Abstract
Purpose
To test the potential of water-soluble divalent cationic salts to inhibit acylation of octreotide encapsulated in poly(D,L-lactic-co-glycolic acid)-star (PLGA) microspheres.
Methods
The divalent cationic salts, calcium chloride and manganese chloride, previously shown to disrupt peptide sorption, were introduced in PLGA microspheres prepared by the double emulsion-solvent evaporation method. Peptide stability was monitored by reversed-phase high performance liquid chromatography (RP-HPLC) and identified by liquid chromatography coupled with mass spectrometry (LC-MS) during microsphere degradation under physiological conditions for four weeks. Microsphere morphology and salt content were examined by scanning electron microscopy (SEM) and inductively coupled plasma-optical emission spectroscopy (ICP-OES), respectively.
Results
Addition of divalent cationic salts solely to the organic phase provided marginal acylation inhibition. However, upon addition of the salt inhibitors to both the primary emulsion and the outer water phase resulted in improved drug and salt encapsulation efficiency as well as significantly decreased salt leaching and octreotide acylation. After 28 days, the extent of acylation inhibition afforded by divalent cations was > 58% relative to 13 % for the NaCl control group.
Conclusions
Divalent cationic salts are suitable class of stabilizers of peptide acylation in PLGA microspheres and this study provides an important formulation approach to maximize stabilizer potency.
Keywords: Peptide stability, Acylation, Sorption, Divalent cations, Controlled release
INTRODUCTION
Linear and star homopolymers of lactic acid (PLAs) and copolymers of lactic acid and glycolic acids (PLGAs) are one class of only a few biodegradable polymers (1, 2), which have been widely used in US-FDA-approved products for the prolonged release of peptides and proteins (3-5). However, the stability of the drug during microencapsulation, storage, and release in vivo has emerged as one of the most significant obstacles in the development of PLGA depots for these drugs (2, 6-8). For proteins and acid-labile peptides, the acidic microclimate within the matrix during the polymer degradation (4, 9, 10) and presence of moisture during the storage (11, 12) have been reported to be particularly deleterious. Another implicated instability mechanism during release incubation is related to hydrophobic surfaces both inside and outside of PLA and PLGA devices, which favor peptide and protein adsorption (13-16).
Although small peptide drugs are often resistant to the aforementioned instability mechanisms, peptides with reactive amines may interact with PLGA, resulting in acylation (17). The formation of acylated peptide impurities poses another potential obstacle to the successful delivery of bioactive substances from PLGA, as peptide (or potentially protein) acylation could have detrimental effects, such as loss of activity, immunogenicity and toxicity (18, 19). Peptide acylation has been shown to be affected by polymer composition and solution pH (20, 21). Murty et al. (21) studied the effect of polymer composition, molecular weight and release medium buffer on the acylation of octreotide. Microspheres composed of polymers with greater lactic acid content displayed a significantly lower percentage of impurities owing to the steric hindrance to nucleophilic attack provided by the additional methyl group. Peptide structural effects also play a role in peptide reactivity and acylation (17).
Several strategies have been proposed for preventing the acylation reactions. Lucke et al. (22) investigated whether use of PLGA diblock copolymers containing PEG would reduce peptide acylation since PEG could in principle reduce the accumulation of degradation products and peptide adsorption to the PLGA surface. However, combination of PEG with PLA did not show acylation inhibition in degrading polymer microspheres. Na et al. (23) presented a new PEGylation strategy to prevent the acylation of octreotide. PEGylated octreotide was more stable than native octreotide likely because of the steric hindrance of the PEG strand. However di-PEGylation may result in significant loss of biological activity to certain peptides. Topp et al. (24, 25) also investigated the effects of various excipients such as proton sponge, magnesium hydroxide, ammonium acetate and magnesium acetate on a hexapeptide chemical stability including acylation. However, these excipients did not reduce acylation.
It has been shown that the interaction of peptide with PLGA polymers involves an initial electrostatically driven adsorption of the peptide to the polymer, followed by an acylation reaction and subsequent release of acylated peptide (23). We therefore investigated whether the use of water-soluble divalent cationic salts could overcome the unwanted peptide acylation by simply suppressing the initial sorption process. In our previous study (26), divalent cations, such as Ca2+, Sr2+, Ni2+ and Mn2+ were found to effectively inhibit the sorption and acylation of octreotide in the presence of PLGA particles due to competition with dicationic octreotide for ionic interactions with carboxyl groups of free-acid end-group PLGA.
Octreotide acetate, a synthetic octapeptide, is administered parenterally for treatment of acromegaly and multiple intestinal diseases (27, 28). This somatostatin analogue has been commercially formulated in a depot form for intramuscular administration on a monthly basis. This study investigated the potential of water-soluble divalent cationic salts to inhibit octreotide acylation in the commonly desired injectable depot, PLGA microspheres, during in vitro incubation under physiological conditions. Octreotide acetate. How to best introduce the salts into microspheres to obtain the highest competitive inhibition was also examined.
MATERIAL AND METHODS
Materials
Octreotide acetate (batch number 98104) and star-type PLGA (batch number 01160141) were provided by Novartis Pharmaceutical Corp. Methylene chloride, acetonitrile (HPLC grade), tetrahydrofuran (THF) and trifluoroacetic acid (TFA) were purchased from Aldrich Chemical. Polyvinyl alcohol (PVA, 88 mol% hydrolyzed, Mw 25000) was obtained from Polysciences, Inc. Anhydrous manganese(II) (MnCl2), and calcium (CaCl2) chlorides were obtained from Sigma. Yttrium 1000 ppm ICP standard solution, calcium 1000 ppm ICP standard solution and manganese 1000 ppm ICP standard solution were provided by GFS Chemicals Company. All other chemicals were of analytical grade or higher and used without further purification.
