalamus, and locus coeruleus, NPY receptors are localized on or impact the function of neurons expressing GABA, glutamate, corticotropin-releasing factor (CRF), and norepinephrine (NE) (Grove and et al, 2000, Dimitrov and et al, 2007, Giesbrecht and et al, 2010, Rostkowski and et al, 2009, Illes et al., 1993, Eaton et al., 2007). It has been hypothesized that NPY serves as a functional “brake” to tone down the excitatory effects of pro-stress neurotransmitters such as CRF and NE (Sah and Geracioti, 2013, Eaton et al., 2007, Heilig and et al, 1994). This hypothesis is supported by studies demonstrating that NPY is frequently contained within the same neuroanatomical brain structures as CRF and NE, and the function of NPY is often physiologically and behaviorally opposite to pro-stress neurotransmitters (reviewed in (Kask and et al, 2002, Sah and Geracioti, 2013, Sajdyk et al., 2004)). Although clear interactions between NPY and pro-stress systems in the regulation of stress-related emotionality still need to be established, it is likely that the balance of these neuropeptides and transmitters in stress-related circuits plays a pivotal role in mediating resilience to stress-associated responses discussed in this review.
3. NPY in stress-related psychiatric disorders: insight from human studies
3.1. Stress and anxiety
Human studies have identified associations between NPY and stress resilience. In healthy human subjects, plasma NPY levels have been shown to rise in response to stress (Morgan 3rd and et al, 2001, Morgan 3rd and et al, 2000, Morgan 3rd and et al, 2002). For example, when military soldiers underwent an interrogation model of extreme psychological stress to mimic the captive experience of prisoners of war, higher levels of NPY following interrogation were present in soldiers displaying lower psychological distress or belonging to special operations forces (Morgan 3rd and et al, 2000, Morgan 3rd and et al, 2002). NPY levels were positively associated with feelings of dominance and self-confidence, and superior performance under interrogation stress (Morgan 3rd and et al, 2001, Morgan 3rd and et al, 2000, Morgan 3rd and et al, 2002).
Genetic variants of the preproNPY gene have been associated with differential stress responses and emotionality (Mickey and et al, 2011, Zhou and et al, 2008). Specific NPY haplotypes have been correlated to postmortem levels of NPY mRNA in the brain, plasma NPY concentrations, and brain activity in response to stressful challenges (Zhou et al., 2008). Individuals possessing a genotype associated with low NPY expression report more negative emotional experiences during a painful stressor, exhibit greater amygdalar reactivity in response to threat-related facial images, and exhibit low stress resilience compared to high NPY genotype carriers (Mickey and et al, 2011, Zhou and et al, 2008). Haplotype-driven NPY expression is also inversely correlated to trait anxiety in healthy individuals (Zhou et al., 2008).
Studies in humans with stress-related psychiatric disorders have also revealed a role for NPY in resilience (Eaton et al., 2007, Morales-Medina et al., 2010, Sah and et al, 2009, Rasmusson and et al, 2000a, Morgan 3rd and et al, 2003), although the evidence stems primarily from populations with PTSD and depression. Rodent studies have provided a wealth of evidence for NPY in resilience to anxiety (see below), but few human studies have been conducted to determine the profile of NPY in generalized anxiety, obsessive compulsive, social anxiety, and panic disorders. One study found an association between a single-nucleotide polymorphism of the NPY gene and increased risk for generalized anxiety disorder in individuals exposed to high stress (Amstadter et al., 2010). Genetic variants of the Y5 receptor gene have been significantly associated with panic disorder (Domschke et al., 2008). Elevated plasma NPY was detected in a study of individuals with panic disorder, in which the authors suggest that an increase in NPY may be compensatory to buffer enhanced sympathetic activation in this disorder (Boulenger et al., 1996). Other studies have not detected differences in NPY levels between healthy controls and persons with obsessive compulsive, social anxiety, or panic disorders (Stein and et al, 1996, Altemus and et al, 1999), or have failed to identify genetic associations between NPY and anxiety disorders (Lindberg et al., 2006).
3.2. Depression
Clinical investigations have revealed that the plasma and CSF of depressed individuals contain decreased concentrations of NPY compared to healthy controls (Hashimoto and et al, 1996, Heilig and et al, 2004, Hou and et al, 2006, Nilsson and et al, 1996, Widerlov and et al, 1988). Additional studies have shown lower NPY in clinically depressed patients with a history of suicide attempts compared to healthy persons, and that NPY levels are lowest in individuals with a recent suicide attempt (Westrin et al., 1999). Likewise, low NPY immunoreactivity has been found in postmortem brain tissue of suicide victims, with the most robust reductions in NPY occurring in the brains of persons with a history of depression (Widdowson et al., 1992). Low levels of NPY mRNA expression are also found in persons with bipolar disorder (Caberlotto and Hurd, 1999, Kuromitsu and et al, 2001). Genetic variants of the preproNPY gene have been associated with resilience or vulnerability to depression (Heilig and et al, 2004, Wang and et al, 2013, Sjoholm and et al, 2009). For instance, a genetic polymorphism resulting in higher levels of mature NPY appears to be protective against depression despite exposure to environmental risk factors (Sjoholm et al., 2009), and the presence of this polymorphism is less frequent in depressed patients (Heilig et al., 2004). In another study, a genotype associated with low NPY expression was found to be overrepresented in persons with major depression compared to healthy controls (Mickey et al., 2011). Interestingly, antidepressant strategies are associated with parallel elevations in NPY and decreases in corticotropin-releasing hormone (CRH), thereby supporting peptidergic interactions in the mechanisms underlying clinically efficacious treatments for depression. For example, CSF levels of NPY are elevated in depressed patients following electroconvulsive therapy, while levels of corticotropin-releasing hormone decrease concurrently (Mathé and et al, 1995, Nikisch and Mathe, 2008). Increased NPY after treatment with the selective serotonin reuptake inhibitor citalopram is associated with a reduction in depression severity and the levels of CRH (Nikisch et al., 2005).
3.3. Post-traumatic stress disorder (PTSD)
Reduced concentrations of cerebrospinal and plasma NPY have been reported in both individuals with PTSD and those who have been exposed to traumatic stress (Sah and et al, 2009, Rasmusson and et al, 2000a, Morgan 3rd and et al, 2003). NPY is inversely related to PTSD symptomology, with low NPY correlating specifically to the presence of intrusion symptoms (Sah et al., 2014). Higher NPY is predicative of PTSD symptom improvement and shows a positive association with coping following a traumatic event (Yehuda et al., 2006). Aberrant NPY and norepinephrine function have been linked in PTSD. Yohimbine, an antagonist of the presynaptic α2-adrenergic receptor that increases norepinephrine levels, elicits panic attacks and exacerbates the core symptoms of PTSD (Bremner et al., 1997). Yohimbine has also been shown to stimulate increases in plasma NPY and levels of the norepinephrine metabolite MHPG (3-methyl-4-hydroxy-phenyl-glycol) in healthy subjects. However, yohimbine-stimulated increases in NPY are significantly blunted in persons with PTSD (Rasmusson and et al, 2000a, Rasmusson and et al, 1998). Additionally, baseline concentrations of plasma NPY correlated negatively to yohimbine-induced increases in MHPG in the same study (Rasmusson et al., 2000). This correlation suggests that low basal levels of NPY were associated with an exaggerated increase in MHPG following yohimbine (Rasmusson et al., 2000). Both basal and yohimbine-stimulated levels of NPY were negatively correlated to scores on a combat-exposure scale, indicating that greater combat exposure was associated with blunted levels of NPY (Rasmusson et al., 2000).
4. Potential therapeutic applications of NPY: evidence from animal models
Pathological responses to stress manifest in behaviors that include enhanced anxiety, arousal, and fear. In this section, we review the findings in animal models utilized to examine these three behavioral responses, as well as the effects of NPY in rodent models of PTSD and depression-like behavior. Examples provided in the text are summarized in Table 1.
Table 1.
