Abstract
The Arizona Sky Island Arthropod Project (ASAP) is a new multi-disciplinary research program at the University of Arizona that combines systematics, biogeography, ecology, and population genetics to study origins and patterns of arthropod diversity along elevation gradients and among mountain ranges in the Madrean Sky Island Region. Arthropods represent taxonomically and ecologically diverse organisms that drive key ecosystem processes in this mountain archipelago. Using data from museum specimens and specimens we obtain during long-term collecting and monitoring programs, ASAP will document arthropod species across Arizona's Sky Islands to address a number of fundamental questions about arthropods of this region. Baseline data will be used to determine climatic boundaries for target species, which will then be integrated with climatological models to predict future changes in arthropod communities and distributions in the wake of rapid climate change. ASAP also makes use of the natural laboratory provided by the Sky Islands to investigate ecological and genetic factors that influence diversification and patterns of community assembly. Here, we introduce the project, outline overarching goals, and describe preliminary data from the first year of sampling ground-dwelling beetles and ants in the Santa Catalina Mountains.
Introduction
The outcome of millions of years of mountain building, the 7250 km long North American Cordillera or “Western Cordillera” runs from northern Alaska to southern Mexico. This great cordillera, the spine of the North American continent, has but one break, a low saddle between the Rocky Mountains/Colorado Plateau and the Sierra Madre Occidental, which forms a biogeographic barrier between the montane biotas of temperate and tropical North America (Heald 1951; Marshall 1957; McLaughlin 1986, 1995; Warshall 1995; Bowers and McLaughlin 1996). The Madrean Sky Islands are the isolated mountain ranges that span this Cordilleran Gap. Sometimes called the “Madrean Province” or “Madrean Archipelago,” these mountain ranges are a unique subset of the Basin and Range Province and cover about 168,400 sq. km. (∼77,700 sq. km. in the United States).
Although many of the plants and some of the animals of the Sky Island Region have been well studied, little is known about the arthropods (e.g., insects, isopods, millipedes, mites, spiders, scorpions, etc.). Yet, these ecologically diverse organisms drive key ecosystem processes such as pollination, litter decomposition, nutrient recycling, and soil aeration, and they are important food for reptiles, birds, and small mammals. Despite their ecological importance, arthropods are poorly known because most are small, live in opaque habitats where observation is difficult, and few taxonomists have specialized on these groups in the Sky Island Region (Behan-Pelletier and Newton 1999). Using data from museum specimens and specimens we obtain during long-term collecting and monitoring programs, ASAP will document arthropod species across Arizona's Sky Islands to address a number of fundamental questions about the arthropods of this region, including the following:
-
What arthropods inhabit Arizona's Sky Islands? How are these species distributed within the region?
One of the greatest resources for this project is the University of Arizona Insect Collection (UAIC), which contains over two million research specimens, 83% of which are identified to species level, mostly from the Sonoran Desert Region, its Sky Islands, and adjacent biomes of northwestern Mexico. In the last few decades, UAIC researchers have participated in long-term surveys in the Santa Catalina, Baboquivari, Mule, Huachuca, Chiricahua, and Waterman Mountains, and other montane areas; as well as in impact studies of the Mt. Graham telescope site in the Pinaleño Mountains and the Rosemont Mine site in the Santa Rita Mountains. Locality data from UAIC specimens will be incorporated into a specimen-level database for the ASAP Project; this database also will be networked with other arthropod museums in the southwestern United States.
Species diversity of the Sky Island Region is also being documented through a series of collecting expeditions. Throughout the project, we will conduct surveys of the arthropod fauna using a variety of collecting methods including light traps, malaise traps, pitfall traps, and hand collecting methods. All specimens from the ASAP project will be accessioned into the UAIC. They will be identified to species, and specimen-level data will be maintained in the UAIC specimen-level database. Representatives of many species will be preserved in a frozen tissue collection in -20 °C freezers and all other specimens collected will be mounted on points/pins and deposited in the UAIC pinned collection or preserved in 80% ethyl alcohol and archived for future work.
-
What are the biogeographic affiliations and phylogeographic relationships of the Sky Island arthropods? Have arthropods diversified or radiated within the Sky Island Region?
The distribution and/or elevation of Madrean Sky Island species has repeatedly shifted as a result of cyclical climate changes during the Quaternary (Martin 1963; Betancourt and others 1990; Van Devender and Spaulding 1990; Davis and Brown 1988; Van Devender 2002). Several studies have focused on the biogeography of species in the Arizona Sky Island Region including plants, arthropods, birds, lizards, and mammals (Brown 1971; Downie 2004; Linhart and Permoli 1994; McCord 1995; Sullivan 1994; Slentz and others 1999; Barber 1999a,b; Maddison and McMahon 2000; Masta 2000; Boyd 2002; Smith and Farrell 2005a,b; McCormack and others 2008; Tennessen and Zamudio 2008; Ober and others 2011). Most of these studies have documented significant morphological variation or genetic structure among separate subspecies or populations endemic to different mountain ranges within the Sky Island Region. However, none of these studies have used broad taxonomic sampling in the context of phylogeographic analyses to look at the geographic origin of the Sky Island fauna. In ASAP, we will infer the evolutionary relationships and estimate divergence times using molecular sequence data from fast-evolving mitochondrial and nuclear genes. From these data, we will reconstruct the evolutionary and biogeographic history of the Sky Island arthropod species, and look for emergent patterns that may reveal phylogeographic origins of the fauna and radiations of species groups among the mountain ranges.