Acid content in PLGA
Acid number (AN), a measure of the acid content directly related to the number of free carboxylic acid functionalities, was determined by titration, applying a modified method reported in the literature (23). About 100 mg of polymer were dissolved in 20 ml of acetone/THF (1:1) mixture. The solution was immediately titrated with 0.01 M methanolic potassium hydroxide to a stable pink end point. Phenolphthalein methanol solution (0.1 wt%) was used as an indicator and 20 ml of acetone/THF (1:1) without polymer was used as a control. The acid number of the star-type PLGA was 4.2 mg KOH/g PLGA.
Preparation of microspheres
Microspheres were prepared using a double emulsion-solvent evaporation method (29). Briefly, octreotide acetate was dissolved in 100 μl of water or divalent cationic salt (CaCl2 or MnCl2) aqueous solution. The solution was mixed with 2 ml of PLGA methylene chloride solution 500 mg/ml (with or without suspension of CaCl2 or MnCl2, which ground and sieved through a 90 μm screen (Newark Wire Cloth Company, Clifton, NJ)). Efforts to sieve salts below 20 μm were unsuccessful because of the affinity of the salt particles to the stainless steel sieve. However, the majority of former sieved particles were viewed to be smaller than 20 μm by scanning electron microscopy (SEM) (data not shown), allowing successful encapsulation of the salts (see table 1). The resulting mixture was homogenized at 10,000 rpm using a homogenizer (VirTis Company, Gardiner, NY) for 1 min in an ice bath. The emulsion stabilizer (2 ml of 1% PVA solution) was added and the mixture was vortexed with a Vortex Genie 2TM (Scientific Industries, Bohemia, NY) at maximum speed for 45 s. The formed W1/O/W2 emulsion was immediately transferred to 100 ml 0.3% PVA aqueous solution (with or without 2.5 wt% CaCl2 or MnCl2) under stirring at a constant rate. As described below and in Table 1, various amounts of sieved MnCl2 or CaCl2 salt were added to the internal water (W1), external water (W2), and/or organic (O) phases. After evaporation of methylene chloride (3 h), the microspheres were collected by sieve and washed repeatedly with deionized water. Finally, the microspheres were lyophilized for 24 h and stored in a desiccator at 4 °C before further investigation. Three different methods of salt addition were carried out in this paper: 1) addition of 25 mg NaCl, CaCl2 or MnCl2 to the organic phase (O); 2) simultaneous addition of 25 mg and 2.5 g of CaCl2 or MnCl2 to the organic (O) and external water (W2) phases, respectively; and 3) addition of 5 mg CaCl2 or MnCl2 to the internal (W1) and 2.5 g to the external (W2) water phases simultaneously.
Table 1.
Formulation conditions and loading data for octreotide-loaded microspheres with salt as additives a
| Formul ation# |
Salt additive |
O mg |
W1
mg |
W2
g |
LTS
w/w% |
LAS
w/w% |
EES
% |
LTD w/w% |
LAD
w/w% |
EED
% |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | - | - | - | - | 3.38 | 2.74±0.01 | 80.9±0.3 |
| 2 | NaCl | 25 | - | - | 2.36 | 0.62±0.01 | 26.2±0.6 | 3.39 | 2.30±0.06 | 67.8±1.9 |
| 3 | CaCl2 | 25 | - | - | 2.36 | 1.35±0.13 | 57.2±5.6 | 3.39 | 2.34±0.12 | 68.9±3.4 |
| 4 | MnCl2 | 25 | - | - | 2.36 | 1.22±0.01 | 51.6±0.4 | 3.39 | 2.42±0.02 | 71.3±0.7 |
| 5 | CaCl2 | 25 | - | 2.5 | 2.36 | 2.21±0.13 | 93.6±5.5 | 3.44 | 3.37±0.11 | 98±3.0 |
| 6 | MnCl2 | 25 | - | 2.5 | 2.36 | 2.34±0.07 | 99.0±3.0 | 3.44 | 3.43±0.07 | 99.8±2.2 |
| 7 | CaCl2 | - | 5 | 2.5 | 0.48 | 0.52±0.02 | 108±4.1 | 3.37 | 3.12±0.11 | 92.5±3.9 |
| 8 | MnCl2 | - | 5 | 2.5 | 0.48 | 0.36±0.01 | 74±2.0 | 3.44 | 3.09±0.05 | 89.9±1.7 |
| 9 | NaCl | 25 | - | 2.5 | 2.36 | 0.91±0.02 | 38.6±0.8 | 3.44 | 2.84±0.28 | 82.6±8.4 |
| 10 | NaCl | - | 4b | 2.5 | 0.38 | 0.25±0.01 | 64.8±2.2 | 3.37 | 2.42±0.10 | 71.9±3.0 |
Definitions:
O, W1, W2 = salt added to oil, internal water, and external water phase;
LTS, LTD = theoretical loading of salt and octreotide, respectively;
LAS, LAD = actual loading of salt determined by ICP-OES and octreotide, respectively;
EES, EED=encapsulation efficiency of salt and octreotide, respectively
: 4 g instead of 5 g NaCl was used in W1 for formulation 10 is because 5 g NaCl salt out the octreotide
Morphology of microspheres
The morphology of microspheres after freeze-drying was analyzed by scanning electron microscopy (SEM). Microspheres samples were sputter-coated with gold for analysis by SEM (Hitachi S3200 Variable Pressure SEM, Tokyo, Japan).
