Abstract
Background
Dopamine (DA) has been shown to play a central role in regulating motivated behavior and encoding reward. Chronic drug abuse elicits a state of hypodopaminergia in the mesocorticolimbic (MCL) system in both humans and preclinical rodent models of addiction, including those modeling alcohol use disorders (AUD).
Methods
Working under the hypothesis that reductions in the bioavailability of DA play an integral role in the expression of the excessive drinking phenotype, the COMT inhibitor Tolcapone was used as a means to amplify cortical DA efflux and drinking behaviors were then assessed. Sucrose and ethanol consumption were measured in P and Wistar rats in both a free choice drinking protocol and a novel cued access protocol.
Results
Tolcapone attenuated the consumption of ethanol, and to a lesser extent sucrose, in P rats in the cued access protocol, while no effect was observed in the free choice drinking protocol. Tolcapone also decreased ethanol consumption in high drinking Wistar rats. A follow-up experiment using the DA agonist D-amphetamine (AMPH) showed no change in ethanol consumption.
Conclusions
Collectively, these data suggest that COMT inhibitors may be capable of alleviating the extremely motivating or salient nature of stimuli associated with alcohol. The hypothesis is put forth that the relative specificity of Tolcapone for cortical DA systems may mediate the suppression of the high seeking/drinking phenotype.
Keywords: alcohol preferring rat, catechol-O-methyltransferase, dopamine, prefrontal cortex, Tolcapone
Introduction
Drugs of abuse are hypothesized to highjack the function of neural circuits that encode motivational and rewarding signals (Koob and LeMoal, 2001). The mesocorticolimbic (MCL) dopamine (DA) system plays a central role in motivated and reward-related behaviors (Kiyatkin, 1995) and is directly targeted by multiple drugs of abuse, including alcohol (Rossetti et al., 1992). Changes in DA receptor expression and the bioavailability of DA have been identified as both a predisposing factor for and consequence of drug abuse (Volkow et al., 1997; Engleman et al., 2006). Moreover, persistent reductions in the bioavailability of DA and DA neuron activity are observed during withdrawal (Wang et al., 2012), which can be alleviated by alcohol administration (Martinez et al., 2005). In sum, numerous streams of evidence converge to suggest that, in addiction, the DA system is “profoundly dysregulated” (Volkow et al., 2007). While a number of pharmacotherapies exist that target this system, they have yet to provide a viable treatment option (George et al., 2002).
Environmental stimuli that are associated with, or explicitly signal, the availability of alcohol can powerfully evoke alcohol seeking and consummatory behaviors (Field et al., 2008; Ryan et al., 2010). When individuals diagnosed with, or at risk for, an alcohol use disorder (AUD) are presented with drug-related stimuli, an enhanced hemodynamic response is observed across the MCL system, especially in the prefrontal cortex (PFC) (Grusser et al., 2004; Myrick et al., 2004; Kareken et al., 2004; 2010). Moreover, enhanced DA efflux to alcohol-paired cues is observed in preclinical rodent models (Melendez et al., 2002) as well as addicted and heavy drinking individuals (Volkow et al., 2006; Oberlin et al., 2013) thus suggesting a role for DA in encoding drug-related stimuli.
The DA transporter regulates the bioavailability of DA in a number of limbic and mesencephalic structures, however, the majority of cortical DA is metabolized by the enzyme catechol-O-methyltransferase (COMT) and taken up by the norepinephrine transporter (Mazei et al., 2002). A functional polymorphism of COMT exists where a substitution of methionine (Met) in place of valine (Val) at codon 158 affects the thermostability and activity of the enzyme leading to differences in the bioavailability of PFC DA (Lotta et al., 1995). The relationship between the bioavailability of DA and cognitive performance is hypothesized to follow an inverted-U shaped function (Williams and Goldman-Rakic, 1995). In accordance with this theory, allelic differences in COMT activity are associated with altered performance of behaviors such as executive function (Farrell et al., 2012), cognition, and sensation seeking (Mattay et al., 2003; Yacubian and Buchel, 2009). Moreover, interactions between allelic variations in COMT and Tolcapone treatment are observed in a number of cognitive behaviors (Farrell et al., 2012). An association is also observed between allelic variations in COMT and alcohol drinking behavior (Tammimäki et al., 2008; Hendershot et al., 2012) as well as the propensity to relapse (Wojnar et al., 2009; but see Köhnke et al., 2003; Foroud et al., 2007).
Alcohol preferring rats (P rats) provide a validated preclinical rodent model of AUD (Murphy et al., 2002). These rodents were selectively bred for alcohol preference and model at risk human populations for excessive drinking, such as individuals with a family history of alcoholism (Froehlich, 2010). Additionally, P rats display pronounced deficits in basal extracellular DA levels in the PFC compared to their progenitor strain, Wistar rats (Engleman et al., 2006). P rats exhibit extremely high levels of ethanol seeking (Czachowski and Samson, 2002) as well as greater resistance to extinction and a more robust cue-induced reinstatement relative to alcohol non-preferring (NP) rats (Ciccocioppo et al., 2001), suggesting an enhanced response to alcohol paired cues. Considering the central role DA plays in integrating alcohol-paired cues and seeking behaviors and the altered PFC DA phenotype of P rats, we hypothesized that increasing the bioavailability of DA would suppress the excessive drinking phenotype of the P rat. To this end, Tolcapone and AMPH were used as separate approaches to enhance DA efflux. Brain penetrant COMT inhibitors, such as Tolcapone and Entacapone, have been used extensively as a noninvasive means to increase the bioavailability of DA (Kaakkola, 2000). Interestingly COMT inhibitors have not been observed to increase basal dopamine but rather accentuate evoked release (Tunbridge et al., 2004; Lapish et al., 2009). On the other hand, AMPH stimulates the release of catecholamines, especially DA, throughout the brain via its actions as a weak base (Sulzer et al., 2005). The current study assessed the effects of Tolcapone and AMPH on drinking in Wistar and P rats in a free choice drinking and a novel cued access protocol.
Materials & Methods
Subjects
Alcohol naïve adult male Wistar rats (Harlan, Indianapolis, IN) and Alcohol preferring (P) rats (71–74th generation; Indiana University, IN) were used for all experiments. Animals weighed approximately 250–300g upon arrival and were individually housed in a climate controlled room on a 12-hour reversed light/dark cycle. All animals were handled one week prior to experimental testing and were approximately 12 weeks of age at the start of testing. All procedures were approved by the IUPUI School of Science Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guide for the care and use of laboratory animals.
Intermittent access drinking protocol (IAP)
A modified two-bottle free choice drinking protocol as described by Simms et al. (2008) was implemented to expose animals to the liquid solutions later used during conditioning and to initiate consumption. Subjects were Wistar and P rats divided into either 2% sucrose (N=16/strain) or 20% ethanol (N=20/strain) groups. At the start of the dark cycle, all water bottles were replaced with two bottles, one containing water and the other containing either sucrose or ethanol. Fluid intake of both water and sucrose or ethanol was measured 30 minutes and 24 hours after placement of bottles. Bottles were removed 24 hours after bottle placement, and replaced with the initial water bottle. Animals had access to their respective solutions for 24 hr 3 d/wk (M, W, F) over the course of a 4 week period. Animals were weighed immediately before bottle placement to calculate grams of sucrose or ethanol intake per kilogram of body weight.