-
What are the over-arching drivers that structure arthropod communities in the Sky Islands?
The spatial arrangement of similar, isolated habitats repeated on numerous mountain ranges in the Sky Island Region provides natural replication for ecological studies. We will utilize the natural laboratory provided by the Sky Islands to investigate ecological and genetic factors that influence diversification and patterns of community assembly. In particular we will address the following questions: (1) How are species distributed along elevational gradients? (2) Are the patterns correlated with plant communities/biomes, soil pH, precipitation, temperature, and/or humidity? (3) Do high elevation arthropod communities differ in species composition between isolated mountain ranges? (4) Do larger tracks of woodland or forest harbor a greater diversity of arthropods? To address these questions, ASAP makes the first effort to extensively and quantitatively sample the arthropod fauna for a broad swath of the Madrean Sky Islands. It will provide baseline data on the abundance, diversity, and community structure along elevation gradients throughout the region for this taxonomically and ecologically diverse group of organisms.
-
How might montane arthropod communities respond to rapidly changing climate conditions?
The Madrean Sky Islands provide a model system for investigating the effects of rapidly changing climate on the biodiversity of isolated landscapes. Sky Islands show a stronger effect of isolation than other types of isolated habitats (Watling and Donnelly 2006). Effects of climate change are already evident here (increased fire, drought, pest outbreaks, and invasive species). Additional temperature increases of as little as a few degrees could push Sky Island plant and arthropod species/communities to higher elevations, reducing their habitable area and potentially causing local extinctions of endemic taxa and evolutionarily unique lineages. Projections from global climate models suggest that temperatures in the Southwest will increase by 3-6 °C over the next century (Kupfer and others 2005; Gutzler and Robbins 2010; Overpeck and Udall 2010; Dominguez and others 2010; IPPC 2001, 2007; CLIMAS 2012). Predictions of whether precipitation will increase or decrease are still in conflict, and confidence in precipitation estimates is low at this time. Models used to examine how plant communities may respond to projected climate change indicate that increases in temperature will lead to upslope movement of communities, leading to an increased area of Desertscrub (western Sky Islands) or Desert Grassland (eastern Sky Islands) and a decrease in the area occupied by Mixed Conifer Forest, the biome situated at the top of the highest mountains (Kupfer and others 2005). However, increased precipitation might slow upslope movements by lessening growth constraints imposed by arid conditions. If climates continue to warm as projected, species will need to shift their present distributions or adapt to warmer conditions rapidly.
Anthropogenic activities are contributing to increased levels of atmospheric CO2 and these higher levels are leading to increased global temperatures and changes in the hydrologic cycle, thus causing or contributing to climate change (IPCC 2001,2007). Several reviews have highlighted that little is known about the current and predicted responses to climate change for most arthropod groups (Coviella and Trumble 1999; Hughes 2003). Because important ecosystem processes, such as litter decomposition and nutrient cycling, are driven by ground-dwelling arthropods (Powers and others 2009; Yang and Chen 2009), ecosystem responses and subsequent feedbacks to atmospheric and climate changes may depend on how soil arthropod communities respond to these perturbations (e.g., reduced decomposition rates will decrease feedbacks to global carbon (C) input, while increased decomposition rates will increase feedbacks to the C cycle; Bardgett and others 2008). As such, understanding the response of these species and communities to climate change is critical if we are to predict how such perturbations are going to alter both biodiversity and the functioning of ecosystems.
While natural communities may be able to adapt to changing climates to some extent, via shifts in the distribution of their local or geographic ranges, paleontological data suggest that communities do not respond to climate change as entities (Coope 1995; Jablonski and Sepkoski 1996). Rather, species responses tend to be idiosyncratic, resulting in the development of new assemblages and associations (Graham and Grimm 1990; Voight and others 2003). These results underscore the need for field-based inventories to better understand current diversity and distribution patterns, which in turn establishes a baseline for future comparisons and ecological studies to track how species and communities respond to future climate change in the Southwest.
We will use data acquired from our own collecting and from specimens housed in museum collections including the UAIC to determine climatic boundaries for target species; species-specific boundaries will then be integrated with climatological models to predict changes in arthropod communities and distributions in the wake of potential rapid climate change. To date, the scientific community knows almost nothing about how arthropod species and communities will respond to climate change. But because changes in both species composition and abundances of arthropods can influence important ecosystem processes, it is vital to understand how arthropods species and communities will respond to climate change and how arthropods influence ecosystem processes. Additionally, because climate change is predicted to lead to a widespread reorganization of species and community patterns, it is important to document the biogeography of Sky Island arthropod species now, to determine patterns of endemism and identify species at risk of extinction.