Drug loading and encapsulation efficiency
Microspheres were characterized for drug content, encapsulation efficiency and initial impurity content by an organic solvent extraction procedure using methylene chloride and acetate buffer. The microspheres were dissolved in 1 ml methylene chloride and the octreotide was extracted with 0.1 M acetate buffer (pH 4.0). Each formulation was analyzed in triplicate. Octreotide acetate concentration was determined by high performance liquid chromatography (HPLC). HPLC conditions are described below.
Ca2+ and Mn2+ content in the microspheres
The content of divalent cations in the microspheres was analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES, Perkin-Elmer Optima 2000 DV with Winlab software). Microspheres were first dissolved in methylene chloride and then extracted as previously described. The buffer solution was diluted with water and 1 ppm Yttrium added as an internal standard. The final aqueous solution was used for ICP-OES analysis. Each sample was analyzed in triplicate.
In vitro release and stability of octreotide in PLGA microspheres
Release and stability kinetics of octreotide encapsulated in PLGA microspheres was carried out in PBST release medium, consisting of PBS (7.74 mM Na2HPO4, 2.26 mM NaH2PO4, 137 mM NaCl, and 3mM KCl, pH 7.4) with 0.02 wt% Tween 80 at 37°C. Microspheres (6-12 mg) were placed in 1.0 ml of PBST. The samples were continuously agitated at constant rate in a shaker set at 37°C. At pre-selected time points (1, 3, 7, 14, 21, 28 days), the release medium was completely removed. The microspheres were resuspended with 1 ml of fresh buffer by vortexing and were placed onto a shaker or lyophilized for further analysis after removing the release medium at specified time points (7, 14, 21 and 28 days). The content of both octreotide impurities resulting from acylation and divalent cations in the microspheres was determined by HPLC and ICP-OES, respectively. Each formulation was examined in triplicate.
Analysis of octreotide solutions by RP-HPLC
Octreotide acetate was analyzed by reversed phase high performance liquid chromatography (RP-HPLC, Waters Alliance, Midford, MA) using a Nova Pak C-18 column (3.9×150 mm, Waters). A gradient elution method was utilized with mobile phase A: 0.1% TFA in acetionitrile and B: 0.1% TFA in water. The linear gradient was 25% to 35% A in 10 minutes with a flow rate of 1.0 mL/min. UV absorbance was measured at 215 nm.
Detection of acylated octreotide products by HPLC-MS
The octreotide solutions were also analyzed by HPLC-mass spectrometry (negative-ion ESI-MS, Thermo Fisher). Octreotide and its degradation products were separated on a linear gradient of 5-55% 0.1% TFA in acetionitrile over 50 min with a flow rate of 0.5 mL/min. The column used was Nova Pak C-18 column (3.9×150 mm, Waters). UV absorbance was measured at 215 nm.
RESULTS AND DISCUSSION
Determination of octreotide impurities
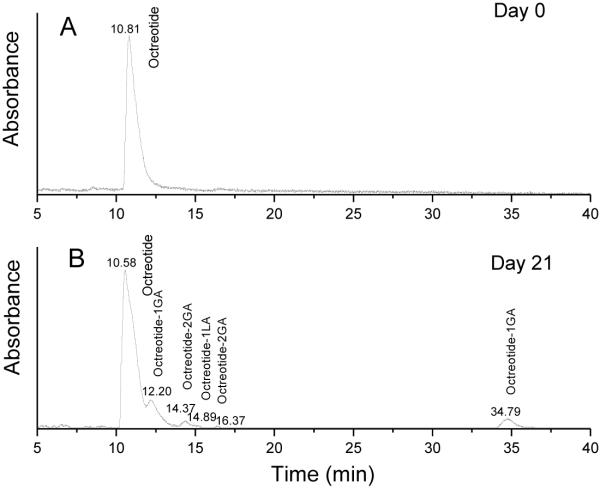
Octreotide acetate was encapsulated in microspheres using a modified double emulsion-solvent evaporation method without any additives (Formulation 1 in Table 1). To obtain information on the chemical structure of non-released peptide in microspheres, the peptide extracted from microspheres incubated at specified time points (days 7, 14, 21 and 28) was analyzed by HPLC-MS. Octreotide extracted from the microspheres before incubation in PBST served as control. Only one peak was detected in the LC-MS spectrum at the retention time of native octreotide acetate (10.81 min, Figure 1A) before microsphere incubation in PBST, indicating that the peptide was stable during microencapsulation and freeze-drying. However, after microspheres were incubated in PBST at 37 °C, octreotide related degradants were formed. Besides the native octreotide peak at 10.58 min, additional peaks appeared in the spectrum at the later retention times of 12.20 min, 14.37 min, 14.89 min, 16.37 min and 34.79 min. Figure 1B displays representative LC-MS chromatograms obtained from buffer extract of microspheres after 21 days of incubation. The molecular masses and the expected structure are listed in Table 2. LC-MS analysis revealed that the existence of derivatives with a molecular mass increased by 72, 58 and 116 amu. Those molecular mass changes were indicative of an acylation of peptide amino-groups by single lactic and glycolic acid units, and two glycolic acid units, respectively. This presence of multiple peaks on the chromatogram with 1 or 2 glycolic acid units each also revealed that octreotide has multiple acylation sites (23). Nucleophilic functional groups such as the primary amino groups present in the N-terminus and lysine residue have been shown to be the major targets for peptide acylation (17, 19, 30). The individual peaks, octreotide and octreotide degradated products in HPLC chromatogram were identified through LC-MS based on the retention times.