Behavioral observations following pharmacological interventions or genetic manipulations of the NPY system.
| Rodent models | Pharmacological intervention or genetic manipulation | Route of administration/region | Direction of behavioral effect | Reference |
|---|---|---|---|---|
| Anxiety–like Behavior | NPY | i.c.v. BLA, CeA Hippocampus Lateral Septum LC |
Decrease | (Broqua and et al, 1995, Heilig and et al, 1989, Britton and et al, 1997, Heilig and et al, 1992) (Primeaux and et al, 2005, Sajdyk et al., 1999, Heilig and et al, 1993) (Lin and et al, 2010, Thorsell and et al, 2000) (Trent and Menard, 2011) (Kask et al., 1998) |
| NPY knockout | Increase | (Bannon et al., 2000) | ||
| Y1R agonists | i.c.v. CeA Hippocampus |
Decrease | (Britton and et al, 1997, Sorensen and et al, 2004) (Heilig and et al, 1993, Lyons and Thiele, 2010) (Olesen et al., 2012) |
|
| Y1R antagonists | i.c.v. PAG LC Hypothalamus CeA |
Increase No effect |
(Kask et al., 1998b, Kask et al., 1998a, Kask et al., 1998b, Kask et al., 1998c, Kask and et al, 1999) (Kask et al., 1998) |
|
| Y1R knockout | Increase | (Karl et al., 2006, Longo and et al, 2014, Bertocchi and et al, 2011) | ||
| Y2R agonist | i.c.v. LC Lateral septum BLA (high dose) |
No effect Decrease |
(Broqua and et al, 1995, Heilig and et al, 1989, Britton and et al, 1997, Sorensen and et al, 2004) (Kask et al., 1998) (Trent and Menard, 2013) (Sajdyk et al., 2002) |
|
| Y2R antagonists | CeA | Decrease | (Kallupi et al., 2013) | |
| Y2R knockout | Global, BLA or CeA GABAergic neurons in CeA |
Decrease Increase |
(Tasan and et al, 2010, Tasan and et al, 2009, Painsipp et al., 2008, Painsipp and et al, 2008, Tschenett and et al, 2003) (McCall et al., 2013) |
|
| Y4R knockout | Decrease | (Tasan and et al, 2009, Painsipp and et al, 2008) | ||
| Y5R agonist | i.c.v. | Decrease | (Sorensen et al., 2004) | |
| Y5R antagonist | BLA | Decrease | (Sajdyk et al., 2002) | |
| Arousal | NPY | i.c.v., BLA CeA |
Decrease No effect |
(Broqua and et al, 1995, Gilpin and et al, 2011, Gutman and et al, 2008) (Gutman et al., 2008) |
| NPY knockout | Increase | (Sudakov et al., 2001) | ||
| Y1R agonists | i.c.v. | Decrease | (Broqua et al., 1995) | |
| Y2R | i.c.v. | No effect | (Broqua et al., 1995) | |
| Y2R knockout | Increase | (Sudakov et al., 2001) | ||
| Fear | NPY | i.c.v., Amygdala | Decrease | (Gutman and et al, 2008, Lach and de Lima, 2013, Fendt and et al, 2009, Pickens and et al, 2009) |
| NPY knockout | Increase | (Verma et al., 2012) | ||
| Y1R agonists | i.c.v. | Decrease | (Lach de Lima, 2013) | |
| Y1R antagonists | i.c.v. Amygdala |
Block NPY effects Increase |
(Lach de Lima, 2013) (Gutman et al., 2008) |
|
| Y1R knockout | Increase | (Fendt et al., 2009) | ||
| Y2R knockout | No effect | (Verma et al., 2012) | ||
| Depression–like Behavior | NPY | i.c.v., Hippocampus | Decrease | (Redrobe and et al, 2005, Stogner and Holmes, 2000, Redrobe and et al, 2002, Ishida and et al, 2007) |
| Y1R agonists | i.c.v. | Decrease | (Redrobe et al., 2002) | |
| Y1R antagonists | i.c.v. | Block NPY effects | (Redrobe et al., 2002) | |
| Y1R knockout | Increase | (Karlsson et al., 2008) | ||
| Y2R antagonists | i.c.v. | Decrease | (Redrobe et al., 2002) | |
| Y2R knockout | Decrease | (Painsipp et al., 2008) | ||
| Y4R knockout | Decrease | (Tasan and et al, 2009, Painsipp and et al, 2008) | ||
| Depression Models (OBX or FSL) | NPY | Decrease (OBX) | (Goyal et al., 2009) | |
| Y1R agonist | i.c.v. | Decrease (OBX) | (Goyal et al., 2009) | |
| Y2R agonist | i.c.v. | Increase (OBX) | (Morales-Medina et al., 2012) | |
| Y2R antagonist | i.c.v. | Decrease (OBX) | (Morales-Medina et al., 2012) | |
| Y5R antagonist | i.c.v. | Decrease (FSL) | (Walker et al., 2009) | |
| PTSD models (PSS and SPS | NPY | Intranasal, Hippocampus | Decrease anxiety, arousal, fear, depression-like behaviors | (Cohen and et al, 2012, Serova and et al, 2013, Serova and et al, 2014) |
| Y1R antagonist | Hippocampus | Increase anxiety, arousal | (Cohen et al., 2012) |
4.1. Anxiety
Genetic rodent models and pharmacological studies have provided insight into the anxiolytic properties of NPY in multiple paradigms of anxiety-like behavior (Kask and et al, 2002, Sajdyk et al., 2004). NPY deficiency is associated with an anxiogenic phenotype in rodents (Bannon et al., 2000), and highly anxious rats are more sensitive to the anxiolytic actions of NPY (Sudakov et al., 2001). Intracerebroventricular (i.c.v.) administration of NPY decreases anxiety-like behavior in the elevated plus maze, Vogel's drinking conflict test (Broqua and et al, 1995, Heilig and et al, 1989), and other operant conflict tasks (Britton and et al, 1997, Heilig and et al, 1992). Site specific-studies have revealed the amygdala, locus coeruleus, lateral septum, and hippocampus as regions that are involved in the anxiolytic properties of NPY (Lin and et al, 2010, Thorsell and et al, 2000, Primeaux and et al, 2005, Sajdyk et al., 1999, Heilig and et al, 1993, Kask et al., 1998a, Kask et al., 1998b, Kask et al., 1998c, Trent and Menard, 2011). For example, infusion of NPY into the basolateral amygdala decreases social anxiety (Sajdyk et al., 1999), produces anti-conflict effects via the central nucleus of the amygdala (Heilig et al., 1993), and decreases anxiety upon injection into the locus coeruleus (Kask et al., 1998a, Kask et al., 1998b, Kask et al., 1998c). The effects of NPY may be related to interactions with CRF signaling, as NPY attenuates anxiety and avoidance behavior induced by CRF and CRF agonists upon i.c.v. or direct delivery into subregions of the amygdala (Ide and et al, 2013, Sajdyk et al., 2006, Britton and et al, 2000). An interaction with norepinephrine systems has also been implicated, as pretreatment with idazoxan, an α2-adrenergic receptor antagonist, blocks the anxiolytic effects of NPY (Heilig et al., 1989).
The receptor subtypes mediating the anxiolytic properties of NPY are currently under investigation. Studies largely support a role for the activation of Y1R in the attenuation of anxiety-like behavior. For example, the anxiolytic effects of NPY are absent in mice lacking the Y1R (Karlsson and et al, 2008, Heilig, 1995), and Y1R knockout mice exhibit an anxiogenic phenotype (Karl et al., 2006, Longo and et al, 2014). Selective knockout of Y1R from excitatory forebrain neurons also results in increased anxiety (Bertocchi et al., 2011). Centrally administered Y1R agonists are anxiolytic in a number of behavioral paradigms (Britton and et al, 1997, Sorensen and et al, 2004), while site-specific examinations implicate the central nucleus of the amygdala and hippocampus as regions of Y1R-mediated anxiolysis (Heilig and et al, 1993, Olesen and et al, 2012, Lyons and Thiele, 2010). Administration of Y1R antagonists centrally or into the periaqueductal grey produces anxiogenic effects (Kask et al., 1998a, Kask et al., 1998b, Kask et al., 1998c), but has no reported effects when delivered into the locus coeruleus, hypothalamus, or central nucleus of the amygdala (Kask et al., 1998a, Kask et al., 1998b, Kask et al., 1998c). The lack of effect in these regions may be due to their low level of expression of Y1R (Kask et al., 2002). Central blockade of Y1R is also sufficient to elicit conditioned place aversion, supporting the notion that Y1R are necessary for endogenous anxiolytic actions of NPY (Kask et al., 1999). Y1R are found to be preferentially expressed on pyramidal cells in the basolateral amygdala (Rostkowski et al., 2009), therefore it is likely that Y1R mediate anxiolysis here by influencing glutamatergic input to the central nucleus of the amygdala and subsequent output to the brainstem (Gilpin et al., 2011).