The ASAP project will monitor distributions of Sky Island arthropod species over the next 20-30 years to see if there are detectable shifts in species distributions as climate changes. We will use ecological niche models and phylogeographic analyses in combination to assess how arthropods have responded to past changes in climate by shifting distributions and/or adapting in situ, which in turn will help us predict how species will likely respond to increasing isolation and future climate change. Ultimately, we aim to identify species in the Sky Island Region that might be at risk of extirpation or extinction as the Southwest warms, and to identify locations of refugia from past glaciation events that may be areas of high allelic diversity that merit conservation attention. We further predict that effects of climate change may be seen in this region of the country and on these isolated mountains before most other areas, and that by studying the effects of climate change here we can make better predictions as to what will happen elsewhere later in time.
Purpose of This Paper
The purpose of this paper is to describe the study system of the ASAP, including our concept of the Sky Island Region and its biomes, and to present preliminary data derived from our collections in the Santa Catalina Mountains during the first year of the project. In initial phase of the project we are documenting the ground-dwelling arthropods of the Santa Catalinas, and the first year's sampling (2011) has been completed. The project will be expanded to other ranges in southern Arizona, and eventually to selected ranges in Mexico and to other arthropods (not just ground-dwelling taxa). Data collected in early years of the project will provide a baseline for future/continuing surveys designed to elucidate how populations, species, and communities change as climate changes in the Southwest.
Biogeographic Parameters of the Study
Boundaries and Concept of the Sky Island Region
When the term “Madrean Sky Islands” was first coined in the 1950s, it referred to those ranges possessing strong shared elements with the Sierra Madre Occidental (the “Madrean” flora). In fact, the term “Madrean Sky Islands” is not a perfect descriptor of the region, because many northern (e.g., Rocky Mountain or Petran) (Brown and others 1979; Brown 1994) species reach their southernmost range limits here, just as many southern (e.g., Madrean) species reach their northernmost range limits in this region. Although we are not suggesting a name change here, conceptually it might be more accurate to think of these mountain ranges collectively as “Cordilleran Gap Sky Islands” and for the ASAP project we broaden the definition of the Madrean Sky Islands to include all ∼65 of the isolated mountain ranges spanning the Cordilleran Gap (that are high enough to have Oak Woodlands) regardless of the degree of shared Madrean flora (fig. 1). We, therefore, include the northern Pinal and Superstition Mountains (Arizona) and the eastern Big and Little Hatchet and Alamo Hueco Mountains (New Mexico), which are traditionally not included on maps of the Madrean Sky Islands. We define the northern boundary of the Madrean Sky Island Region as the Salt River Valley; north of this lie the Mazatzal and Bradshaw Mountains, and other ranges of Arizona's Transition Zone that grade into the base of the escarpment of the Colorado Plateau's Mogollon Rim. The southernmost limit of the Sky Island Region is somewhat arbitrarily set at 29° N latitude (about the latitude of Hermosillo, Sonora). So, the northernmost Sky Islands are the Pinals and Superstitions; the westernmost Sky Islands are Sierra el Humo (in Sonora) and the Baboquivari Mountains (in Arizona); the easternmost are the Alamo Hueco and Big Hatchet Mountains of New Mexico; and the southernmost Sky Island is the Sierra Mazatán 70 km east of Hermosillo, Sonora.
Figure 1.
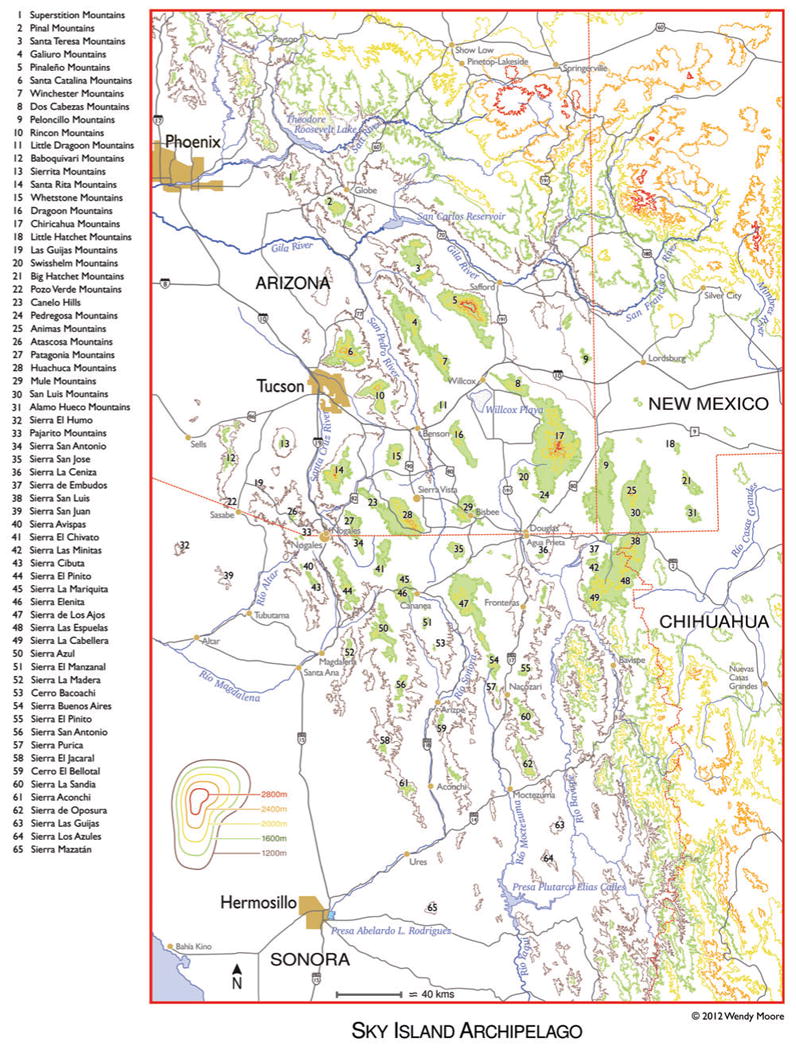
Elevation map of the Sky Island Region with 65 Sky Island mountains labeled. Green shading indicates approximate area above 1600 m (5250 ft), roughly the elevation at which oak woodlands first begin, although there is considerable variation in this due to latitude, slope and aspect, rainfall patterns, etc. (Map based on cartographic GIS research by Joel Viers/Lirica.)