Figure 1.
HPLC chromatograms of octreotide in microspheres incubated in PBST for 0 day (A) and 21 days (B) detected at 215 nm.
Table 2.
HPLC-MS of octreotide recovered from PLGA microspheres incubated in PBST for 21 days
| Observed mass | Expected structure |
|---|---|
| 1019 | Octreotide |
| 1077 | Octreotide-1GA |
| 1135 | Octreotide-2GA |
| 1091 | Octreotide-1LA |
GA: glycolic acid, LA: lactic acid
Effect of adding divalent cationic salts to the organic phase during microsphere preparation
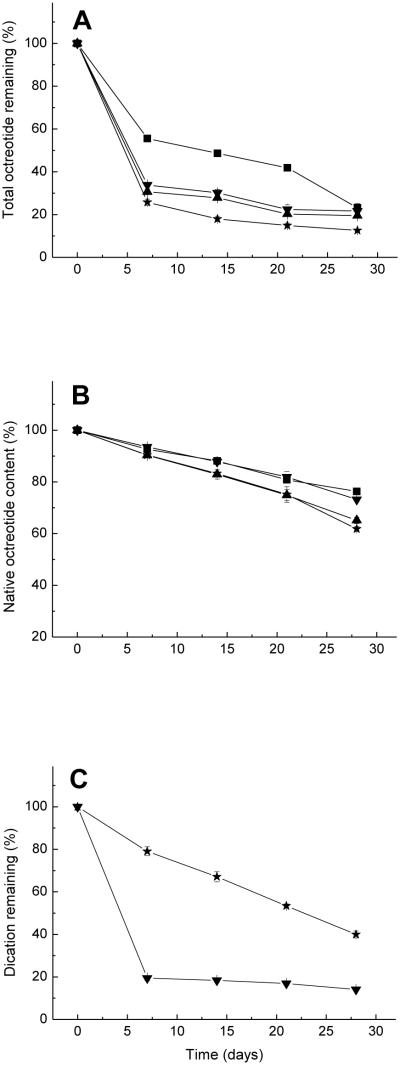
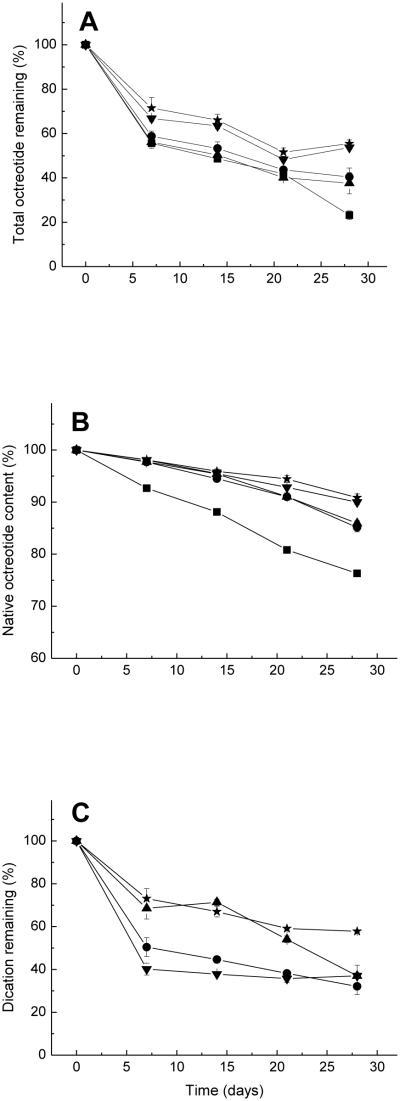
Two water-soluble divalent cationic salts, CaCl2 and MnCl2, which showed promise as acylation inhibitors in our previous study (26), were co-incorporated in the microspheres with octreotide by adding the ground and sieved salts to PLGA methylene chloride solution (organic phase). The encapsulation conditions are listed in Table 1 (formulations 3 and 4). Two additional formulations, one without salt and one made by adding NaCl to the organic phase (formulation 1 and 2, respectively) were used as controls. Figures 2A-C show the total peptide remaining, native octreotide content, and divalent cation remaining in microspheres, respectively, during in vitro incubation over 28 days. The native peptide content decreased as acylation increased with incubation time in all formulations. The addition of CaCl2 and MnCl2 to the organic phase alone did not improve the stability of octreotide in microspheres. In fact, adding MnCl2 to the organic phase resulted in slightly more acylation than the NaCl control.
Figure 2.