The function of Y2R in anxiety is allegedly opposite of the Y1R subtype; however conflicting reports demonstrating both anxiogenic and anxiolytic effects mediated by Y2R make the role of this subtype in anxiety less clear. Y2R are generally considered NPY autoreceptors and evidence for their pre-synaptic localization has been demonstrated in humans and rodents (Stanic and et al, 2011, Caberlotto and et al, 1998). Central administration of Y2R agonists have failed to alter anxiety-like behavior in a number of studies (Broqua and et al, 1995, Heilig and et al, 1989, Britton and et al, 1997, Sorensen and et al, 2004). However, agonism of Y2R in the locus coeruleus and lateral septum produces anxiolytic effects, whereas Y2R are required for NPY-mediated anxiolysis in the hippocampus (Kask et al., 1998a, Kask et al., 1998b, Kask et al., 1998c, Trent and Menard, 2013, Smialowska and et al, 2007). Y2R agonism in the basolateral amygdala has bidirectional effects on anxiety in the social interaction test, with low agonist doses generating anxiety and high doses decreasing anxiety (Sajdyk et al., 2002). A recent study indicates that knockout of the Y2R in GABAergic neurons located in the central nucleus of the amygdala was anxiogenic specifically in female mice (McCall et al., 2013). Contrasting reports indicate that Y2R antagonism in the central nucleus of the amygdala is anxiolytic (Kallupi et al., 2013), and that ablation of Y2R in either the basolateral or central nucleus of the amygdala produces an anxiolytic phenotype (Tasan et al., 2010). Global deletion of Y2R reduces anxiety in the elevated plus maze, light–dark, open-field, and marble burying tests (Tasan and et al, 2009, Painsipp et al., 2008, Painsipp and et al, 2008, Tschenett and et al, 2003), and Y2R deficient mice exhibit reduced neuronal activation upon exposure to an anxiogenic environment (Nguyen et al., 2009). Taken together, this evidence suggests that Y2R may function in a regionally specific and neurochemically selective fashion.
The Y4R and Y5R also have putative roles in rodent anxiety-like behavior. Similar to Y2R mutant mice, deletion of the Y4R also reduces anxiety-like behavior in a number of rodent paradigms (Tasan and et al, 2009, Painsipp and et al, 2008). Knockout of the Y4R with the Y2R enhances the anxiolytic phenotype observed following deletion of either receptor alone (Tasan et al., 2009). Finally, pharmacological studies indicate that Y5R ligands may have promising anxiolytic properties. A Y5R antagonist blocked the anxiolytic effects of a Y2R agonist in the basolateral amygdala (Sajdyk et al., 2002), while i.c.v. delivery of a Y5R agonist produced anxiolytic effects (Sorensen et al., 2004). Y5R can form heterodimers with Y1R (Gehlert et al., 2007), and these receptor subtypes are colocalized in the basolateral amygdala, hippocampus, and hypothalamus (Wolak and et al, 2003, Longo and et al, 2014, Oberto and et al, 2007, Fetissov et al., 2004). Y1 and Y5 receptors act synergistically in the regulation of energy homeostasis (Mashiko et al., 2009). Although the combined effects of Y1 and Y5 receptor agonists have not been tested in the context of anxiety thus far, the notion of co-activating these receptors could be valuable in the development of pharmacotherapeutics for enhanced anxiolytic effects.
4.2. Arousal
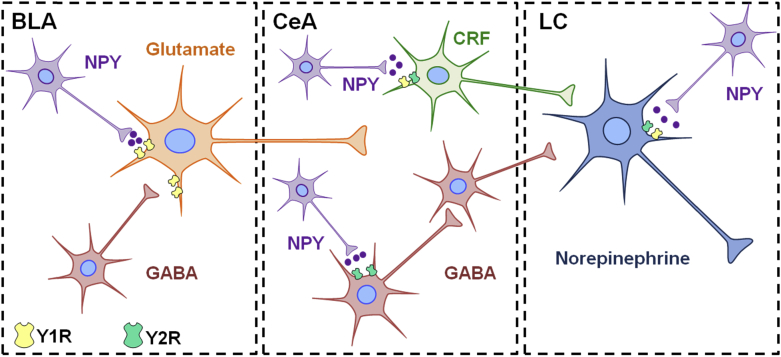
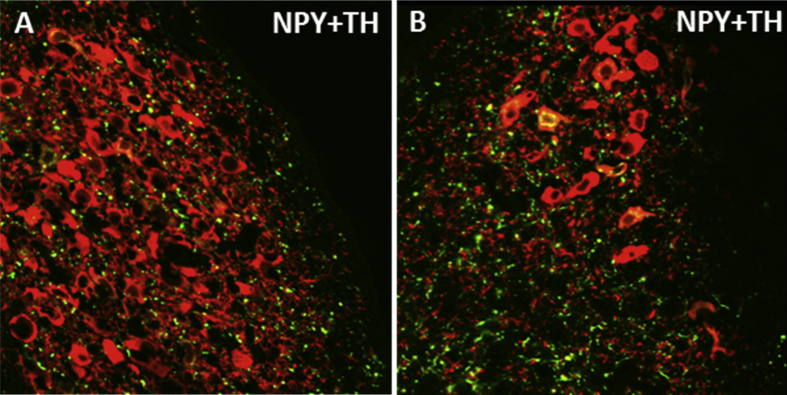
Hypervigilance is a characteristic symptom of stress-related psychiatric disorders that may reflect dysregulation of brain arousal systems. Startle responses can be measured in rodents using loud acoustic tones, and can be enhanced in fear-potentiated startle, a paradigm in which startle is tested in an environment previously paired with footshocks. Central administration of NPY inhibits both basal acoustic startle and fear-potentiated startle in rodents (Broqua and et al, 1995, Gilpin and et al, 2011, Gutman and et al, 2008). Another study demonstrated that NPY infusion into the basolateral, but not central nucleus, of the amygdala mimics the effects of NPY on acoustic startle and fear-potentiated responses (Gutman et al., 2008). Central administration of a Y1R agonist attenuates fear-potentiated startle, whereas a Y2R agonist was reported to have no effect (Broqua et al., 1995). In genetically modified rodents, knockout of NPY or Y2R enhances acoustic startle (Bannon et al., 2000), whereas deletion of the Y1R yields impaired habituation of startle responses (Karl et al., 2010). These studies indicate a role for NPY in the modulation of startle and potential for NPY as a therapeutic for hyperarousal in stress-related psychiatric disorders. However the receptor subtypes and brain regions dictating NPY-induced resilience to this behavioral response remain unclear. The NE system originating in the locus coeruleus (LC) is a brainstem region contributing to arousal responses (Samuels and Szabadi, 2008, Sara and Bouret, 2012), thus NPY may mediate arousal behavior by directly acting in the LC or by influencing brain regions upstream. Fig. 1 demonstrates putative neurochemical interactions and circuitry that may influence the function of the LC-NE system and arousal behavior. NPY inhibits the firing rate of NE neurons in the LC, and potentiates the effect of NE on presynaptic autoinhibition of neuronal firing (Illes et al., 1993, Finta et al., 1992). This electrophysiological evidence suggests that NPY may act to restrain the activity of noradrenergic neurons, which may have important implications for stress-psychiatric diseases in which the LC-NE system is disrupted. In combination with anatomical evidence demonstrating rich NPY innervation of the LC (Smialowska, 1988) (shown in Fig. 2),these studies suggest that NPY may play an important role in the regulation of noradrenergic stress responses and arousal via NE circuitry.
Fig. 1.
Putative modulation of arousal behavior by NPY within stress–integrative circuitry. Excitatory glutamatergic (Glu) projections from the basolateral amygdala (BLA) activate the central nucleus of the amygdala (CeA) in response to stress. Subsequent activation of afferents expressing corticotropin-releasing factor (CRF) leads to enhanced activity of norepinephrine (NE) neurons in the locus coeruleus (LC), which then project to and activate regions of the forebrain to regulate arousal behavior. Putative interactions of NPY with stress responsive regions are shown. Activation of Y1 receptors on Glu neurons in the BLA may decrease activation of the CeA in response to stress (Rostkowski et al., 2009). NPY may suppress noradrenergic activation in the LC via Y2R located on NE neurons (Illes et al., 1993, Finta et al., 1992), or suppress Y2R-expressing GABAergic interneurons in the CeA leading to disinhibition of GABA output to the LC (not shown) (Gilpin, 2012). Alternatively, we hypothesize that NPY axon terminals may directly interact with CRF neurons in the CeA to suppress the activity of the LC-NE system in response to stress.
Fig. 2.