Nearly all of the Madrean Sky Islands exceed 1525 m in elevation, or if falling short of that, they are connected by woodland habitat to an adjacent higher range and thus form part of a Sky Island complex. The Sierra el Humo reaches an elevation of 1650 m and has ∼1036 ha of Oak Woodland (Flesch and Hahn 2005). Two small ranges to the west of Sierra el Humo have oak patches, but they do not exceed 1370 m elevation and do not have sizeable Oak Woodlands (Sierra El Cobre, 1350 m; Sierra El Duranzo, 1210 m). The Sierra Mazatán reaches only 1545 m, but supports a large area of Oak Woodland (∼3626 ha, with five oak species) at its highest elevations, and it is surrounded by a “sea” of foothills thornscrub (Flesch and Hahn 2005; Dimmitt and others 2011).
The northernmost Sky Island ranges (the Pinal and Superstition Mountains) largely lack common Madrean species such as the silverleaf oak (Quercus hypoleucoides) that is so common in more southern ranges of Arizona. Instead, they harbor woodland of northern species such as Gambel oak (Quercus gambelii) the only winter-deciduous oak in the Sky Island Region, and Palmer oak (Quercus palmeri). The Pinals, which reach over 2380 m in elevation, support a large Petran Montane Pine Forest, surrounded by well-developed Oak Woodlands that connect to the woodlands of the Superstitions, the latter having only a few patches of ponderosa pine (the highest peak in the Superstitions is 1900 m).
In addition to these 65 ranges (fig. 1), several other small ranges in Mexico are apparently high enough to support Oak Woodlands, notably a few east of the Sierra Mazatán. However, plant data for these ranges are too sparse to draw firm conclusions at this time. We do not consider ranges with only scattered oak patches but lacking true Oak Woodland— such as Arizona's Tucson Mountains (Wasson Peak, 1429 m)— to be Sky Islands. Similarly, many of the small mountains in Sonora, west of the Sierra Madre Occidental, are high enough to support scattered patches of oaks, especially on their north-facing slopes (e.g., Sierra Cucurpe), but in general these are not large or contiguous encinals (Oak Woodlands). To the east, we include all of the ranges of appropriate elevation and isolation west of the Continental Divide, plus three east of the divide (Big and Little Hatchets and Alamo Hueco in New Mexico). Some authors have included the far eastern Cedar Mountains of New Mexico and others have included the Sierra San Javier, 130 km east-southeast of Hermosillo. Although included on some Sky Island maps, we do not include Sonora's huge Sierra el Tigre mountain complex (i.e., Sierras El Tigre, San Diego, el Oso, and los Pilares de Teras) because the woodlands of those ranges connect to the Sierra Madre Occidental south of Huachinera (via the Mesa Los Tabachines, Sierra El Gato, and Sierra Los Tules), which connect to the Sierras at elevations over 1525 m, effectively making the Sierra El Tigre complex a “woodlands peninsula” of the Sierra Madre Occidental. This said, it is known that these woodland connections have been greatly diminished by drought, fires, and tree cutting and in the near future this “peninsula” may become an “island” as it is surrounded on the other three sides by the Río Bavispe. Nevertheless, for historical biogeographic purposes, the El Tigre mountain complex is part of the Sierra Madre. South of the huge Sierra el Tigre complex in Sonora, thornscrub surrounds most of the ranges south to the Sierra de Mazatán.
The “Apachian Floristic District” (of McLaughlin 1995) and the “Apachian Subprovince” (of the larger Madrean Floristic Province) largely coincide with our definitions of the Madrean Sky Island Region. The 12.146-million ha “Apache Highlands Ecoregion” (of The Nature Conservancy) somewhat exceeds our view of the Sky Island Region in that it extends northwest through the Verde River and Big Chino Valleys of central Arizona to the Mogollon Rim, and south to include the northernmost region of the Sierra Madre Occidental.