Kinetics of total octreotide remaining (A), native octreotide content (B) and divalent cation remaining (C) after incubation of PLGA microspheres in PBST at 37°C. ■ Formulation 1, no additive was added to organic phase; ▲Formulation 2, adding 25 mg NaCl to organic phase; ▼Formulation 3, adding 25 mg CaCl2 to organic phase; and ✩Formulation 4, adding 25 mg MnCl2 to organic phase
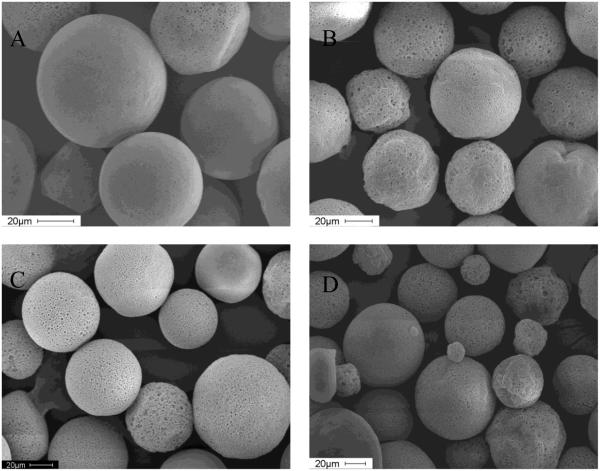
SEMs of microspheres containing the divalent cationic salts and NaCl added to the organic phase showed that all microspheres were highly porous (Figures 3B-D), undoubtedly owing to the osmotic gradient created by the salt in the polymer solution and the resulting water influx from the outer aqueous phase (31). Microspheres without salt additive had only few pores (Figure 3 A). Porous microspheres allow for rapid release of acidic PLGA oligomers, which typically raises the microclimate pH within eroding microspheres (32). Since acylation has been shown to be accelerated by high concentrations of oligomers in solution, as well as by acidic conditions (19, 20), more porous microspheres were expected to slow down and suppress the acylation of octreotide. In addition, acylation in microspheres containing water-soluble divalent cationic salts might be prevented by reducing sorption of octreotide to PLGA (26). However, porosity caused by salt in PLGA microspheres did not help prevent acylation.
Figure 3.
Scanning electron micrographs of microspheres prepared without salt (A, Formulation 1), and adding 25 mg NaCl (B, Formulation 2), CaCl2 (C, Formulation 3) and MnCl2 (D, Formulation 4) to the organic phase.
We also examined the availability of the salt inhibitors during release by monitoring their retention in the polymer (Figure 2C). Interestingly, extensive leakage of Ca2+ was recorded in the porous microspheres with the original inhibitor content dropping to just 19.4% of the original loading within 7 days. The loss of Mn2+ was not as rapid, possibly due to coordination of the dication with carboxyl or hydroxyl groups in PLGA. Also, the presence of salts within highly porous microspheres can result in accelerated water uptake, which is necessary for acylation. Topp et al. (33, 34) found that water influx into the dosage form provided a solvent medium effect for chemical reaction of a hexapeptide formulated in poly(vinyl pyrrolidone) and poly(vinyl alcohol) matrices. Göpferich et al. also suggested that the presence of water is a prerequisite of acylation and elevated levels of moisture provide sufficient protein mobility for reaction (19, 35).
Therefore, adding divalent cationic salts to the organic phase is expected to have both positive and negative effects on acylation. All salts increased the porosity, water uptake and thus acylation reactivity. However, divalent cations are also expected to inhibit the sorption of peptide to microspheres and acylation. Thus, the addition of NaCl, which increases porosity and water uptake, but is a poor inhibitor of octreotide sorption, resulted in higher acylation. The use of divalent cations, such as CaCl2, provided greater inhibition of octreotide sorption, which apparently overcompensated for the salt effect on porosity and water uptake. It is possible that the inhibitory effect of MnCl2 on octreotide sorption does not overcompensate for its effect on factors that accelerate acylation, explaining its unexpectedly weak stabilization effect.
Adding salts to the organic phase also had substantial effect on peptide release behavior. Figure 2A displays of the kinetics of total octreotide remaining in the microspheres. Since octreotide is unstable in phosphate buffer (36), the octreotide remaining was used to evaluate the release behavior. Based on the assumption that the molar extinction coefficients of octreotide and octreotide acylated products at 215 nm were equivalent, the amount of the acylated peptides remaining in the microspheres was calculated from the peak areas of the UV chromatograms at 215 nm. With the formulations containing salt in the organic phase, only 25-35% total octreotide remained within the microspheres at 7 days, after which the amount of octreotide remaining did not change up to 28 days. The rapid release of octreotide within one week is likely caused by the diffusion through microspheres’ pores before pore closure ensues (29).
Acylation inhibition by divalent cations
From the above results, the inclusion of divalent cations might decrease the acylation of octreotide in PLGA microspheres. However, high porosity caused by salt increased the initial burst and water uptake, opposing the inhibitory effect on acylation. We therefore sought to reduce the porous structure of microspheres. The use of salt in the external aqueous phase is known to reduce the difference in the osmotic pressure between the internal and the external aqueous phase and particle porosity, which has been shown in many previous studies (37-40).
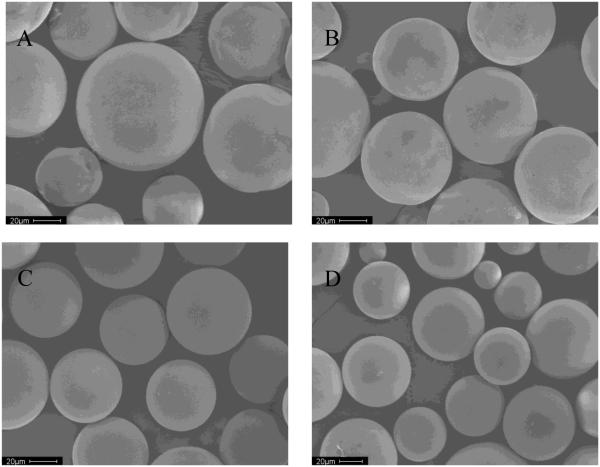
Therefore, to minimize dication inhibitor loss, we made another four formulations of microspheres, adding the divalent cationic salts, calcium or manganese chlorides, both to the organic phase or inner water phase, and to the outer water phase (formulations 5-8 in Table 1). SEMs of the microspheres (Figure 4) show that addition of corresponding divalent cationic salts to outer water phase led to microspheres with a smooth surface without noticeable pores. Also, both drug encapsulation efficiency and salt encapsulation efficiency were increased because of the increased osmotic pressure in outer water phase (see Table 1).