Two-way cued access protocol (2CAP)
Upon completion of IAP, animals were transitioned to the 2CAP, a Pavlovian conditioning-based protocol. Subjects were a subset of Wistar and P rats previously exposed to either 2% sucrose (N=8/strain) or 20% ethanol (N=10/strain) in the IAP. Animals weighed approximately 400–500 g at the start of conditioning. Conditioning sessions were conducted in modular two compartment operant chambers divided by a movable guillotine door (20.3 cm × 15.9 cm × 21.3 cm; Med Associates, St Albans, VT), which remained open for all conditioning sessions. All chambers were equipped with white noise generators, infrared photocell beams, stimulus lights, and retractable graduated cylinder tubes fitted with stainless steel spouts containing double ball bearings. At the beginning of each conditioning trial, a stimulus light (CS) 16.5 cm above the retractable sipper was illuminated for two seconds and after a one second inter-stimulus interval, ten seconds of access to either sucrose or ethanol (US) was granted via retractable sipper tube. Location of the CS/US was randomized across trials within a session between the left and right compartments of the chamber. Each conditioning session was composed of 40 trials of CS/US pairings. The inter-trial intervals (ITI) ranged from 20–180 seconds, with a mean of 90 seconds. Daily sessions lasted approximately 71 minutes. All animals received 12 conditioning sessions (2CAP-same) prior to treatment. To confirm that animals were using the CS to predict the location of the US, all animals had four additional days of conditioning where the CS/US spatial relationship was reversed (2CAP-opposite). In these subsequent four sessions, the relationship between the CS and US was altered such that a CS on the right side of the chamber predicted availability of the US on the left side and vice versa. In each of these experiments, time spent at the sipper was measured using infrared photocell beams and solution intake (g/kg) was calculated using mls consumed and body weights.
Blood ethanol concentration (BEC)
On day five, at approximately trial 25 (~ 40 minutes), the conditioning session was prematurely stopped and animals were removed from the chambers. Blood samples were then collected from the tail to measure BEC. Bloods were centrifuged and a 5 µl plasma sample was analyzed using an AMI analyzer (Analox, Instruments, Lunenburg, MA).
Tolcapone and 2CAP
To assess the role of inhibiting the COMT enzyme on conditioned drinking and approach behavior, animals received counterbalanced ip injections of Tolcapone (Valeant Pharmaceuticals; 17.0 or 30.0 mg/kg and vehicle) one hour prior to testing after 12 days of 2CAP.
AMPH and 2CAP
To assess differences in global DA modulation relative to PFC DA specificity, a separate group of ethanol P rats (N=8) received 4 weeks of IAP immediately followed by 2CAP, as described previously. Counterbalanced ip injections of AMPH (Sigma; 0.15 mg/kg) or vehicle were administered 20 minutes prior to testing after 12 days of 2CAP.
Tolcapone and free choice drinking
The effects of Tolcapone on free choice drinking were assessed in a separate group of sucrose (N=7/strain) and ethanol (N=8/strain) animals who did not have any experience with the 2CAP task. Animals had four weeks of intermittent two-bottle drinking as described previously. Afterwards, animals had two additional weeks of the IAP in which they received drug or vehicle injections. On days where animals had access to solutions, all animals received an ip injection of Tolcapone (0, 3.0, 17.0, 30.0 mg/kg; balanced design) one hour prior to two-bottle placement. Intake was measured 30 minutes and 24 hours after placement of bottles. Sucrose or ethanol intake (g/kg) was calculated using solution consumed and body weights.
Data analysis and statistics
Statistical analyses were performed in R (http://www.r-project.org) and Matlab (Mathworks, Natick, MA). All group effects were first assessed by analysis of variance testing (ANOVA) and Tukey’s post-hoc was used for all multiple comparison procedures except where stated otherwise. Pearson’s correlation was used to determine the strength of the relationship between ethanol intake (g/kg) and BEC. To control for ethanol induced changes in locomotor activity and vigilance, time at sipper data were analyzed using the mean of the first 20 trials for each animal in a session. Data for time at the sipper across each trial are presented as a smoothed time-series via a moving average kernel with a span of 3 trials. All group stats were performed on non-smoothed data.
Results
Quantification of sucrose and ethanol intake during the IAP
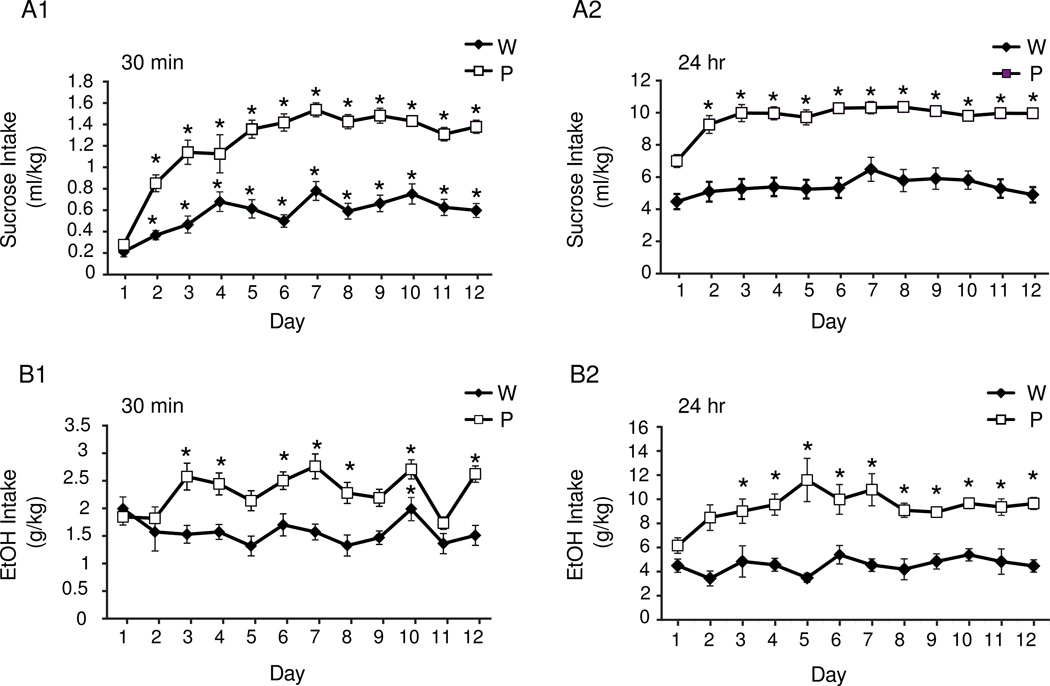
After the first day, P rats consumed more sucrose than Wistars in the initial 30 min of the IAP [Two-way repeated measures ANOVA: strain × day interaction, F(11,330)=6.938, p<0.0001; Figure 1, A1]. P rats also consumed more sucrose than Wistars at the 24 hour reading [Two-way repeated measures ANOVA: strain × day interaction, F(11,329)=2.352, p=0.008; Figure 1, A2]. A Two-way repeated measures ANOVA with 2 levels of strain and 12 levels of day also revealed a significant main effect of day in P rats at the 24 hour reading [F(11,180)=5.516, p<0.0001]. P rats consumed more total fluid [main effect of strain, F(1,24)=35.6, p<0.0001; Figure S2A] but not water [F(1,24)=0.01, p=0.922; Figure S2C], indicating that increased total fluid consumption observed in P rats is driven by greater sucrose intake. Together these data show that P rats increase their sucrose intake over days, especially at the 30 min measurement, compared with Wistars throughout the IAP.