Mountain Islands vs. Habitat Islands
A mountain range in this region is traditionally defined as a “Sky Island” if it is high enough to include Oak Woodland habitat and is not connected by Oak Woodlands to the Cordilleran ranges of the Rocky Mountains/Colorado Plateau or the Sierra Madre Occidental (Heald 1951; Dimmitt and others 2011). Using this definition, we recognize ∼65 mountain ranges that should be designated as Sky Islands, 32 of which are in the United States (table 1).
Table 1.
Alphabetical list of the 32 Madrean Sky Islands in the United States, with elevations of highest peaks.
| Mountain Range (State) | Elevation (ft) |
Elevation (m) |
|---|---|---|
| Alamo Hueco Mountains (NM) | 6159 | 1877 |
| Animas Mountains (NM) | 8642 | 2634 |
| Atascosa Mountains (AZ) | 6422 | 1957 |
| Baboquivari Mountains (AZ) | 7734 | 2357 |
| Big Hatchet Mountains (NM) | 8366 | 2550 |
| Canelo Hills (AZ) | 5861 | 1786 |
| Chiricahua Mountains (AZ) | 9763 | 2976 |
| Dos Cabezas Mountains (AZ) | 8354 | 2546 |
| Dragoon Mountains (AZ) | 7523 | 2293 |
| Caliuro Mountains (AZ) | 7663 | 2336 |
| Huachuca Mountains (AZ) | 9466 | 2885 |
| Las Guijas Mountains (AZ) | 4665 | 1422 |
| Little Dragoon Mountains (AZ) | 6732 | 2052 |
| Little Hatchet Mountains (NM) | 6247 | 1904 |
| Mule Mountains (AZ) | 7360 | 2243 |
| Pajarito Mountains (AZ) | 5460 | 1664 |
| Patagonia Mountains (AZ) | 7221 | 2201 |
| Pedregosa Mountains (AZ) | 6540 | 1993 |
| Peloncillo Mountains (AZ, NM) | 6931 | 2113 |
| Pinal Mountains (AZ) | 7848 | 2392 |
| Pinaleño Mountains (AZ) | 10724 | 3269 |
| Pozo Verde Mountains (AZ) | 4701 | 1433 |
| Rincon Mountains (AZ) | 8664 | 2641 |
| San Luis Mountains (NM, CHI) | 8268 | 2520 |
| Santa Catalina Mountains (AZ) | 9157 | 2791 |
| Santa Rita Mountains (AZ) | 9456 | 2882 |
| Santa Teresa Mountains (AZ) | 8282 | 2524 |
| Sierrita Mountains (AZ) | 6190 | 1887 |
| Superstition Mountains (AZ) | 6266 | 1900 |
| Swisshelm Mountains (AZ) | 7185 | 2190 |
| Whetstone Mountains (AZ) | 7711 | 2350 |
| Winchester Mountains (AZ) | 6921 | 2110 |
In reality, 18 of the 32 named mountain ranges north of the border are connected to at least one other range by contiguous Oak Woodland and thus these 32 ranges can be classified into 21 distinct Sky Island complexes (table 2). Many of the Sonoran Sky Islands are connected to one another by Oak Woodland also, and, in the future, with improved information these could be combined into mountain complexes as we've done in table 2. The 21 U.S. mountain complexes that are connected by Oak Woodland can be thought of, ecologically and biogeographically as 21 “Oak Woodland islands” or “habitat islands.” Many of these mountain ranges are high enough to also harbor higher elevation biomes (e.g., Pine-Oak Woodlands, Chaparral, Pine Forest). However, to date, we have not determined how many contiguous vs. isolated landscapes of these higher elevation biomes exist. These concepts have considerable relevance for the biogeography of arthropods that inhabit these montane biomes.
Table 2.
The twenty-one Oak Woodland “habitat islands” (or Madrean Sky Island complexes) in the United States.
| Mountain ranges connected by contiguous Oak Woodland habitat | |
|---|---|
| 1. | Superstition and Pinal Mountains |
| 2. | Santa Teresa Mountains |
| 3. | Pinaleño Mountains |
| 4. | Santa Cataiina Mountains |
| 5. | Rincon Mountains |
| 6. | Sierrita Mountains |
| 7. | Baboquivari and Pozo Verde Mountains |
| 8. | Santa Rita Mountains |
| 9. | Galiuro and Winchester Mountains |
| 10. | Dos Cabezas, Chiricahua, Pedrogosa, and Swisshelm Mountains |
| 11. | Little Dragoon Mountains |
| 12. | Dragoon Mountains |
| 13. | Mule Mountains |
| 14. | Whetstone Mountains |
| 15. | Peloncillo Mountains (AZ and NM) |
| 16. | Atascosa, Pajarito, Las Guijas Mountains (and Sierras Las Avispas and Cíbuta in Sonora) |
| 17. | Huachuca and Patagonia Mountains and Canelo Hills (and Sierra San Antonio in Sonora) |
| 18. | Animas Mountains (NM) |
| 19. | Big Hatchet and Little Hatchel Mountains (NM) |
| 20. | Alamo Hueco Mountains (NM) |
| 21. | San Luis Mountains (NM) (and Sierras San Luis, Las Espuelas, La Caballena, Embudos, and Minitas, in Sonora and Chihuahua) |
In addition, some Sky Island mountain ranges actually comprise two or more subranges, even though maps and common usage typically use the name of only the largest of these. For example, the Baboquivari Mountains actually comprise four ranges—the Coyote, Quinlan, Pozo Verde, and Baboquivari Mountains—separated from one another by distinct valleys. But, popular usage refers to them collectively as the Baboquivari Mountains. This situation is especially troublesome in Mexico where we have tried to determine the most commonly used name for such ranges. Many Mexican ranges also have more than one name due to local usage, etc., and we have relied on the INEGI (Instituto Nacional de Estadística y Geografía) 1:250,000 maps of Sonora and Chihuahua for these names. We used USGS and INEGI information, and a Garmin 62S GPS device, for estimating elevation data.