Figure 4.
Scanning electron micrographs of microspheres adding divalent cationic salts as additives
A (Formulation 5): 25 mg and 2.5 g CaCl2 was added to O and W2 respectively; B (Formulation 6): 25 mg and 2.5 g MnCl2 was added to O and W2 respectively; C (Formulation 7): 5 mg and 2.5 g CaCl2 was added to W1 and W2 respectively; and D (Formulation 8): 5 mg and 2.5 g MnCl2 was added to W1 and W2 respectively.
W1: internal water phase, O: organic phase; W2: external water phase.
Figures 5A and 5B show the kinetics of total peptide and native octreotide fraction remaining the microspheres incubated in PBST 37°C. The amount of octreotide released from microspheres prepared by adding divalent cationic salts to the outer water phase as well as the organic or inner water phase was reduced relative to microspheres made without salt in the outer water phase. Decreasing microsphere porosity reduced the amount of peptide available for rapid release through the fluid-filled pores.
Figure 5.
Kinetics of total octreotide remaining (A), native octreotide content (B) and divalent cation remaining (C) after incubation of PLGA microspheres in PBST at 37°C.
■Formulation 1, no additive was added; ●Formulation 5, 25 mg and 2.5 g CaCl2 was added to O and W2 respectively; ▲Formulation 6, 25 mg and 2.5 g MnCl2 was added to O and W2 respectively; ▼Formulation 7, 5 mg and 2.5 g CaCl2 was added to W1 and W2 respectively; and ✩Formulation 8, 5 mg and 2.5 g MnCl2 was added to W1 and W2 respectively.
W1: internal water phase, O: organic phase; W2: external water phase.
Compared to the microspheres without any additives, addition of divalent cationic salts both to the organic and outer water phases, or both to the inner and outer water phases, largely increased the stability of octreotide in microspheres. After adding CaCl2, native peptide remaining in the microspheres decreased with time slightly (Figure 5B), which is consistent with CaCl2 content in microspheres (Figure 5C). Adding corresponding divalent cationic salts to the outer water phase helped to retard the leaching of dications in the microspheres. After 7 days incubation in PBST, Ca2+ content dropped to 50.4% with salt addition to the outer water phase compared to 19.4% without salt addition. Although addition of corresponding MnCl2 to outer water phase did not strongly increase the MnCl2 content in microspheres with time, it did decrease the water uptake of microspheres and acylation.
DeLuca et al. (23) indicated that carboxyl end groups of PLGA and generated carboxylic acid end groups of degraded PLGA play an important role in the sorption and ionic interaction with octreotide. At physiologic pH, most α- and ε-amino groups of octreotide are ionized. Therefore, the co-encapsulated divalent cationic salts can outcompete for the adsorption sites and ionic interaction between carboxyl end groups of PLGA relative to positively charged animo groups of octreotide (26). As the polymer degrades and medium pH slightly decreases, the primary amine of the Lys side chain is highly protonated and most N-terminal amine groups are ionized too. However, water uptake of microspheres increases and divalent cations are released from the microspheres gradually. Therefore, the inhibition of adsorption and acylation is expected to decrease.
The inhibition of octreotide acylation by divalent cationic salts is shown in Table 3. It is interesting to note that the microspheres with divalent cationic salts added to both to the inner and outer water phases (Formulations 7 and 8) display slightly improved inhibition relative to microspheres prepared by adding salts both to the organic phase and outer water phase (Formulations 5 and 6), even though salt loading in the former is much lower than the latter. This might be explained by the higher density of the Formulations 7 and 8 microspheres which may have decreased water uptake and peptide reactivity.
Table 3.
Extent of acylation inhibition (%) afforded by divalent cations
| Time (days) |
Formulation # | |||
|---|---|---|---|---|
| 5 | 6 | 7 | 8 | |
| 7 | 68.5±2.4 | 69.1±1.3 | 73.7±3.8 | 74.2±2.6 |
| 14 | 53.6±1.3 | 61.5±4.1 | 62.0±2.5 | 65.7±3.7 |
| 21 | 53.2±3.8 | 53.3±3.1 | 62.7±0.8 | 70.9±3.6 |
| 28 | 37.3±3.7 | 40.5±2.2 | 57.8±2.0 | 61.4±3.0 |
Inhibition extent was calculated by [acylated octreotide content in microspheres without additive(%) – acylated octreotide content in microsphere with salt additive(%)]/acylated octreotide content in microspheres without additive(%)
In order to confirm that it is the divalent cations instead of the increased density of microspheres, which is responsible for the reduced acylation of octreotide in PLGA microspheres, we also performed the same experiments except instead NaCl (Formulations 9 and 10) was equivalently replaced for the divalent chloride salts. As seen in Table 4, this replacement resulted in microspheres that were more dense than microspheres without any salts. However, acylation was not decreased nearly so strongly as that afforded by CaCl2 and MnCl2. This result is consistent with sorption inhibition results reported in our previous paper (26). However, although the MnCl2 content remaining in microspheres was typically higher than that of CaCl2, the inhibition caused by MnCl2 was not much higher than CaCl2 as expected. This modest stabilization afforded by Mn2+ may have been due to 1) water uptake of microspheres incorporated with MnCl2 is much higher than with CaCl2 (e.g., wet weight increase of low Mw free-acid end-group PLGA (50/50) millicylinders with MnCl2 (26) was found to be 15-55% more than millicylinders with CaCl2 during 1-14 days incubation in PBST at 37°C (data not shown)); and 2) coordination of Mn2+ with carboxyl or hydroxyl groups in PLGA might weaken the absorption and acylation to octreotide; and 3) divalent cations could act as a catalyst for hydrolysis of PLGA, increasing pore formation (41).