Figure 1.
The volume of sucrose (A) and ethanol (B) consumed at 30 minutes (1) and 24 hours (2) post bottle placement during the IAP. All animals in the ethanol groups drink > 1.0 g/kg within 30 minutes (B1), indicating that both P’s and Wistars achieve pharmacologically relevant levels of intoxication in this protocol. Values are means + SEM. * p <0.05, Bonferroni comparisons, days different from day 1.
At the 30 min reading P rats consumed more ethanol than Wistars [Two-way repeated measures ANOVA: main effect of strain, F(1,38)=27.66, p<0.001; Figure 1, B1]. At the 24-hour reading a similar pattern was also observed, where P rats also drank more than the Wistars [Two-way repeated measures ANOVA: main effect of strain, F(1,37)=54.34, p<0.001; Figure 1, B2]. Wistars consumed more total fluid [Two-way repeated measures ANOVA: main effect of strain, F(1,25)=10.926, p=0.002; Figure S1B] and water [Two-way repeated measures ANOVA: main effect of strain, F(1,31)=92.47, p<0.0001; Figure S2D] than P rats. Additionally, there was a reduction in water intake over days in both strains [Two-way repeated measures ANOVA: main effect of day, F(11,455)=10.289, p<0.0001; Figure S2D].
Intake and behavioral profile of sucrose rats in 2CAP
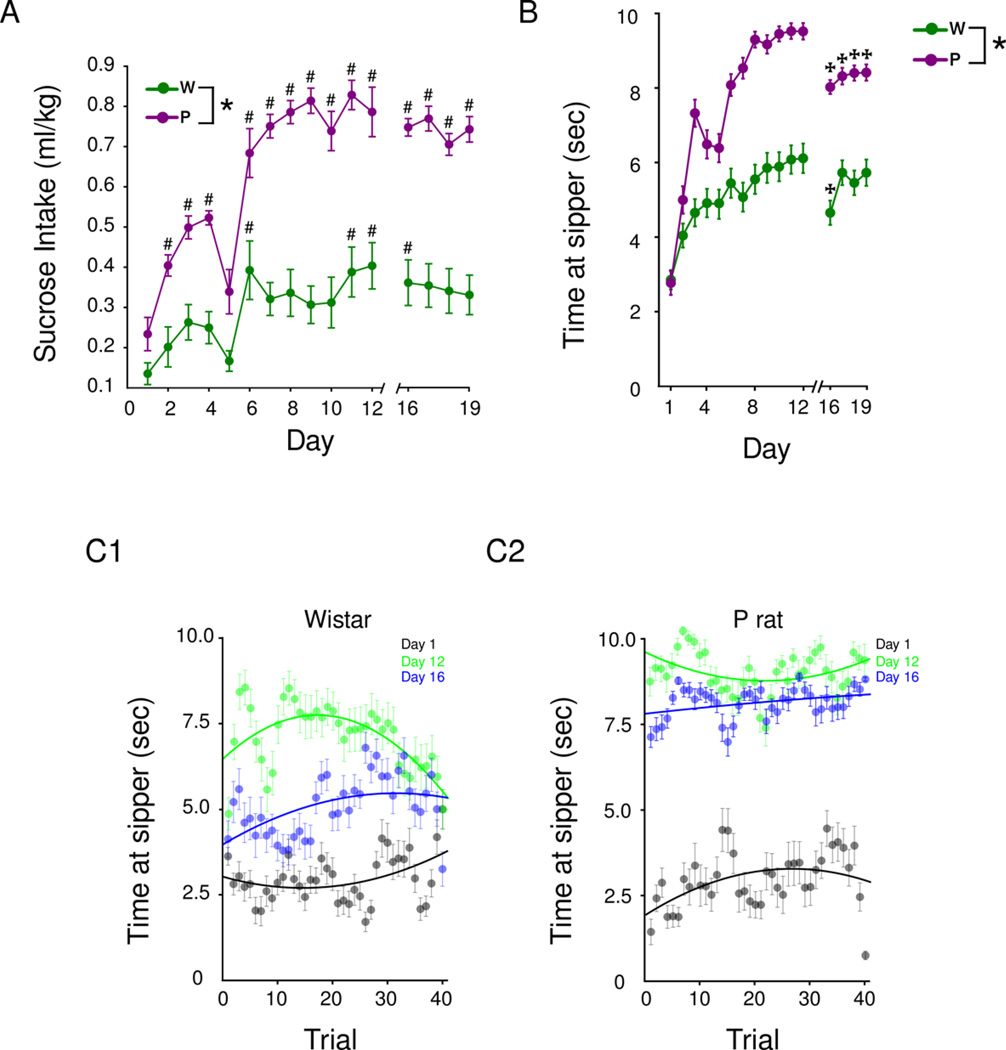
Each strain progressively consumed more sucrose throughout the 2CAP task relative to day 1. However, P rats exhibited a larger increase than Wistars [Two-way repeated measures ANOVA (day (1–12) × strain): strain × day interaction, F(18,252)=5.78, p<0.0001; Figure 2A]. This pattern was also reflected in their behavior where the time at the sipper increased [Two-way repeated measures ANOVA day (1–12) × strain: main effect of day, F(15,5088)=33.87, p<0.001) throughout 2CAP-same (Figure 2B). On a given day, the trial-to-trial pattern of sucrose intake was relatively stable within strains, which was measured as time spent at the sipper during each trial (Figure 2C). However, P rats consistently spent more time at the sipper during each trial [Three-way repeated measures ANOVA (day (1,12,16) × strain × trial (1–40): main effect of strain, F(1,5088)=516.61, p<0.001]. These data demonstrate that both strains in the sucrose groups learn that the CS predicts the US in the 2CAP, as evidenced by greater time at the sipper following presentation of the CS, and thereby increase their sucrose intake over days.
Figure 2.
Sucrose consumption and associated behavioral measures during 2CAP. P rats drink more sucrose in the 2CAP protocol than Wistars (A). In panel B, the mean time spent at the sipper for the first 20 trials is shown; alterations in each measure are observed on day 16 where the switch from 2CAP-same to 2CAP-opposite occurs. In panels C1–2, the time spent at the sipper for each trial in each line is shown on three days. Note the time spent at the sipper is relatively stable across trials for sucrose (c.f. Figure 3C). Values are means +SEM. * p<0.05, main effect of strain, # p<0.05 Post hoc Bonferroni comparisons, days different from day 1,✠ p<0.05 Post hoc Bonferroni comparisons, days different from day 12.