Biomes of Arizona's Sky Islands
Ecologists generally agree that there are about a dozen major plant community types that occur worldwide, e.g., tundra/alpine, temperate broadleaf forests, Mediterranean woodlands/shrublands, tropical rain forests, coniferous forests, temperate and tropical Grasslands, temperate and tropical savannahs, tropical dry forests, Chaparral, savannahs, and deserts. Examples of all but the first four are found in the Madrean Sky Island Region. We refer to these as “biomes” in this study.
Here, we follow the Whittaker and Niering (1968b), Whittaker and others (1968), and Niering and Lowe (1985) studies of the vegetation of the Santa Catalina Mountains in recognizing the following biomes for the Sky Islands of Arizona: Desertscrub, Desert Grassland, grazing disturbed Grassland, Oak-Grassland, Oak Woodland, Pine-Oak Woodland, Chaparral, Pine Forest, and Mixed Conifer Forest. Whittaker and Niering (1965, 1968) listed the signature species for each of these biomes in their now classic diagrams depicting the biomes of the Santa Catalina Mountains. Not all of the Madrean Sky Islands are high enough to have all of these biomes, and many lack Pine Forest and/or Mixed Conifer Forest, especially in Mexico. However, all eight of these biomes occur in the Santa Catalina Mountains.
The elevational ordering of these biomes, and the strong Madrean component of most of the Sky Islands, were first formally described by Forrest Shreve early in the twentieth century (Shreve 1915, 1919, 1922, 1936, 1951). Joseph Marshall's 1957 study of the birds of Pine-Oak Woodlands of the border region also described the stacking of biotic communities on each island mountain from the Mogollon Rim to the Sierra Madre, and it was Marshall who defined the “Madrean Archipelago” as those mountains with Mexican Pine-Oak Woodlands in the Cordilleran Gap. In 1951, Weldon Heald, studying and living in the Chiricahua Mountains, coined the evocative and descriptive phrase “Sky Islands” for these ranges. Also in the 1960s, the pioneering ecological studies of Robert Whittaker, William Niering, and Charles Lowe described the botany of these ranges in growing detail. Comprehensive descriptions of Arizona's Sky Island biomes can also be found in the forthcoming book A Natural History of the Santa Catalina Mountains, Arizona, with an Introduction to the Madrean Sky Islands (Moore and Brusca, in press, Arizona-Sonora Desert Museum Press).
ASAP Surveys in the Santa Catalina Mountains
Catalinas Elevational Gradients
Situated 140 km north of the United States-Mexico border, the Santa Catalina Mountains are one of the best-known Sky Islands. To assess the diversity and distribution of ground-dwelling arthropods along elevation and environmental gradients, 66 sampling sites were identified in recognizable biomes along the elevation gradients of the southern and northern sides of the Santa Catalina Mountains, along the Mt. Lemmon Highway (south side), the Control Road (north side), and on Mt. Lemmon and Mt. Bigelow (fig. 2). These same elevation gradients along the same roads were studied botanically by Lowe (1961), Whittaker and Niering (1964, 1965, 1968a,b, 1975), Niering and Lowe (1985), and Whittaker and others (1968). Whittaker and others (1968) established 30 0.1-ha quadrats (20×50 m) for their plant censuses. Our study used 66 belt transects, each 0.02 ha in size (2×100 m) for species-X-abundance plant censuses. Neither the study by Whittaker and others (1968) nor our initial study examined riparian or “wet canyon” sites; i.e., all transect sites established during the first year of the project are upland sites. In total we established 28 sites each on the southern and northern slopes of the Catalina Mountains and 10 mixed conifer sites on Mt. Lemmon and Mt. Bigelow.
Figure 2.