NPY innervation of the rat locus coeruleus. NPY fibers (green) innervating the nuclear core (A) and the ventral dendritic region (B) of the locus coeruleus are shown. Noreprinephrine neurons in the locus coeruleus are represented by staining of the biosynthetic enzyme tyrosine hydroxylase (TH) (red). Colocalization of NPY and TH can be observed in cell bodies and fibers (yellow). NPY and TH in this high magnification image were visualized by immunofluorescence and confocal laser microscopy. Tissues were obtained from a non-colchicine treated Sprague–Dawley rat, which may contribute to the minimal observation of NPY synthesizing neurons in this image. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.3. Fear
Recent rodent studies suggest that NPY may be useful in the treatment of psychiatric diseases such as PTSD, which is heavily characterized by behavioral sequelae associated with fear. NPY has been found to influence multiple fear-related behaviors including the acquisition, incubation, expression, and extinction of conditioned fear. For example, i.c.v. administration of NPY or a Y1R agonist inhibits freezing behavior in both the acquisition and consolidation phases of fear conditioning, and these effects are blocked by pretreatment with a Y1R antagonist (Lach de Lima, 2013). Y1R may not be necessary for the cued-expression of fear, as intra-amygdalar administration of NPY robustly decreases the expression of conditioned fear, but these effects are not replicated by Y1R agonists and are not blocked by pretreatment with a Y1R antagonist (Fendt et al., 2009). In this particular study, Y1R knockout mice showed slight elevations in freezing behavior during fear conditioning, but did not show an enhanced phenotype upon testing for the cued-expression of fear compared to wildtype mice (Fendt et al., 2009). In addition, NPY was still capable of reducing the cued-expression of fear in these Y1R deficient mice, suggesting that the Y1R may not be involved in this phase (Fendt et al., 2009). NPY can suppress the long-term incubation of conditioned fear, while delivery of NPY prior to extinction training attenuates freezing and enhances retention of extinguished fear memories (Gutman and et al, 2008, Lach and de Lima, 2013, Pickens and et al, 2009). Y1R antagonism blocks NPY-induced reductions in freezing and blockade of amygdalar Y1R leads to deficient extinction retention (Gutman and et al, 2008, Lach and de Lima, 2013). Consistent with pharmacological studies, NPY knockout mice display accelerated acquisition of conditioned fear, excessive recall of fear, and impaired fear extinction (Verma et al., 2012). Interestingly, deletion of the Y1R has moderately similar effects, whereas knockout of the Y2R has no effect on fear (Verma et al., 2012). However, double Y1R and Y2R knockout mice exhibit a remarkably similar phenotype to NPY deficient mice, indicating that both receptor subtypes do play a role in aspects of fear conditioning (Verma et al., 2012). In an inescapable footshock paradigm, interactions between the NPY and CRF systems were evident as increased amygdalar CRFR1 and decreased Y1R mRNA were found concurrently in animals displaying enhanced freezing time, and all of these effects were reversed in parallel following re-exposure to the footshock-paired environment (Hendriksen et al., 2012). Indirect evidence for NPY interactions with norepinephrine was obtained using auditory fear conditioning, in which centrally administered NPY and a Y1R agonist blunted fear-induced tachycardia (Tovote et al., 2004). These effects were blocked by a Y1R antagonist (Tovote et al., 2004).
4.4. Rodent models of depression
NPY is implicated in depression-like behavior and produces antidepressant effects. For example, central administration of NPY dose-dependently reduces immobility and increases swimming time in the forced swim test (Redrobe and et al, 2005, Stogner and Holmes, 2000, Redrobe and et al, 2002), a screening paradigm for pharmacological anti-depressant activity. Y1R agonists and Y2R antagonists also produce anti-depressant effects in forced swim (Redrobe et al., 2002), whereas Y1R antagonists block the anti-depressant effects of NPY (Redrobe et al., 2002). Intra-hippocampal infusion of NPY has anti-depressant properties in a learned helplessness paradigm, which is blocked by co-administration of a Y1R, but not a Y2R antagonist (Ishida et al., 2007). Y1R knockout mice display increased immobility in the forced swim test, indicative of a depression-like phenotype (Karlsson et al., 2008). Both Y2R and Y4R knockout mice exhibit reduced depression-like behavior in the tail suspension test, another common screening assay for antidepressant potential (Tasan and et al, 2009, Painsipp et al., 2008, Painsipp and et al, 2008). Knockout of both Y2R and Y4R results in augmented anti-depressant effects compared to single-knockout of either receptor (Tasan et al., 2009). Anti-depressant strategies including imipramine and electroconvulsive stimuli increase NPY immunoreactivity or receptor mRNA and binding sites, respectively (Heilig and et al, 1988, Madsen and et al, 2000). The anti-depressant properties of NPY may be mediated through interactions with the serotonin system, as administration of a tryptophan hydroxylase inhibitor blocked the anti-depressant effects of NPY in the forced swim test (Redrobe et al., 2005).
The Flinders-sensitive line (FSL) is a transgenic model of depression in which abnormalities in NPY, serotonin, and catecholaminergic systems have been identified (Overstreet and et al, 2005, Serova and et al, 1998). Depression-like behavior has been associated with impaired hippocampal neurogenesis, and enhanced NPY and serotonin activities been shown to increase cell proliferation in the dentate gyrus of the hippocampus (Husum et al., 2006). Hippocampal and amygdalar NPY immunoreactivity is lower in FSL rats compared to Flinders-resistant controls (Jimenez Vasquez and et al, 2000, Jimenez-Vasquez et al., 2000, Zambello and et al, 2008), and aging is associated with exacerbated loss of hippocampal NPY immunoreactivity in the FSL line (Husum et al., 2006). In FSL rats, Y5R antagonism produces anti-depressant effects in the forced swim test (Walker et al., 2009). Electroconvulsive stimuli and the selective serotonin reuptake inhibitor fluoxetine increase NPY mRNA or immunoreactivity in the hippocampus and hypothalamus, and upregulate amygdalar Y1R binding sites in FSL rats (Caberlotto and et al, 1998, Caberlotto and et al, 1999). Exercise and escitalopram are associated with similar alterations in hippocampal NPY and Y1 receptor mRNA (Bjornebekk et al., 2010). NPY has also been examined in olfactory bulbectomized rats (OBX), which are utilized as a rodent model due to depression-like disruptions in behavior, physiology, and neurochemistry (Song and Leonard, 2005, Kelly et al., 1997). Anti-depressant effects are observed following chronic treatment with NPY, a Y1R agonist, and a Y2R antagonist in OBX rats (Goyal and et al, 2009, Morales-Medina and et al, 2012a). In contrast, chronic administration of a Y2R agonist enhanced depression-like behavior in OBX rats in the forced swim test (Morales-Medina et al., 2012).
Future studies investigating the efficacy of NPY in depression-like behavior induced by chronic psychosocial stress using the resident-intruder model of social defeat would be interesting. Social defeat reproduces behavioral and physiological indices of depression including disruption of CRF and NE systems (Wood and et al, 2010, Wood, 2014, Chaijale and et al, 2014, Chaijale and et al, 2013, Russo and et al, 2012), and would likely yield important information regarding the role of NPY in depressive behavior and disorders.
4.5. Rodent models of PTSD
Several rodent models of PTSD indicate that NPY expression in the brain following stress may be associated with susceptibility to PTSD-associated impairments. For example, rats displaying extreme anxiety and arousal following exposure to predator scent stress (PSS) had lower NPY protein levels in the cortex, amygdala, hippocampus, and periaqueductal grey compared to rodents that were less impaired or to unstressed controls (Cohen et al., 2012). Injection of NPY into the hippocampus 1 h after PSS reduced the development of anxiety-like behavior, hyperarousal, and cue-elicited freezing. Additionally, NPY administration reduced the prevalence of an extreme behavioral response (Cohen et al., 2012).
Delivery of NPY to the brain by intranasal (IN) infusion has been used to examine its efficacy in the single prolonged stress (SPS) model of PTSD (Serova and et al, 2013, Laukova and et al, in press, Serova and et al, 2014). Intranasal NPY can elevate CSF concentrations to a range that reduces anxiety behavior after i.c.v. administration, while also reaching multiple stress responsive brain regions and leaving plasma NPY levels unchanged (Serova and et al, 2013, Laukova and et al, in press). Pretreatment with IN NPY slowed the development of immobility during the forced swim portion of SPS, and reduced the induction of gene expression of the NE biosynthetic enzymes, tyrosine hydroxylase and dopamine beta hydroxylase, in the locus coeruleus shortly after SPS (Serova et al., 2013). SPS-induced increases in plasma corticosterone and ACTH were also attenuated by IN NPY, suggesting either less activation or more rapid recovery of the hypothalamic-pituitary-adrenal (HPA) axis (Serova et al., 2013). Intranasal NPY administered prior to or immediately after SPS led to pronounced and long-lasting effects on the development of behavioral, neuroendocrine, and molecular impairments associated with PTSD. NPY greatly attenuated, and in many cases prevented, increases in anxiety, hyperarousal, and depression-like behavior observed 1–2 weeks after exposure to traumatic stress (Serova et al., 2013). NPY prevented SPS-triggered induction of CRF, glucocorticoid receptor (GR), and FKBP5 mRNAs and the reduction in phosphorylated-GR in the mediobasal hypothalamus (Laukova et al., in press). NPY also increased the expression and phosphorylation of GR in the hippocampus (Laukova et al., in press). These studies suggest that early intervention with intranasal NPY may prevent dysregulation of the HPA axis by restoring proper negative feedback inhibition by GR. Intranasal NPY also attenuated long-term changes in the central noradrenergic system induced by SPS, including the development of increased sensitization of the LC to re-experiencing the forced swim (Serova et al., 2013). Taken together, PSS and SPS studies indicate that a single treatment with NPY near the time of the traumatic stress could provide long-lasting resilience to the development of PTSD and co-morbid impairments such as depression. Moreover, recent work also suggests that NPY may be efficacious as a treatment once PTSD-like symptoms have already manifested. Rats given IN NPY one week after SPS, when PTSD-like symptoms have manifested, exhibit anxiety-like behavior similar to unstressed controls up to 2 days later (Serova et al., 2014). Rats administered NPY after SPS also had reduced depression-like behavior (Serova et al., 2014). Further studies are necessary to determine if intranasal NPY reverses other impairments associated with PTSD, as well as the duration and sustainability of the improvements.