To further assess if each strain used the CS to predict the availability of the sucrose US, the spatial relationship between the CS and US was changed on day 16 such that the stimulus light (CS) on the opposite side of the active sipper was illuminated (2CAP-opposite). Changing this spatial relationship led to decreased time spent at sipper on day 16 in both strains [Bonferroni comparisons, days different from day 12, p<0.05; Figure 2B]. While Wistars’ time at sipper returned to levels observed in 2CAP-same, P rats remain impaired [Bonferroni comparisons, days different from day 12, p<0.05; Figure 2B]. This change in approach behavior was not influenced by administration of Tolcapone prior to the switch since animals who received saline or no injection still show a significant reduction in time at sipper (Figure S3).
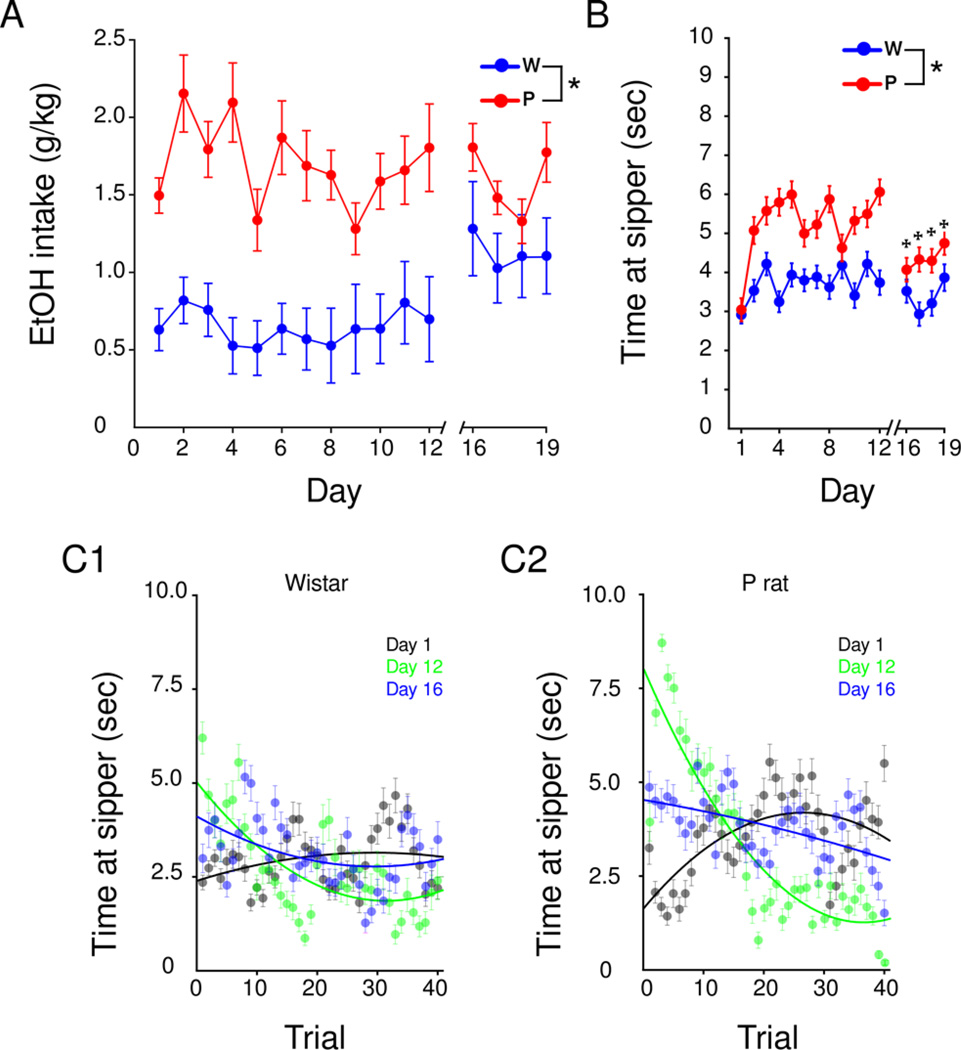
Intake and behavioral profile of ethanol rats in 2CAP
No consistent, daily change in ethanol intake was observed throughout the 2CAP task in either strain (Figure 3A). A linear regression comparing 12 days of IAP and 12 days of 2CAP was performed. IAP intake explained a significant portion of variance in 2CAP intake, R2=0.10, F(1,238)=26.48, p<0.001, significantly predicted intake during 2CAP, =1.28, t(238)=12.75, p<0.0001, and as seen in the IAP sessions, P rats drank more ethanol than Wistars overall [Two-way repeated measures ANOVA: main effect of strain, F(18,324)=3.67, p<0.0001]. During 2CAP, 100% of P rats (M=100, SD=0) and approximately 35% of Wistars (M=35, SD=26) consumed ethanol to levels shown to be discriminated by the oral route (>1.0 g/kg, Hodge et al., 2001) at least once per week. On day 1, progressive increases in the time spent at the sipper with each trial were observed in both P rats and Wistars (black line, Figure 3C), while on day 12 (green line, Figure 3C) a progressive decrease was observed in P rats [Two-way repeated measures ANOVA day (1,12,16) × trial (1–40): trial × day interaction, F(1,796)=49.28, p<0.0001; Figure 3C] but not Wistars [Two-way repeated measures ANOVA day (1,12,16) × trial (1–40): F(1,796)=0.01, p=0.053]. This transition reflects a change in drinking patterns where animals are able to use the CS to predict the active sipper and then maximize ethanol intake in the first ~10–20 trials of the 2CAP. Thus, while P rats’ between session drinking shows no effect of day on intake, within session drinking assessed by time at sipper varies between trials over days. On day 16, after the change in the spatial relationship between the CS and US, the initial spike in time at the sipper in the first ~20 trials of the 2CAP was not as robust as observed on day 12 [Two-way repeated measures ANOVA day (1,12,16) × trial (1–40): trial × day interaction, F(1,796)=15.55, p<0.0001; Figure 3C], thus providing evidence that these animals use the CS to predict the location of the ethanol US. Similar to sucrose animals, ethanol P rats remain impaired during 2CAP-opposite whereas Wistars’ time at sipper was not altered by the switch from 2CAP-same to 2CAP-opposite [Bonferroni comparisons, days different from day 12, p<0.05; Figure 3B].
Figure 3.
Ethanol consumption and associated behavioral measures during 2CAP. P rats drink more ethanol (A) and spend more time at the sipper (B) than Wistars in the 2CAP. Transitioning from 2CAP-same to 2CAP-opposite (day 16) reduced time at sipper in P rats (B). In contrast to sucrose reinforced animals, P rats developed a binge-like pattern of ethanol intake where they spent more time at the sipper in the early trials of 2CAP-same (C2). This binge-like pattern was not observed on day 16 when the switch to 2CAP-opposite occurred. Values are means +SEM. * p<0.05, main effect of strain, ✠ p<0.05 Post hoc Bonferroni comparisons, days different from day 12.