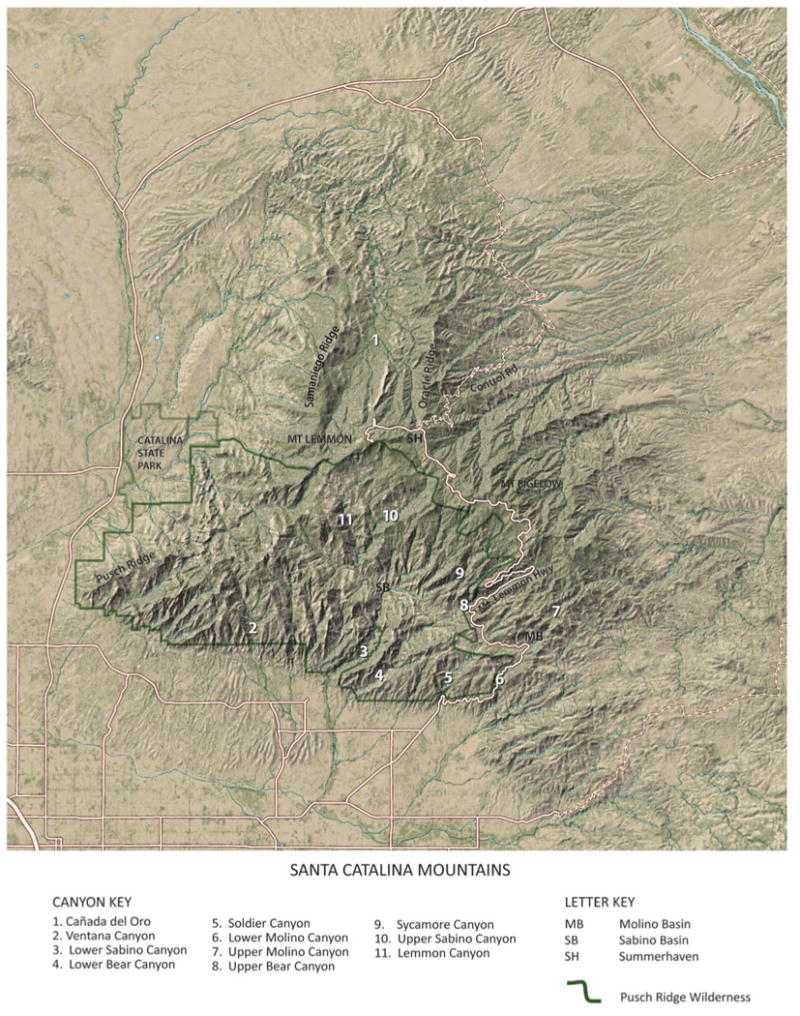
The Santa Catalina Mountains from space showing location of major topographic features as well as the Mt. Lemmon Highway and the Control Road.
While past workers have been in agreement about the over-arching sense of plant species turnover and plant community change with elevation, they have agreed only partly in where to draw lines separating these biomes, and what to call them. Forrest Shreve, in his benchmark 1915 paper, separated the slopes of the Catalina Mountains into three broad zones based on the biogeographic roots of the predominant plants: Desertscrub, “encinal” (dominated by Madrean species), and “forest” (dominated by Rocky Mountain/Petran species). Shreve's idea was compelling, but it does not work well for all the other Sky Islands, especially those on the fringes of the Sky Island region whose plant community affinities are not so clear-cut. It also largely ignored the grasses and the importance of Grassland habitat on the Sky Islands. In a detailed series of papers by Whittaker, Niering, and Lowe, Shreve's ideas were refined into something very close to what we use in this paper. Our scheme is a slight alteration of the scheme presented in Niering and Lowe's 1985 publication, and it is based on vegetation analyses of 66 transect sites in the Catalina Mountains established by the ASAP Project (table 3, fig. 2).
Table 3. Summary of the 66 Catalina transects.
| Biome type a | Eleavation and Aspect b | Transect Coordinates c | Comments [transect field code identifier] |
|---|---|---|---|
| Desertscrub (S) | 1045m/3428ft S (170°) | 32.30978 N, 110.73497 W | Classic Arizona Upland (Soldier Canyon Trail) [CAT-DS-S-01] |
| Desertscrub (S) | 1133m/3717ft S (180°) | 32.31060 N, 110.71922 W | Arizona Upland with some grassland species (Babad Do'ag Trail). Large patches of Lehmann lovegrass in area. [CAT-DS-S-03] |
| Desertscrub (S) | 1159m/3802ft SE (144°) | 32.31163 N, 110.71733 W | Arizona Upland with some grassland species (Babad Do'ag Trail). Large patches of Lehmann lovegrass in area. [CAT-DS-S-04] |
| Desertscrub (S) | 1172m/3845ft NE to S (65° to 170°) | 32.31366 N, 110.71221 W | Arizona Upland with some grassland species (east of Babad Do'ag Trail). Transect slope faces toward Molino Canyon. [CAT-DS-S-05] |
| Desertscrub (S) | 1160m/3806ft S (175°) | 32.31263 N, 110.71693 W | Arizona Upland with some grassland species (Babad Do'ag Trail). Large patches of Lehmann lovegrass in area. [CAT-DS-S-06] |
| Oak-Grassland (S) | 1384m/4541ft NNW (340°) | 32.33543 N, 110.7044 W | Along Arizona Trail, near Molino Basin Campground area. Some oaks in this area burned in 2003 Aspen Fire (regrowing from root crowns). [CAT-GL-S-01] |
| Oak-Grassland (S) | 1400m/4593ft E (90°) | 32.33604 N, 110.70673 W | Along Arizona Trail, near Molino Basin Campground area. Some oaks in this area burned in 2003 Aspen Fire (regrowing from root crowns). [CAT-GL-S-02] |
| Oak-Grassland (S) | 1492m/4895ft | 32.33690 N, 110.7180 W | Near Gordon Hirabayashi Picnic area. [CAT-GL-S-03] |