Table 4.
Comparison of kinetics of native octreotide content (%) in microspheres for various formulations
| Time (days) |
Formulation | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 7 | 92.7±0.5 | 97.7±0.2 | 97.8±0.1 | 98.1±0.3 | 98.1±0.2 | 94.4±0.2 | 93.4±0.9 |
| 14 | 88.1±0.3 | 94.5±0.2 | 95.4±0.3 | 95.5±0.3 | 95.9±0.4 | 91.6±0.9 | 91.2±0.2 |
| 21 | 80.8±0.4 | 91.0±0.7 | 91.1±0.6 | 92.9±0.1 | 94.4±0.7 | 88.3±0.8 | 87.6±0.4 |
| 28 | 76.3±0.4 | 85.2±0.9 | 85.9±0.5 | 90.0±0.5 | 90.9±0.7 | 79.4±0.3 | 79.2±0.7 |
Adding divalent cationic salts to outer water phase resulted in improved microsphere characteristics including a dense morphology, an increased drug and salt encapsulation efficiency, and a reduced initial burst release, dication leakage, and acylation of peptide in microspheres.
CONCLUSION
The peptide acylation pathway is initiated upon sorption to PLGA, which is primarily driven by electrostatic interactions. The divalent cationic salts, calcium chloride and manganese chloride, which compete with the peptide for the ionic interaction with PLGA, were successfully incorporated into PLGA microspheres. By minimizing microsphere surface porosity and divalent divalent cations loss, octreotide acylation was strongly inhibited by the CaCl2 and MnCl2. Therefore, divalent cationic salts can be effectively used to improve the stability of acylation-labile peptides in PLGA microspheres.
ACKNOWLEGMENTS
This study was supported by Novartis Pharma AG and NIH HL 68345.
REFERENCES
- 1.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 2.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 3.Jiang WL, Schwendeman SP. Stabilization and controlled release of bovine serum albumin encapsulated in poly(D, L-lactide) and poly(ethylene glycol) microsphere blends. Pharm. Res. 2001;18:878–885. doi: 10.1023/a:1011009117586. [DOI] [PubMed] [Google Scholar]
- 4.Zhu GZ, Schwendeman SP. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: Mechanism of stabilization by basic additives. Pharm. Res. 2000;17:351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]
- 5.Jiang WL, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliver. Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat. Biotechnol. 2000;18:24–25. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 7.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 8.Houchin ML, Topp EM. Chemical degradation of peptides and proteins in PLGA: A review of reactions and mechanisms. J. Pharm. Sci. 2008;97:2395–2404. doi: 10.1002/jps.21176. [DOI] [PubMed] [Google Scholar]
- 9.Estey T, Kang J, Schwendeman SP, Carpenter JF. BSA degradation under acidic conditions: A model for protein instability during release from PLGA delivery systems. J. Pharm. Sci. 2006;95:1626–1639. doi: 10.1002/jps.20625. [DOI] [PubMed] [Google Scholar]
- 10.Kang JC, Schwendeman SP. Comparison of the effects of Mg(OH)(2) and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(D,L-lactide-co-glycolide) implants. Biomaterials. 2002;23:239–245. doi: 10.1016/s0142-9612(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu WR, Langer R, Klibanov AM. Moisture-Induced Aggregation of Lyophilized Proteins in the Solid-State. Biotechnol. Bioeng. 1991;37:177–184. doi: 10.1002/bit.260370210. [DOI] [PubMed] [Google Scholar]
- 12.Schwendeman SP, Costantino HR, Gupta RK, Siber GR, Klibanov AM, Langer R. Stabilization of Tetanus and Diphtheria Toxoids against Moisture-Induced Aggregation. P. Natl. Acad. Sci. USA. 1995;92:11234–11238. doi: 10.1073/pnas.92.24.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotts G, Sah H, Park TG. Adsorption determines in-vitro protein release rate from biodegradable microspheres: Quantitative analysis of surface area during degradation. J. Controlled Release. 1997;47:101–111. [Google Scholar]
- 14.Calis S, Jeyanthi R, Tsai T, Mehta RC, DeLuca PP. Adsorption of Salmon-Calcitonin to Plga Microspheres. Pharm. Res. 1995;12:1072–1076. doi: 10.1023/a:1016278902839. [DOI] [PubMed] [Google Scholar]
- 15.Oscarsson S. Factors affecting protein interaction at sorbent interfaces. J. Chromatogr. B. 1997;699:117–131. doi: 10.1016/s0378-4347(97)00224-7. [DOI] [PubMed] [Google Scholar]
- 16.Welzel PB. Investigation of adsorption-induced structural changes of proteins at solid/liquid interfaces by differential scanning calorimetry. Thermochim. Acta. 2002;382:175–188. [Google Scholar]
- 17.Na DH, Youn YS, Lee SD, Son MW, Kim WB, DeLuca PP, Lee KC. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. J. Controlled Release. 2003;92:291–299. doi: 10.1016/s0168-3659(03)00366-3. [DOI] [PubMed] [Google Scholar]
- 18.Murase S, Yumoto N, Petukhov MG, Yoshikawa S. Acylation of the alpha-amino group in neuropeptide Y(12-36) increases binding affinity for the Y-2 receptor. J. Biochem. 1996;119:37–41. doi: 10.1093/oxfordjournals.jbchem.a021213. [DOI] [PubMed] [Google Scholar]
- 19.Lucke A, Kiermaier J, Gopferich A. Peptide acylation by poly(alpha-hydroxy esters) Pharm. Res. 2002;19:175–181. doi: 10.1023/a:1014272816454. [DOI] [PubMed] [Google Scholar]