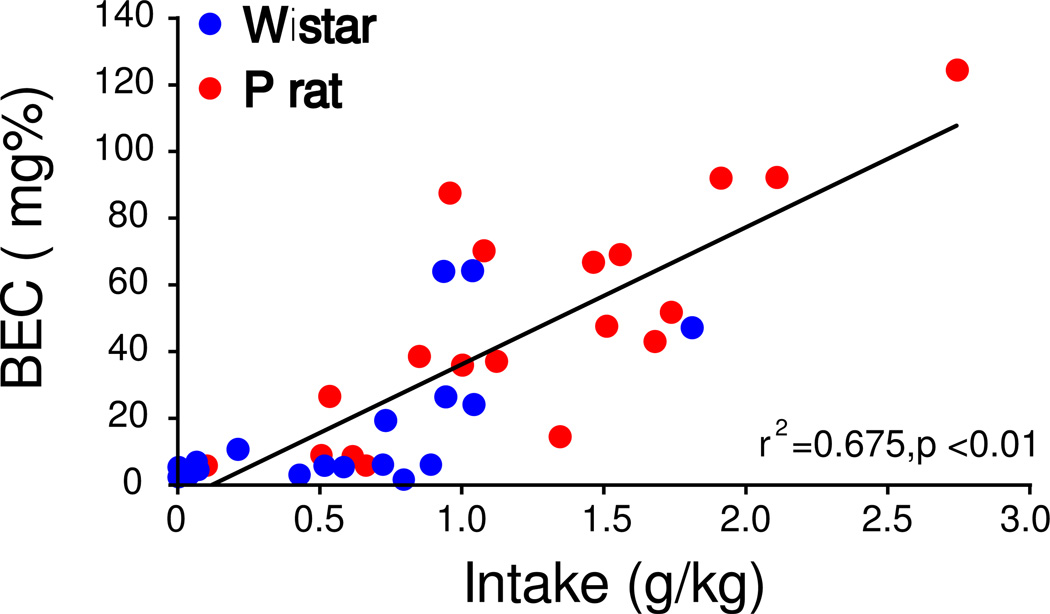
Quantification of BEC in 2CAP
A significant relationship between ethanol intake (g/kg) and BEC, r2=0.675, p<0.01 was observed (Figure 4A), indicating that ethanol intake reliably predicts BEC in the 2CAP protocol. A linear regression was performed to predict BEC’s based on intake during each 2CAP session. Based on these data, ~100% of P rats (M=98, SD=5) and ~ 40% of Wistars (M=35, SD=26) drink to pharmacologically relevant levels (>40 mg%) and ~50% of P rats (M=53, SD=15) and ~ 15% of Wistars (M=15, SD=6) drink to binge levels (>80 mg%; NIAAA, 2004) at least once a week. Time at sipper on the day when bloods were taken (day 5, brown line, Figure 4B) was less than day 12 (green line, Figure 4B, trial × day interaction, F(1,196)=7.88, p=0.006) in the first 10 trials suggesting that animals were not yet at optimal responding in the 2CAP task when blood samples were taken, and leaves open the possibility that higher BECs might have been observed on subsequent days.
Figure 4.
BEC is positively correlated with intake in the 2CAP protocol (A). The time spent at the sipper for both Wistars (B1) and P rats (B2) is shown for the day bloods were collected (day 5, brown line) and the last day of 2CAP-same for reference (day 12, green line).
The effect of Tolcapone and AMPH on intake and behavior in 2CAP
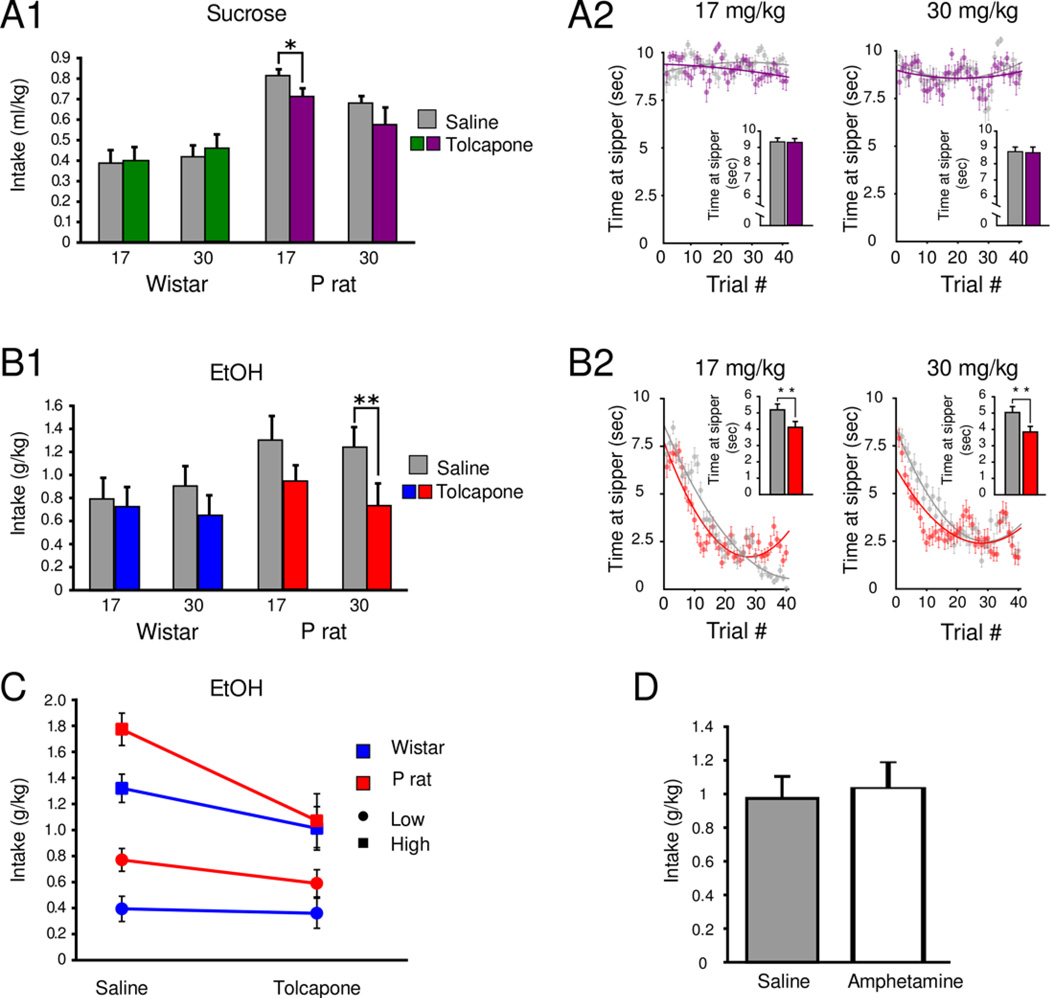
A factorial ANOVA of intake by strain, dose and treatment in the sucrose group revealed a main effect of strain, F(1,40)=27.63, p<0.001, showing that P rats consumed more sucrose than Wistars in general. Based on this finding, data were stratified by strain and two ANOVAs with Bonferroni corrected alpha levels adjusted for 2 comparisons (P rat and Wistar) were used to assess intake by treatment and dose within each strain. There was a main effect of treatment in P rats [F(1,20)=4.67, p=0.014] but not Wistars [p=0.68]. In the P rats, the observed decrease in sucrose consumption was observed following the 17.0 mg/kg dose [p=0.022, Bonferroni corrected planned comparison; Figure 5, A1]. Similarly, in the ethanol group, a factorial ANOVA of intake by strain, dose and treatment revealed a main effect of strain [F(1,40)=6.57, p=0.014], which prompted subsequent Bonferroni corrected two-way ANOVAs of intake by treatment and dose in each strain. There was a main effect of treatment in P rats [F(1,20)=10.01, p=0.019] but not Wistars [p=0.33]. A decrease in intake was observed in ethanol drinking P rats following the 30.0 mg/kg dose [p=0.007, Bonferroni corrected planned comparison, Figure 5, B1].