5. Therapeutic implications
The examples presented herein demonstrate that pharmacological interventions targeting the NPY system display much promise for the treatment of numerous stress-related psychiatric disorders. Future pharmacotherapeutic studies should consider targeting the central NPY system in stress-related emotionality and resilience. The preponderance of data suggests that NPY itself has significant therapeutic potential as a mediator of stress resilience. There are two major challenges associated with the development of NPY as a drug for psychiatric disorders; it is a peptide and it has a broad range of activities that may result in undesirable side-effects. The attractiveness and challenges of peptide therapeutics for CNS disorders has recently been reviewed (McGonigle, 2012). Peptides do not accumulate in tissues and are effectively metabolized by endogenous enzymes; therefore they have limited potential for drug–drug interactions. However, peptides have short half-lives and several methods have been introduced to prolong their stability in vivo. Encouragingly, as demonstrated in rodent models (Serova and et al, 2013, Laukova and et al, in press, Serova and et al, 2014), NPY may confer long-lasting benefits for stress resilience despite its short half-life.
Although this review has concentrated on the beneficial effects of NPY in the CNS, NPY also has multiple actions in the periphery (Hirsch and Zukowska, 2012, Held and et al, 2006, Pedrazzini et al., 2003). For example, NPY is a co-transmitter in sympathetic nerves, plays a role in vascular tone, and contributes to cardiovascular remodeling (Zukowska-Grojec, 1995, Edvinsson and et al, 1984, Schuerch and et al, 1998, Abe et al., 2007). Rodent studies have demonstrated NPY-induced disruption of metabolic homeostasis, as chronic NPY administration in rodents leads to abnormal baroreflex sensitivity, abdominal obesity, and dyslipidemia (Xie et al., 2012). NPY release from sympathetic nerves also stimulates fat angiogenesis, macrophage infiltration, and proliferation and differentiation of new adipocytes leading to abdominal obesity and a metabolic syndrome in rodents (Kuo et al., 2007). NPY also plays a role in bone physiology, gastrointestinal function, and cancer progression (Brothers and Wahlestedt, 2010). Peripheral administration of NPY may result in undesirable side effects on these physiological processes, increasing the value and necessity for strategies of NPY administration to the brain. Moreover, peptides do not typically cross the blood–brain barrier unless carried by specific transporters. Although no such transporter is known to exist for NPY, studies have shown that NPY can enter the brain to some extent (Kastin and Akerstrom, 1999).
Intranasal (IN) infusion represents a clinically relevant and non-invasive approach for the delivery of NPY to the brain. IN administration allows peptides to rapidly and directly enter the CNS via intracellular neuronal olfactory and extracellular trigeminal-associated pathways bypassing the blood–brain barrier to affect multiple sites within the brain (Dhuria et al., 2010, Ionescu and et al, 2012, Thorne and et al, 1995, Thorne and et al, 2004). As demonstrated in rodent models (Serova and et al, 2013, Laukova and et al, in press, Serova and et al, 2014), NPY delivered to the brain by IN infusion has beneficial effects on stress-related emotionality and pathology, which is likely achieved by influencing NPY responsive systems in all regions regulating stress responses. A potential disadvantage of IN infusion is the lack of selective targeting and potential for CNS-mediated side effects. For example, NPY is also a powerful orexigenic agent and regulates circadian rhythms (Brothers and Wahlestedt, 2010, Gehlert, 1999). Although not used for stress-related implications, studies have administered NPY by IN infusion in humans (Lacroix and Mosimann, 1996, Lacroix and et al, 1996, Cervin and et al, 1999, Hallschmid and et al, 2003, Hallschmid and et al, 2004). One small clinical trial aimed to test the effect of IN NPY on mood and anxiety (NCT 00748956) (U.S. National Institutes of Health., 2000a, U.S. National Institutes of Health., 2000b) while another is currently underway to investigate the safety of IN NPY using a dose escalation in PTSD (NCT 01533519) (U.S. National Institutes of Health., 2000a, U.S. National Institutes of Health., 2000b). To date no side effects have been reported. The viability of this route of administration makes it much more feasible to consider clinical proof of concept studies for severe stress-related disorders such as PTSD, for which there are no truly effective treatments and the initiating stress is often known. In the event that CNS-mediated side effects prove to be significant obstacles to the chronic use of NPY as a therapeutic, it is possible that the selective activation or inhibition of individual receptor subtypes may be a safer yet still effective alternative. There is already considerable preclinical data demonstrating the therapeutic potential of Y1R agonists and Y2R antagonists for the treatment of stress-related disorders and these targets clearly merit additional study.
6. Future directions
Elucidating the neuroanatomical interactions of the NPY system with other neurotransmitters and peptides within stress-integrative circuitry would greatly advance our knowledge regarding the role of NPY in stress resilience and emotionality in future studies. In addition, future studies should consider the impact of sex differences on NPY-mediated effects. Human and rodent studies indicate that females may be more vulnerable to stress and stress-related psychiatric diseases than males (Bangasser and Valentino, 2014). Psychiatric symptomology and treatments responses also vary based on sex (Kokras and Dalla, 2014). Future studies examining the efficacy of NPY on stress and emotionality in females with direct comparisons to males would advance our understanding of sex differences in stress resilience. Neuroanatomical and molecular studies conducted across sexes would reveal potential mechanisms underlying effective coping to stress and intervention strategies for stress-induced psychiatric diseases.
Acknowledgements
This work was supported by DA09082 (EJV) from the National Institutes of Health and DM102281(ELS) from US Army, Department of Defense Medical Research and Development Program.
Contributor Information
Nicole M. Enman, Email: nicole.enman@drexelmed.edu.
Esther L. Sabban, Email: esther_sabban@nymc.edu.
Paul McGonigle, Email: paul.mcgonigle@drexelmed.edu.
Elisabeth J. Van Bockstaele, Email: elisabeth.vanbockstaele@drexelmed.edu.