- 20.Lucke A, Gopferich A. Acylation of peptides by lactic acid solutions. Eur. J. Pharm. Biopharm. 2003;55:27–33. doi: 10.1016/s0939-6411(02)00138-8. [DOI] [PubMed] [Google Scholar]
- 21.Murty SB, Goodman J, Thanoo BC, DeLuca PP. Identification of chemically modified peptide from poly(d,l-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS PharmSciTech. 2003;4:392–405. doi: 10.1208/pt040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucke A, Fustella E, Tessmar J, Gazzaniga A, Gopferich A. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. J. Controlled Release. 2002;80:157–168. doi: 10.1016/s0168-3659(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 23.Na DH, DeLuca PP. PEGylation of octreotide: I. Separation of positional isomers and stability against acylation by Poly(D,L-lactide-co-glycolide) Pharm. Res. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- 24.Houchin ML, Heppert K, Topp EM. Deamidation, acylation and proteolysis of a model peptide in PLGA films. J. Controlled Release. 2006;112:111–119. doi: 10.1016/j.jconrel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Houchin ML, Neuenswander SA, Topp EM. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide. J. Controlled Release. 2007;117:413–420. doi: 10.1016/j.jconrel.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sophocleous AM, Zhang Y, Schwendeman SP. A new class of inhibitors of peptide sorption and acylation in PLGA. J. Controlled Release. doi: 10.1016/j.jconrel.2009.03.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szilagyi A, Shrier I. Systematic review: the use of somatostatin or octreotide in refractory diarrhoea. Aliment. Pharmacol. Ther. 2001;15:1889–1897. doi: 10.1046/j.1365-2036.2001.01114.x. [DOI] [PubMed] [Google Scholar]
- 28.McKeage K, Cheer S, Wagstaff AJ. Octreotide long-acting release (LAR) - A review of its use in the management of acromegaly. Drugs. 2003;63:2473–2499. doi: 10.2165/00003495-200363220-00014. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Wang BM, Schwendeman SP. Mechanistic evaluation of the glucose-induced reduction in initial burst release of octreotide acetate from poly(D,L-lactide-co-glycolide) microspheres. Biomaterials. 2004;25:1919–1927. doi: 10.1016/j.biomaterials.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Domb AJ, Turovsky L, Nudelman R. Chemical Interactions between Drugs Containing Reactive Amines with Hydrolyzable Insoluble Biopolymers in Aqueous-Solutions. Pharm. Res. 1994;11:865–868. doi: 10.1023/a:1018985909777. [DOI] [PubMed] [Google Scholar]
- 31.Ravivarapu HB, Lee H, DeLuca PP. Enhancing initial release of peptide from poly(d,l-lactide-co-glycolide) (PLGA) microspheres by addition of a porosigen and increasing drug load. Pharm. Dev. Tehcnol. 2000;5:287–296. doi: 10.1081/pdt-100100543. [DOI] [PubMed] [Google Scholar]
- 32.Zhu GZ, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat. Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 33.Lai MC, Hageman MJ, Schowen RL, Borchardt RT, Laird BB, Topp EM. Chemical stability of peptides in polymers. 2. Discriminating between solvent and plasticizing effects of water on peptide deamidation in poly(vinylpyrrolidone) J. Pharm. Sci. 1999;88:1081–1089. doi: 10.1021/js9802289. [DOI] [PubMed] [Google Scholar]
- 34.Lai MC, Hageman MJ, Schowen RL, Borchardt RT, Topp EM. Chemical stability of peptides in polymers. 1. Effect of water on peptide deamidation in poly(vinyl alcohol) and poly(vinyl pyrrolidone) matrixes. J. Pharm. Sci. 1999;88:1073–1080. doi: 10.1021/js980227g. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim MA, Ismail A, Fetouh MI, Gopferich A. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. J. Controlled Release. 2005;106:241–252. doi: 10.1016/j.jconrel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Wang BA, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J. Controlled Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 37.Pistel KF, Kissel T. Effects of salt addition on the microencapsulation of proteins using W/O/W double emulsion technique. J. Microencapsulation. 2000;17:467–483. doi: 10.1080/026520400405723. [DOI] [PubMed] [Google Scholar]
- 38.Jiang G, Thanoo BC, DeLuca PP. Effect of osmotic pressure in the solvent extraction phase on BSA release profile from PLGA microspheres. Pharm. Dev. Technol. 2002;7:391–399. doi: 10.1081/pdt-120015040. [DOI] [PubMed] [Google Scholar]
- 39.De Rosa G, Quaglia F, Bochot A, Ungaro F, Fattal E. Long-term release and improved intracellular penetration of oligonucleotide-polyethylenimine complexes entrapped in biodegradable microspheres. Biomacromolecules. 2003;4:529–536. doi: 10.1021/bm025684c. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann J, Bodmeier R. The Effect of Particle Microstructure on the Somatostatin Release from Poly(Lactide) Microspheres Prepared by a W/O/W Solvent Evaporation Method. J. Controlled Release. 1995;36:63–71. [Google Scholar]
- 41.Fredenberg S, Reslow M, Axelsson A. Effect of divalent cations on pore formation and degradation of Poly(D,L-lactide-co-glycolide) Pharm. Dev. Technol. 2007;12:563–572. doi: 10.1080/10837450701560588. [DOI] [PubMed] [Google Scholar]