Figure 5.
Tolcapone suppresses ethanol intake and drinking behavior in the 2CAP protocol. In sucrose animals, Tolcapone (17 mg/kg) suppresses sucrose consumption in P rats with no effect in Wistars (A1). No changes in time at sipper were detected in sucrose animals after Tolcapone (A2). Tolcapone (30 mg/kg) suppresses ethanol consumption in P rats with no effects observed in Wistars (B1). Both doses of Tolcapone decrease time spent at the sipper in P rats (B2). A median spilt was performed in each group of animals and it was observed that 30 mg/kg Tolcapone suppressed drinking in each of the high drinking subgroups while the 17 mg/kg dose only suppressed ethanol consumption in high-drinking animals (C). No measureable effect on ethanol consumption after AMPH injection was observed in a group of ethanol reinforced P rats (D). * p<0.05, ** p<0.01 Bonferroni corrected planned comparison.
Time at sipper was not altered in sucrose-reinforced P rats treated with either dose of Tolcapone (Figure 5, A2). However, in ethanol-reinforced animals, time at sipper decreased [Two-way ANOVA main effect of treatment, F(3,1592)=4.65, p<0.001]. Post hoc testing indicated differences in Tolcapone treated P rats at both doses (Figure 5, B2).
Ethanol animals were split into high and low drinking groups via median split (Table 1). Drinking was suppressed in high drinking, but not low drinking P’s at the 17 mg/kg dose [Factorial ANOVA (group, strain, treatment: group × treatment interaction F(1,16)=7.344, p=0.0155] and both the low and high drinking P and Wistar rats in the 30 mg/kg group exhibited a decrease in drinking [Factorial ANOVA (group, strain, treatment: main effect of treatment, F(1,20)=10.61, p=0.004; Figure 5, C].
A student’s t-test revealed no effect of AMPH on ethanol consumption [t(7)=0.3, p=0.75] or time at sipper t(7) = 0.35, p = 0.73; Figure 5D] in the 2CAP.
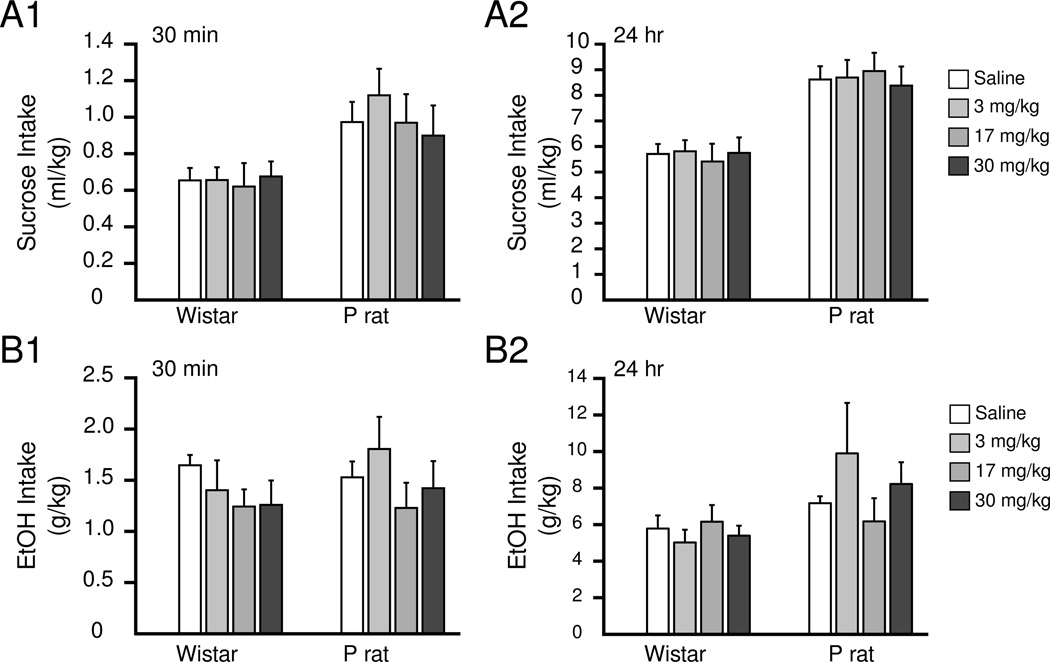
The effect of Tolcapone on intake in IAP
No effect of treatment on intake for either strain in either sucrose [repeated measures ANOVA treatment(0,3,17,30), time (30min, 24hr), strain): F(3,32) >0.177, p>0.52] or ethanol [repeated measures ANOVA treatment(0,3,17,30), time (30min, 24hr), strain): F(3,39)>0.31, p>0.22] at any of the time points measured was observed (Figure 6).
Figure 6.
No measurable changes in ethanol or sucrose intake were detected in the IAP after Tolcapone treatment. Sucrose intake after 30 min and 24 hr is shown in A1 and A2, respectively. Ethanol intake after 30 min or 24 hr is shown in B1 and B2, respectively.
Discussion
An enhanced response to alcohol-paired cues may be a critical aspect of excessive drinking. Working under the hypothesis that the PFC DA system plays a central role in processing alcohol-paired cues, we targeted this system with the COMT inhibitor, Tolcapone. Tolcapone suppressed alcohol intake in a cued access protocol, with no effects in a free-access paradigm. These findings were in contrast to AMPH, which had no effect on ethanol intake in the cued access protocol. These data underscore the importance of tasks that engage drug seeking behaviors and also recommend further assessment of COMT as a potential target capable of suppressing excessive reward seeking.
The primary advantage of using the IAP to induce consumption is that animals will consume 20% ethanol in the absence of any added sweetening, thus the motivation to drink is solely attributable to an association with the solution and its pharmacological properties (Simms et al., 2008). While increases in ethanol intake in Wistars have been reported throughout the IAP (Simms et al., 2008), this was not observed in the current study (Figure 1B). This was likely attributable to high intake levels on the first day of the IAP, which may have impaired our ability to detect a subsequent increase. Importantly, the intakes of the Wistars after 4 weeks of the IAP correspond to intake ranges previously observed at the same time point (Simms et al., 2008). Moreover, in the first 30 min of the IAP, both P rats and Wistars consumed amounts of alcohol shown to be discriminated orally (>1.0 g/kg; Hodge et al., 2001), which suggests that drinking was motivated by the pharmacological properties of ethanol.