References
- Abe K., Tilan J.U., Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr. Top. Med. Chem. 2007;7(17):1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- Adrian T.E. Neuropeptide Y distribution in human brain. Nature. 1983;306(5943):584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- Allen Y.S. Neuropeptide Y distribution in the rat brain. Science. 1983;221(4613):877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Altemus M. Normal CSF oxytocin and NPY levels in OCD. Biol. Psychiatry. 1999;45(7):931–933. doi: 10.1016/s0006-3223(98)00263-7. [DOI] [PubMed] [Google Scholar]
- Amstadter A.B. NPY moderates the relation between hurricane exposure and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2010;27(3):270–275. doi: 10.1002/da.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35(3):309–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon A.W. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868(1):79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Bertocchi I. Regulatory functions of limbic Y1 receptors in body weight and anxiety uncovered by conditional knockout and maternal care. Proc. Natl. Acad. Sci. U. S. A. 2011;108(48):19395–19400. doi: 10.1073/pnas.1109468108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A., Mathe A.A., Brene S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus. 2010;20(7):820–828. doi: 10.1002/hipo.20683. [DOI] [PubMed] [Google Scholar]
- Boulenger J.P. Elevated plasma levels of neuropeptide Y in patients with panic disorder. Am. J. Psychiatry. 1996;153(1):114–116. doi: 10.1176/ajp.153.1.114. [DOI] [PubMed] [Google Scholar]
- Bremner J.D. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch. Gen. Psychiatry. 1997;54(3):246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Britton K.T. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacol. Berl. 1997;132(1):6–13. doi: 10.1007/s002130050313. [DOI] [PubMed] [Google Scholar]
- Britton K.T. Neuropeptide Y blocks anxiogenic-like behavioral action of corticotropin-releasing factor in an operant conflict test and elevated plus maze. Peptides. 2000;21(1):37–44. doi: 10.1016/s0196-9781(99)00169-2. [DOI] [PubMed] [Google Scholar]
- Broqua P. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav. Pharmacol. 1995;6(3):215–222. [PubMed] [Google Scholar]
- Brothers S.P., Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol. Med. 2010;2(11):429–439. doi: 10.1002/emmm.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto L. Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Brain Res. Mol. Brain Res. 1998;59(1):58–65. doi: 10.1016/s0169-328x(98)00137-5. [DOI] [PubMed] [Google Scholar]
- Caberlotto L. Alterations in neuropeptide Y levels and Y1 binding sites in the flinders sensitive line rats, a genetic animal model of depression. Neurosci. Lett. 1999;265(3):191–194. doi: 10.1016/s0304-3940(99)00234-7. [DOI] [PubMed] [Google Scholar]
- Caberlotto L., Hurd Y.L. Reduced neuropeptide Y mRNA expression in the prefrontal cortex of subjects with bipolar disorder. Neuroreport. 1999;10(8):1747–1750. doi: 10.1097/00001756-199906030-00022. [DOI] [PubMed] [Google Scholar]
- Caberlotto L., Fuxe K., Hurd Y.L. Characterization of NPY mRNA-expressing cells in the human brain: co-localization with Y2 but not Y1 mRNA in the cerebral cortex, hippocampus, amygdala, and striatum. J. Chem. Neuroanat. 2000;20(3–4):327–337. doi: 10.1016/s0891-0618(00)00107-1. [DOI] [PubMed] [Google Scholar]
- Cervin A. Functional effects of neuropeptide Y receptors on blood flow and nitric oxide levels in the human nose. Am. J. Respir. Crit. Care Med. 1999;160(5 Pt 1):1724–1728. doi: 10.1164/ajrccm.160.5.9902102. [DOI] [PubMed] [Google Scholar]
- Chaijale N.N. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology. 2013;38(10):1833–1843. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale N.N. Repeated social stress increases reward salience and impairs encoding of prediction by rat locus coeruleus neurons. Neuropsychopharmacology. Aug 11 2014 doi: 10.1038/npp.2014.200. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quidt M.E., Emson P.C. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–II. Immunohistochemical analysis. Neuroscience. 1986;18(3):545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- de Quidt M.E., Emson P.C. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986;18(3):527–543. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Dhuria S.V., Hanson L.R., Frey W.H., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J. Pharm. Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- Dimitrov E.L. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148(8):3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- Domschke K. Chromosome 4q31-34 panic disorder risk locus: association of neuropeptide Y Y5 receptor variants. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008:510–516. doi: 10.1002/ajmg.b.30629. 147b(4) [DOI] [PubMed] [Google Scholar]
- Dumont Y. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J. Neurosci. 1993;13(1):73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J. Comp. Neurol. 1998;402(3):372–384. [PubMed] [Google Scholar]
- Eaton K., Sallee F.R., Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr. Top. Med. Chem. 2007;7(17):1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br. J. Pharmacol. 1984;83(2):519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacol. Berl. 2009;206(2):291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Fetissov S.O., Kopp J., Hokfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38(4):175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Finta E.P., Regenold J.T., Illes P. Depression by neuropeptide Y of noradrenergic inhibitory postsynaptic potentials of locus coeruleus neurones. Naunyn Schmiedeb. Arch. Pharmacol. 1992;346(4):472–474. doi: 10.1007/BF00171093. [DOI] [PubMed] [Google Scholar]
- Gehlert D.R. Co-expression of neuropeptide Y Y1 and Y5 receptors results in heterodimerization and altered functional properties. Biochem. Pharmacol. 2007;74(11):1652–1664. doi: 10.1016/j.bcp.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Gehlert D.R. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33(5):329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- Giesbrecht C.J. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J. Neurosci. 2010;30(50):16970–16982. doi: 10.1523/JNEUROSCI.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N.W. Effects of neuropeptide Y and ethanol on arousal and anxiety-like behavior in alcohol-preferring rats. Alcohol. 2011;45(2):137–145. doi: 10.1016/j.alcohol.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N.W. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46(4):329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S.N. Neuropeptide Y modulates the antidepressant activity of imipramine in olfactory bulbectomized rats: involvement of NPY Y1 receptors. Brain Res. 2009;1266:45–53. doi: 10.1016/j.brainres.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Grove K.L. Neuropeptide Y Y5 receptor protein in the cortical/limbic system and brainstem of the rat: expression on gamma-aminobutyric acid and corticotropin-releasing hormone neurons. Neuroscience. 2000;100(4):731–740. doi: 10.1016/s0306-4522(00)00308-0. [DOI] [PubMed] [Google Scholar]
- Gutman A.R. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J. Neurosci. 2008;28(48):12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M. NPY attenuates positive cortical DC-potential shift upon food intake in man. Psychoneuroendocrinology. 2003;28(4):529–539. doi: 10.1016/s0306-4530(02)00038-0. [DOI] [PubMed] [Google Scholar]
- Hallschmid M. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav. 2004;83(1):55–64. doi: 10.1016/j.physbeh.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Hashimoto H. Plasma neuropeptide Y in patients with major depressive disorder. Neurosci. Lett. 1996;216(1):57–60. doi: 10.1016/0304-3940(96)13008-1. [DOI] [PubMed] [Google Scholar]
- Heilig M. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur. J. Pharmacol. 1988;147(3):465–467. doi: 10.1016/0014-2999(88)90182-3. [DOI] [PubMed] [Google Scholar]
- Heilig M. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacol. Berl. 1989;98(4):524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul. Pept. 1992;41(1):61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Heilig M. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8(4):357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heilig M. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J. Psychiatr. Res. 2004;38(2):113–121. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul. Pept. 1995;59(2):201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- Held K. Neuropeptide Y (NPY) shortens sleep latency but does not suppress ACTH and cortisol in depressed patients and normal controls. Psychoneuroendocrinology. 2006;31(1):100–107. doi: 10.1016/j.psyneuen.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Hendriksen H. Re-exposure and environmental enrichment reveal NPY-Y1 as a possible target for post-traumatic stress disorder. Neuropharmacology. 2012;63(4):733–742. doi: 10.1016/j.neuropharm.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Hirsch D., Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell. Mol. Neurobiol. 2012;32(5):645–659. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res. 2006;1095(1):154–158. doi: 10.1016/j.brainres.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Husum H. Exacerbated loss of cell survival, neuropeptide Y-immunoreactive (IR) cells, and serotonin-IR fiber lengths in the dorsal hippocampus of the aged flinders sensitive line “depressed” rat: Implications for the pathophysiology of depression? J. Neurosci. Res. 2006;84(6):1292–1302. doi: 10.1002/jnr.21027. [DOI] [PubMed] [Google Scholar]
- Ide S. Opposing roles of corticotropin-releasing factor and neuropeptide Y within the dorsolateral bed nucleus of the stria terminalis in the negative affective component of pain in rats. J. Neurosci. 2013;33(14):5881–5894. doi: 10.1523/JNEUROSCI.4278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Finta E.P., Nieber K. Neuropeptide Y potentiates via Y2-receptors the inhibitory effect of noradrenaline in rat locus coeruleus neurones. Naunyn Schmiedeb. Arch. Pharmacol. 1993;348(5):546–548. doi: 10.1007/BF00173217. [DOI] [PubMed] [Google Scholar]
- Ionescu I.A. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology. 2012;37(6):1323–1337. doi: 10.1038/npp.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H. Infusion of neuropeptide Y into CA3 region of hippocampus produces antidepressant-like effect via Y1 receptor. Hippocampus. 2007;17(4):271–280. doi: 10.1002/hipo.20264. [DOI] [PubMed] [Google Scholar]