In the 2CAP task, progressive increases in volume consumed and time at sipper indicate that both strains increasingly seek and drink sucrose (Figure 2). With respect to ethanol intake, the overall amount consumed did not change over days (Figure 3A), but it appears that it was consumed faster each day, which reflects a transition to a binge-like pattern of intake throughout 2CAP-same. Both Wistars and P rats consumed comparable levels of sucrose in 2CAP relative to the IAP. However, only P rats consumed comparable amounts of ethanol in the 2CAP as measured at the 30 min reading of the IAP. Therefore, a compelling case cannot be made that Wistar rats, as a group, actually acquired the 2CAP task with ethanol as their intake values decreased ~1.0 g/kg relative to the 30 min IAP reading, and measurements of time at the sipper were sporadic.
After the transition from 2CAP-same to 2CAP-opposite (Days 16–19), P rats exhibited a reduction in time at sipper in both the sucrose and ethanol groups. The inability of P rats to perform at pre-switch levels may have been a result of their continued approach to the CS, even though it signaled availability of solution on the opposite side of the chamber. Future uses of the 2CAP paradigm will utilize video monitoring in order to accurately quantify the time to approach the sipper that takes into account the location and orientation of the subjects at the moment that the CS is initiated.
While sucrose and ethanol can generate a number of similarities in motivated behavior, the pharmacological/intoxicating effects of ethanol can lead to a number of differences. In contrast to the sucrose group, the trial-to-trial variance in time spent at the sipper on a given day was much different in the ethanol group. With each day, P rats spent progressively more time at the sipper in the first 10–20 trials of 2CAP-same (Figure 3C2). On day 16, after the switch, time at the sipper was altered in P rats in the ethanol group in a consistent manner to that observed with sucrose, albeit less robustly (Figure 3B). Moreover, the binge-like pattern in time at the sipper observed in the early trials of the task was not observed on the day of the switch (day 16, Figure 3C2). Collectively, these data provide further evidence that P rats responded to the alcohol-paired CS and when this relationship was altered it prevented the expression of the binge-like pattern that emerges as animals acquire 2CAP.
Tolcapone reduced consumption in the 2CAP but not the IAP, suggesting that separate neural systems may be engaged during free choice versus cued access behavioral protocols. The critical difference between the IAP and 2CAP is likely the manner by which environmental cues are used to motivate drinking bouts in a limited period of time. Drinking in the IAP is largely self-paced over a 24hr period and does not require the animal to attend to the presentation of a CS prior to solution availability and, as such, the requirement to seek the solution is minimal. In contrast, in the 2CAP, access to the solution is restricted to 10 sec bouts that are each preceded by a CS and requires that the animal engage in repeated seeking behavior prior to each solution presentation. Considering that consumption in the IAP does not heavily engage seeking behaviors, it is possible that Tolcapone retains some specificity for the neural systems supporting seeking behaviors, possibly explaining the lack of effect in the IAP.
Lapish et al., (2009) found that a 30.0 mg/kg dose of Tolcapone amplified PFC DA efflux by ~100% during anticipation and consumption of a small amount of food, which is comparable to the amount of DA released after treatment with a low dose (0.15 mg/kg) of the DA-releasing agent, AMPH (Berridge and Stalnaker, 2002). The findings with Tolcapone in 2CAP, therefore, prompted the assessment of AMPH in 2CAP to examine if the differential manner that the two drugs target the DA system would yield different behavioral outcomes. Because there was no effect of Tolcapone on Wistars, we chose to not use Wistars in the AMPH experiment. Importantly, we found no effect of AMPH treatment on ethanol intake in 2CAP protocol, which is consistent with previous studies assessing ethanol drinking in High Alcohol Preferring mice (Oberlin et al., 2010). The null effects of AMPH in the current experiment may be influenced by a number of factors such as AMPH’s differential effects in various brain regions and neurotransmitters. Importantly, this further underscores the utility of COMT inhibitors in selectively targeting cortical DA.
Both COMT knockout and COMT deficient mice possess elevated extracellular DA levels within the PFC whereas striatal DA levels in these animals are comparable to wild-type mice (Yavich et al., 2007; Käenmäki et al., 2010). Similarly, COMT inhibitors enhance extracellular PFC DA levels (Tunbridge et al., 2004; Lapish et al., 2009) and DA content in the hippocampus with minimal effects on striatal DA (Huotari et al., 1999; Laatikainen et al., 2013). This is likely due to different reuptake mechanisms in cortex and striatum (Mazei et al., 2002; Käenmäki et al., 2010). Based on these observations, we speculate that the ability of Tolcapone, but not AMPH, to suppress drinking in the 2CAP may be mediated by its relative specificity for the cortical DA system. Lastly, Tolcapone does not appear to affect extracellular levels of other catecholamines in the PFC (Tunbridge et al., 2004; Laatikainen et al., 2013).
The P rat was used in the current study for their high ethanol intake and because reduced basal levels of PFC DA are observed in these animals prior to alcohol consumption (Engleman et al., 2006). The fact that ethanol consumption was not completely abolished by Tolcapone, but reduced in P rats such that they were not different from Wistars, suggests that ethanol retains motivational salience, albeit reduced. In this way, Tolcapone may have a “normalizing” effect on P rats via rebalancing DA signaling. P rats will also seek alcohol to a greater extent than other rodent strains suggesting that the incentive motivational properties of ethanol are greater in these animals (Czachowski and Samson, 2002). Phasic increases of DA neuron firing are observed in response to a CS predicting the availability of a reward and scale as the magnitude of the reward increases (Tobler et al., 2005). For this reason, it would be expected that P rats would exhibit larger increases in DA efflux in response to ethanol cues than Wistars. When this is considered in combination with reductions in basal DA observed in the P rat, these two observations suggest that the PFC DA system of the P rat would operate in a very wide dynamic range. Since Tolcapone would prolong the bioavailability of cue-evoked DA released via preventing metabolism, this could increase tone on the D2 receptor and blunt subsequent DA release events (Del Arco and Mora, 2008). It is tempting to speculate that the net effect of Tolcapone would be to reduce the dynamic range of the PFC DA system, thus blunting the motivational salience of alcohol associated conditioned stimuli.
Reduced alcohol consumption observed in the P rat following Tolcapone suggests that inhibition of COMT may provide a therapeutic option capable of reducing the motivational salience of alcohol-paired, and to a lesser extent, sucrose-paired cues. While therapeutic options for AUD exist that target the DA system, most are not viable treatment options considering their dangerous side effect profile (Swift, 2010), which is thought to be mediated largely by effects on the DA system in the ventral midbrain and striatum. COMT inhibitors may provide an attractive therapeutic option for AUD because they provide some level of selectively for the cortical DA system (Yavich et al., 2007; Käenmäki et al., 2010), do not directly target DA receptors, and do not evoke but rather accentuate naturally occurring DA release (Tunbridge et al., 2004). While the current study strongly recommends further exploring COMT as a potential therapeutic target for AUD and possibly other addictions, dangerous side effects have been observed with extended treatment of Tolcapone (Colosimo, 1999) which may prevent its use in this context. However, if novel and safer translational approaches to target COMT or the PFC DA system can be developed, they may provide an effective strategy to mitigate the motivating effects of stimuli associated with drugs of abuse.
Supplementary Material
Acknowledgements
The authors wish to thank Valeant Pharmaceuticals for providing drug to these studies and Maureen Timm for excellent technical assistance. Support for this work was provided by AMBRF (CCL), and NIAAA P60AA007611 (CCL, CLC).
References
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater Discounting of Delayed Rewards in Young Adults with Family Histories of Alcohol and Drug Use Disorders: Studies from the Oklahoma Family Health Patterns Project. Alcoholism: Clinical and Experimental Research. 2011;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Stalnaker TA. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–149. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: Role of genetic ethanol preference. Alcoholism-Clinical and Experimental Research. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Colosimo C. The rise and fall of tolcapone. J Neurol. 1999;246:880–882. doi: 10.1007/s004150050477. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcoholism-Clinical and Experimental Research. 2002;26:1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex–nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val158Met Genotype Determines the Direction of Cognitive Effects Produced by Catechol-O-Methyltransferase Inhibition. Biological Psychiatry. 2012;71:538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Schoenmakers T, Wiers RW. Cognitive processes in alcohol binges: a review and research agenda. Current drug abuse reviews. 2008;1:263. doi: 10.2174/1874473710801030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC. What Aspects of Human Alcohol Use Disorders Can Be Modeled Using Selectively Bred Rat Lines? Substance Use & Misuse. 2010;45:1727–1741. doi: 10.3109/10826084.2010.482424. [DOI] [PubMed] [Google Scholar]
- George TP, Kosten TA, Kosten TR. The potential of dopamine agonists in drug addiction. Expert Opinion on Investigational Drugs. 2002;11:491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Lindgren KP, Liang TB, Hutchison KE. COMT and ALDH2 polymorphisms moderate associations of implicit drinking motives with alcohol use. Addiction Biology. 2012;17:192–201. doi: 10.1111/j.1369-1600.2010.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABAA and NMDA receptors in rats. Psychopharmacology. 2001;154:13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- Huotari M, Gainetdinov R, Männistö PT. Microdialysis Studies on the Action of Tolcapone on Pharmacologically-Elevated Extracellular Dopamine Levels in Conscious Rats. Pharmacology & Toxicology. 1999;85:233–238. doi: 10.1111/j.1600-0773.1999.tb02014.x. [DOI] [PubMed] [Google Scholar]
- Kaakkola S. Clinical Pharmacology, Therapeutic Use and Potential of COMT Inhibitors in Parkinson’s Disease. Drugs. 2000;59:1233–1250. doi: 10.2165/00003495-200059060-00004. [DOI] [PubMed] [Google Scholar]
- Käenmäki M, Tammimäki A, Myöhänen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Männistö PT. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. Journal of Neurochemistry. 2010;114:1745–1755. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O'Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. NeuroImage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O'Connor SJ, Lowe M. Alcohol-Related Olfactory Cues Activate the Nucleus Accumbens and Ventral Tegmental Area in High-Risk Drinkers: Preliminary Findings. Alcoholism: Clinical and Experimental Research. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Functional significance of mesolimbic dopamine. Neuroscience & Biobehavioral Reviews. 1995;19:573–598. doi: 10.1016/0149-7634(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug Addiction, Dysregulation of Reward, and Allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Laatikainen LM, Sharp T, Harrison PJ, Tunbridge EM. Sexually Dimorphic Effects of Catechol-O-Methyltransferase (COMT) Inhibition on Dopamine Metabolism in Multiple Brain Regions. PLoS ONE. 2013;8:e61839. doi: 10.1371/journal.pone.0061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish C, Ahn S, Evangelista L, So K, Seamans J, Phillips A. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology. 2009;202:521–530. doi: 10.1007/s00213-008-1342-1. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of Human Soluble and Membrane-Bound Catechol O-Methyltransferase: A Revised Mechanism and Description of the Thermolabile Variant of the Enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang D-R, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolanchana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences. 2003;100:6189–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Research. 2002;936:58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM. Microdialysis of Dopamine in the Nucleus Accumbens of Alcohol-Preferring (P) Rats During Anticipation and Operant Self-Administration of Ethanol. Alcoholism: Clinical and Experimental Research. 2002;26:318–325. [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li T-K. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior Genetics. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential Brain Activity in Alcoholics and Social Drinkers to Alcohol Cues: Relationship to Craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- NIAAA. Binge drinking defined. NIAAA Newsletters. 2004;3:3. [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacologic Dissociation Between Impulsivity and Alcohol Drinking in High Alcohol Preferring Mice. Alcoholism: Clinical and Experimental Research. 2010;34:1363–1375. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer Flavor Provokes Striatal Dopamine Release in Male Drinkers: Mediation by Family History of Alcoholism. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo A, Fontán A, Mena MA, Herranz A, Casado S, de Yébenes JG. Tolcapone increases plasma catecholamine levels in patients with Parkinson's disease. Parkinsonism & Related Disorders. 2001;7:93–96. doi: 10.1016/s1353-8020(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL. Dramatic Depletion of Mesolimbic Extracellular Dopamine after Withdrawal from Morphine, Alcohol or Cocaine: A Common Neurochemical Substrate for Drug Dependence. Annals of the New York Academy of Sciences. 1992;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Kreiner DS, Chapman MD, Stark-Wroblewski K. Virtual Reality Cues for Binge Drinking in College Students. CyberPsychology, Behavior & Social Networking. 2010;13:159–162. doi: 10.1089/cyber.2009.0211. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent Access to 20% Ethanol Induces High Ethanol Consumption in Long–Evans and Wistar Rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Progress in Neurobiology. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Swift R. Medications acting on the dopaminergic system in the treatment of alcoholic patients. Current Pharmaceutical Design. 2010;16:2136–2140. doi: 10.2174/138161210791516323. [DOI] [PubMed] [Google Scholar]
- Tammimäki A, Fosberg MM, Karayiorgou M, Gogos JA, Männistö PT. Increase in free choice oral ethanol self-administration in catechol-omethyltransferase gene-disrupted male mice. Basic Clin Pharmacol Toxicol. 2008;103:297–304. doi: 10.1111/j.1742-7843.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive Coding of Reward Value by Dopamine Neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-Methyltransferase Inhibition Improves Set-Shifting Performance and Elevates Stimulated Dopamine Release in the Rat Prefrontal Cortex. The Journal of Neuroscience. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Begleiter H, et al. High levels of dopamine d2 receptors in unaffected members of alcoholic families: Possible protective factors. Archives of General Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Wang G, Smith L, Volkow N, Telang F, Logan J, Tomasi D, Wong C, Hoffman W, Jayne M, Alia-Klein N. Decreased dopamine activity predicts relapse in methamphetamine abusers. Molecular Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine Dl receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, M B. Association between Val66Met brain-derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Buchel C. The genetic basis of individual differences in reward processing and the link to addictive behavior and social cognition. Neuroscience. 2009;164:55–71. doi: 10.1016/j.neuroscience.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-Specific Role of Catechol-O-Methyltransferase in Dopamine Overflow within Prefrontal Cortex and Dorsal Striatum. The Journal of Neuroscience. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.