- Jimenez Vasquez P.A. Neuropeptide Y in brains of the flinders sensitive line rat, a model of depression. Effects of electroconvulsive stimuli and d-amphetamine on peptide concentrations and locomotion. Behav. Brain Res. 2000;111(1–2):115–123. doi: 10.1016/s0166-4328(00)00142-x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Vasquez P.A., Overstreet D.H., Mathe A.A. Neuropeptide Y in male and female brains of flinders sensitive line, a rat model of depression. Effects of electroconvulsive stimuli. J. Psychiatr. Res. 2000;34(6):405–412. doi: 10.1016/s0022-3956(00)00036-4. [DOI] [PubMed] [Google Scholar]
- Kallupi M. Neuropeptide Y Y2R blockade in the central amygdala reduces anxiety-like behavior but not alcohol drinking in alcohol-dependent rats. Addict. Biol. 2013;19(5):755–757. doi: 10.1111/adb.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T. Acoustic startle response and sensorimotor gating in a genetic mouse model for the Y1 receptor. Neuropeptides. 2010;44(3):233–239. doi: 10.1016/j.npep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Karl T., Burne T.H.J., Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav. Brain Res. 2006;167(1):87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karlsson R.M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacol. Berl. 2008;195(4):547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Kask A. Neuropeptide Y Y1 receptor antagonist BIBP3226 produces conditioned place aversion in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23(4):705–711. doi: 10.1016/s0278-5846(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Kask A. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002;26(3):259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kask A., Rago L., Harro J. Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13-36 microinjected into vicinity of locus coeruleus in rats. Brain Res. 1998;788(1–2):345–348. doi: 10.1016/s0006-8993(98)00076-6. [DOI] [PubMed] [Google Scholar]
- Kask A., Rago L., Harro J. Anxiogenic-like effect of the NPY Y1 receptor antagonist BIBP3226 administered into the dorsal periaqueductal gray matter in rats. Regul. Pept. 1998:255–262. doi: 10.1016/s0167-0115(98)00076-7. 75-76. [DOI] [PubMed] [Google Scholar]
- Kask A., Rago L., Harro J. NPY Y1 receptors in the dorsal periaqueductal gray matter regulate anxiety in the social interaction test. Neuroreport. 1998;9(12):2713–2716. doi: 10.1097/00001756-199808240-00005. [DOI] [PubMed] [Google Scholar]
- Kastin A.J., Akerstrom V. Nonsaturable entry of neuropeptide Y into brain. Am. J. Physiol. 1999 doi: 10.1152/ajpendo.1999.276.3.E479. 276(3 Pt 1): p. E479-82. [DOI] [PubMed] [Google Scholar]
- Kelly J.P., Wrynn A.S., Leonard B.E. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol. Ther. 1997;74(3):299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kokras N., Dalla C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014;171(20):4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormos V., Gaszner B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides. 2013;47(6):401–419. doi: 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Kuo L.E. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007;13(7):803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Kuromitsu J. Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res. Gene Expr. Patterns. 2001;1(1):17–21. doi: 10.1016/s1567-133x(01)00003-5. [DOI] [PubMed] [Google Scholar]
- Lach G., de Lima T.C. Role of NPY Y1 receptor on acquisition, consolidation and extinction on contextual fear conditioning: dissociation between anxiety, locomotion and non-emotional memory behavior. Neurobiol. Learn Mem. 2013;103:26–33. doi: 10.1016/j.nlm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lacroix J.S. Intranasal administration of neuropeptide Y in man: systemic absorption and functional effects. Br. J. Pharmacol. 1996;118(8):2079–2084. doi: 10.1111/j.1476-5381.1996.tb15647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J.S., Mosimann B.L. Attenuation of allergen-evoked nasal responses by local pretreatment with exogenous neuropeptide Y in atopic patients. J. Allergy Clin. Immunol. 1996;98(3):611–616. doi: 10.1016/s0091-6749(96)70095-7. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Origins of the many NPY-family receptors in mammals. Peptides. 2001;22(3):295–307. doi: 10.1016/s0196-9781(01)00331-x. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38(4):141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Blomqvist A.G., Soderberg C. Evolution of neuropeptide Y and its related peptides. Comp. Biochem. Physiol. C. 1993;106(3):743–752. doi: 10.1016/0742-8413(93)90236-e. [DOI] [PubMed] [Google Scholar]
- Laukova M. Early intervention with intransala NPY prevents single prolonged stress-triggered impairments in hypothalamus and ventral Hippocampus in male rats. Endocrinology. 2014 doi: 10.1210/en.2014-1192. (in press) [DOI] [PubMed] [Google Scholar]
- Lin E.J. Adult-onset hippocampal-specific neuropeptide Y overexpression confers mild anxiolytic effect in mice. Eur. Neuropsychopharmacol. 2010;20(3):164–175. doi: 10.1016/j.euroneuro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Lindberg C. No association between the -399 C > T polymorphism of the neuropeptide Y gene and schizophrenia, unipolar depression or panic disorder in a Danish population. Acta Psychiatr. Scand. 2006;113(1):54–58. doi: 10.1111/j.1600-0447.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Longo A. Conditional inactivation of neuropeptide Y Y1 receptors unravels the role of Y1 and Y5 receptors coexpressing neurons in anxiety. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.009. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Lundberg J.M., Hokfelt T. Multiple co-existence of peptides and classical transmitters in peripheral autonomic and sensory neurons–functional and pharmacological implications. Prog. Brain Res. 1986;68:241–262. doi: 10.1016/s0079-6123(08)60242-3. [DOI] [PubMed] [Google Scholar]
- Lyons A.M., Thiele T.E. Neuropeptide Y conjugated to saporin alters anxiety-like behavior when injected into the central nucleus of the amygdala or basomedial hypothalamus in BALB/cJ mice. Peptides. 2010;31(12):2193–2199. doi: 10.1016/j.peptides.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T.M. Electroconvulsive stimuli enhance both neuropeptide Y receptor Y1 and Y2 messenger RNA expression and levels of binding in the rat hippocampus. Neuroscience. 2000;98(1):33–39. doi: 10.1016/s0306-4522(00)00078-6. [DOI] [PubMed] [Google Scholar]
- Mashiko S. Synergistic interaction between neuropeptide Y1 and Y5 receptor pathways in regulation of energy homeostasis. Eur. J. Pharmacol. 2009;615(1–3):113–117. doi: 10.1016/j.ejphar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Mathé A.A. Effects of electroconvulsive treatment on somatostattn, neuropeptide Y, endothelin, and neurokinin a concentrations in cerebrospinal fluid of depressed patients: a pilot study. Depression. 1995;3(5):250–256. [Google Scholar]
- McCall N.M. Effects of sex and deletion of neuropeptide Y2 receptors from GABAergic neurons on affective and alcohol drinking behaviors in mice. Front. Integr. Neurosci. 2013;7:100. doi: 10.3389/fnint.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle P. Peptide therapeutics for CNS indications. Biochem. Pharmacol. 2012;83(5):559–566. doi: 10.1016/j.bcp.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Mickey B.J. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch. Gen. Psychiatry. 2011;68(2):158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina J.C. Chronic administration of the Y2 receptor antagonist, JNJ-31020028, induced anti-depressant like-behaviors in olfactory bulbectomized rat. Neuropeptides. 2012;46(6):329–334. doi: 10.1016/j.npep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Morales-Medina J.C. Role of neuropeptide Y Y(1) and Y(2) receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology. 2012;62(1):200–208. doi: 10.1016/j.neuropharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Morales-Medina J.C., Dumont Y., Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- Morgan C.A., 3rd Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol. Psychiatry. 2000;47(10):902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Morgan C.A., 3rd Relationship among plasma cortisol, catecholamines, neuropeptide Y, and human performance during exposure to uncontrollable stress. Psychosom. Med. 2001;63(3):412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Morgan C.A., 3rd Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol. Psychiatry. 2002;52(2):136–142. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- Morgan C.A., 3rd Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol. Psychiatry. 2003;54(10):1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- Nguyen N.K. Effect of neuropeptide Y Y2 receptor deletion on emotional stress-induced neuronal activation in mice. Synapse. 2009;63(3):236–246. doi: 10.1002/syn.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikisch G. Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int. J. Neuropsychopharmacol. 2005;8(3):403–410. doi: 10.1017/S1461145705005158. [DOI] [PubMed] [Google Scholar]
- Nikisch G., Mathe A.A. CSF monoamine metabolites and neuropeptides in depressed patients before and after electroconvulsive therapy. Eur. Psychiatry. 2008;23(5):356–359. doi: 10.1016/j.eurpsy.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Nilsson C. Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides. 1996;17(3):359–362. doi: 10.1016/0196-9781(96)00013-7. [DOI] [PubMed] [Google Scholar]
- Oberto A. Expression patterns of promoters for NPY Y(1) and Y(5) receptors in Y(5)RitTA and Y(1)RVenus BAC-transgenic mice. Eur. J. Neurosci. 2007;26(1):155–170. doi: 10.1111/j.1460-9568.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- Olesen M.V. Neuropeptide Y Y1 receptor hippocampal overexpression via viral vectors is associated with modest anxiolytic-like and proconvulsant effects in mice. J. Neurosci. Res. 2012;90(2):498–507. doi: 10.1002/jnr.22770. [DOI] [PubMed] [Google Scholar]
- Overstreet D.H. The flinders sensitive line rat: a selectively bred putative animal model of depression. Neurosci. Biobehav. Rev. 2005;29(4–5):739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Painsipp E. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes. Brain Behav. 2008;7(5):532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Herzog H., Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008;55(1):117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini T., Pralong F., Grouzmann E. Neuropeptide Y: the universal soldier. Cell. Mol. Life Sci. 2003;60(2):350–377. doi: 10.1007/s000180300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens C.L. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience. 2009;164(4):1398–1406. doi: 10.1016/j.neuroscience.2009.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux S.D. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30(9):1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M. Plasma neuropeptide Y (NPY) increases in humans in response to the alpha 2 antagonist yohimbine. Neuropsychopharmacology. 1998;19(1):95–98. doi: 10.1016/S0893-133X(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol. Psychiatry. 2000;47(6):526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Redrobe J.P. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26(5):615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Redrobe J.P. Role of serotonin (5-HT) in the antidepressant-like properties of neuropeptide Y (NPY) in the mouse forced swim test. Peptides. 2005;26(8):1394–1400. doi: 10.1016/j.peptides.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Rostkowski A.B. Cell-specific expression of neuropeptide Y Y1 receptor immunoreactivity in the rat basolateral amygdala. J. Comp. Neurol. 2009;517(2):166–176. doi: 10.1002/cne.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J. Neurobiology of resilience. Nat. Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol. Psychiatry. 2009;66(7):705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R. Cerebrospinal fluid neuropeptide Y in combat veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:277–283. doi: 10.1016/j.psyneuen.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R., Geracioti T.D. Neuropeptide Y and posttraumatic stress disorder. Mol. Psychiatry. 2013;18(6):646–655. doi: 10.1038/mp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T.J. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol. Biochem. Behav. 2002;71(3):419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk T.J., Vandergriff M.G., Gehlert D.R. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur. J. Pharmacol. 1999;368(2–3):143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk T.J., Schober D.A., Gehlert D.R. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43(7):1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk T.J., Shekhar A., Gehlert D.R. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38(4):225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sajdyk T.J., Fitz S.D., Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9(1):21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Samuels E.R., Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr. Neuropharmacol. 2008;6(3):235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S.J., Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Schuerch L.V. Human neuropeptide Y potentiates alpha1-adrenergic blood pressure responses in vivo. Am. J. Physiol. 1998 doi: 10.1152/ajpheart.1998.275.3.H760. 275(3 Pt 2): p. H760-6. [DOI] [PubMed] [Google Scholar]
- Serova L. Altered gene expression for catecholamine biosynthetic enzymes and stress response in rat genetic model of depression. Brain Res. Mol. Brain Res. 1998;63(1):133–138. doi: 10.1016/s0169-328x(98)00270-8. [DOI] [PubMed] [Google Scholar]
- Serova L.I. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Serova L.I. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014;24(1):142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Sjoholm L.K. PreproNPY Pro7 protects against depression despite exposure to environmental risk factors. J. Affect Disord. 2009;118(1–3):124–130. doi: 10.1016/j.jad.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Smialowska M. The effect of intrahippocampal injection of group II and III metobotropic glutamate receptor agonists on anxiety; the role of neuropeptide Y. Neuropsychopharmacology. 2007;32(6):1242–1250. doi: 10.1038/sj.npp.1301258. [DOI] [PubMed] [Google Scholar]
- Smialowska M. Neuropeptide Y immunoreactivity in the locus coeruleus of the rat brain. Neuroscience. 1988;25(1):123–131. doi: 10.1016/0306-4522(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Song C., Leonard B.E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005;29(4–5):627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sorensen G. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J. Neurosci. Res. 2004;77(5):723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- Stanic D. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J. Comp. Neurol. 2006;499(3):357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Stanic D. Characterization of NPY Y2 receptor protein expression in the mouse brain. II. Coexistence with NPY, the Y1 receptor, and other neurotransmitter-related molecules. J. Comp. Neurol. 2011;519(7):1219–1257. doi: 10.1002/cne.22608. [DOI] [PubMed] [Google Scholar]
- Stein M.B. Plasma neuropeptide Y in anxiety disorders: findings in panic disorder and social phobia. Psychiatry Res. 1996;59(3):183–188. doi: 10.1016/0165-1781(95)02776-9. [DOI] [PubMed] [Google Scholar]
- Stogner K.A., Holmes P.V. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur. J. Pharmacol. 2000:387. doi: 10.1016/s0014-2999(99)00800-6. p. R9-10, (2) [DOI] [PubMed] [Google Scholar]
- Sudakov S.K. Differences in genetic predisposition to high anxiety in two inbred rat strains: role of substance P, diazepam binding inhibitor fragment and neuropeptide Y. Psychopharmacol. Berl. 2001;154(4):327–335. doi: 10.1007/s002130000651. [DOI] [PubMed] [Google Scholar]
- Tasan R.O. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158(4):1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan R.O. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J. Neurosci. 2010;30(18):6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne R.G. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692(1–2):278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- Thorne R.G. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc. Natl. Acad. Sci. U. S. A. 2000;97(23):12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul. Pept. 2004;120(1–3):205–214. doi: 10.1016/j.regpep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Trent N.L., Menard J.L. Infusions of neuropeptide Y into the lateral septum reduce anxiety-related behaviors in the rat. Pharmacol. Biochem. Behav. 2011;99(4):580–590. doi: 10.1016/j.pbb.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Trent N.L., Menard J.L. Lateral septal infusions of the neuropeptide Y Y2 receptor agonist, NPY13–36 differentially affect different defensive behaviors in male. Long Evans rats. Physiol. Behav. 2013:20–29. doi: 10.1016/j.physbeh.2012.12.011. 110–111(0) [DOI] [PubMed] [Google Scholar]
- Tschenett A. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J. Neurosci. 2003;18(1):143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Verma D. NPY controls fear conditioning and fear extinction by combined action on Y(1) and Y(2) receptors. Br. J. Pharmacol. 2012;166(4):1461–1473. doi: 10.1111/j.1476-5381.2012.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Institutes of Health; Mount Sinai School of Medicine. Intranasal administration of neuropeptide Y in health male volunteers (NPY). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000a – [cited 2014 Oct 16]. Available from: http://clinicaltrials.gov/show/NCT00748956 NLM Identifier: NCT00748956.
- U.S. National Institutes of Health; Mount Sinai School of Medicine. A dose escalation study of intranasal neuropeptide Y in post-traumatic stress disorder (PTSD). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000b – [cited 2014 Oct 16]. Available from: http://clinicaltrials.gov/show/NCT01533519 NLM Identifier: NCT01533519.
- Wahlestedt C., Ekman R., Widerlov E. Neuropeptide Y (NPY) and the central nervous system: distribution effects and possible relationship to neurological and psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1989;13(1–2):31–54. doi: 10.1016/0278-5846(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Walker M.W. The novel neuropeptide Y Y5 receptor antagonist Lu AA33810 [N-[[trans-4-[(4,5-dihydro[1]benzothiepino[5,4-d]thiazol-2-yl)amino]cyclohexyl]me thyl]-methanesulfonamide] exerts anxiolytic- and antidepressant-like effects in rat models of stress sensitivity. J. Pharmacol. Exp. Ther. 2009;328(3):900–911. doi: 10.1124/jpet.108.144634. [DOI] [PubMed] [Google Scholar]
- Wang Y. A neuropeptide Y variant (rs16139) associated with major depressive disorder in replicate samples from Chinese Han population. PLoS One. 2013:8. doi: 10.1371/journal.pone.0057042. p. e57042, (2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrin A., Ekman R., Traskman-Bendz L. Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur. Neuropsychopharmacol. 1999;9(3):205–211. doi: 10.1016/s0924-977x(98)00026-1. [DOI] [PubMed] [Google Scholar]
- Widdowson P.S., Ordway G.A., Halaris A.E. Reduced neuropeptide Y concentrations in suicide brain. J. Neurochem. 1992;59(1):73–80. doi: 10.1111/j.1471-4159.1992.tb08877.x. [DOI] [PubMed] [Google Scholar]
- Widerlov E. Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J. Psychiatr. Res. 1988;22(1):69–79. doi: 10.1016/0022-3956(88)90030-1. [DOI] [PubMed] [Google Scholar]
- Wolak M.L. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J. Comp. Neurol. 2003;464(3):285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Wood S.K. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151(4):1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K. Individual differences in the neurobiology of social stress: implications for depression-cardiovascular disease comorbidity. Curr. Neuropharmacol. 2014;12(2):205–211. doi: 10.2174/1570159X11666131120224413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F. Long-term neuropeptide Y administration in the periphery induces abnormal baroreflex sensitivity and obesity in rats. Cell. Physiol. Biochem. 2012;29(1–2):111–120. doi: 10.1159/000337592. [DOI] [PubMed] [Google Scholar]
- Yamazoe M. Distribution of neuropeptide Y in the lower brainstem: an immunohistochemical analysis. Brain Res. 1985;335(1):109–120. doi: 10.1016/0006-8993(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Brand S., Yang R.-K. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to Trauma exposure, recovery from PTSD, and coping. Biol. Psychiatry. 2006;59(7):660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Zambello E. Acute stress differentially affects corticotropin-releasing hormone mRNA expression in the central amygdala of the “depressed” flinders sensitive line and the control flinders resistant line rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(3):651–661. doi: 10.1016/j.pnpbp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Zhou Z. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukowska-Grojec Z. Neuropeptide Y. A novel sympathetic stress hormone and more. Ann. N. Y. Acad. Sci. 1995;771:219–233. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]