| Oak-Grassland (S) | 1477m/4845ft SW (245°) | 32.33751 N, 110.72071 W | Near Gordon Hirabayashi Picnic area. Some oaks in this area burned in 2003 Aspen Fire (regrowing from root crowns). [CAT-GL-S-04] |
| Oak-Grassland (S) | 1513m/4964ft NE (25°) | 32.34452 N, 110.71972 W | Near Bug Spring Trailhead. Quite a few chaparral plants at this site; some oaks in this area burned in 2003 Aspen Fire (regrowing from root crowns). [CAT-GL-S-05] |
| Oak-Grassland (S) | 1533m/5030ft NW (315°) | 32.34448 N, 110.71769 W | Near Bug Spring Trailhead. Quite a few chaparral plants at this site; some oaks in this area burned in 2003 Aspen Fire (regrowing from root crowns). [CAT-GL-S-06] |
| Pine-Oak Woodland (S) | 1803m/5915ft N (360°) | 32.37395 N, 110.69254 W | Near Middle Bear Canyon Picnic Area, [CAT-PO-S-01] |
| Pine-Oak Woodland (S) | 2122m/6962ft | 32.38335 N, 110.69482 W | [CAT-PO-S-02] |
| Pine-Oak Woodland (S) | 2183m/7162ft | 32.39205 N, 110.69728 W | Below Rose Canyon Rd. parking area. [CAT-PO-S-03] |
| Pine-Oak Woodland (S) | 2266m/7434ft SW (205°) | 32.40078 N, 110.70033 W | [CAT-PO-S-04] |
| Pine-Oak Woodland (S) | 2422m/7946ft SE (150°) | 32.40951 N, 110.71229 W | Below Visitor's Center. This high Pine-Oak site is transitional to Pine Forest. [CAT-PO-S-05] |
| Pine-Oak Woodland (S) | 2209m/7247ft | 32.4004 N, 110.6909 W | Near San Pedro Vista area, [CAT-PO-S-06] |
| Pine-Oak Woodland (S) | 2030m/6660ft NNW (345°) | 32.37546 N, 110.70695 W | Near Hoo Doo Vista area. [CAT-PO-S-O7] |
| Chaparral (S) | 2052m/6732ft SSE (163°) | 32.36873 N, 110.71519 W | Above Windy Point Vista. Some oaks in this area burned in 2003 Aspen Fire (regrowing from root crowns). [CAT-CH-S-01] |
| Chaparral (S) | 1923m/6309ft S (180°) | 32.37292 N, 110.70142 W | Near Manzanita Vista area. [CAT-CH-S-02] |
| Pine Forest (S) | 2224m/7297ft WNW (285°) | 32.39534 N, 110.69073 W | Near Rose Canyon Rd. parking area. [CAT-P-S-01] |
| Pine Forest (S) | 2386m/7829ft NE (30°) | 32.42924 N, 110.74430 W | Near Sunset Trail parking area. [CAT-P-S-03] |
| Pine Forest (S) | 2324m/7625ft E (100°) | 32.42886 N, 110.75649 W | Marshall Gulch Picnic Area. [CAT-P-S-04] |
| Pine Forest (S) | 2296m/7534ft NE (50°) | 32.42772 N, 110.75578 W | Marshall Gulch Picnic Area. [CAT-P-S-05] |
| Pine Forest (S) | 2447m/8028ft WNW (300°) | 32.42004 N, 110.73919 W | Near Box Camp parking area. [CAT-P-S-06] |
| Pine Forest (S) | 2401m/7877ft WSW (250°) | 32.42699 N, 110.73949 W | Near Butterfly Trailhead. [CAT-P-S-07] |
| Pine Forest (S) | 2394m/7854ft SSW (200°) | 32.43175 N, 110.75110 W | Near Sykes Knob-Inspiration Point. [CAT-P-C-02] |
| Pine Forest (S) | 2463m/8081ft SSW (200°) | 32.41498 N, 110.73312 W | 1 mi. below Box Camp parking area. [CAT-P-C-03] |
| Mixed Conifer Forest (MTN) | 2442m/8012ft WSW (200°) | 32.42227 N, 110.73134W | Bear Wallow area. [CAT-MC-S-01] |
| Mixed Conifer Forest (MTN) | 2452m/8045ft E (90°) | 32.41866 N, 110.73264 W | Bear Wallow area. [CAT-MC-S-02] |
| Mixed Conifer Forest (MTN) | 2514m/8248ft W (270°) | 32.41514 N, 110.72688 W | Near observatory on Mt. Bigelow. [CAT-MC-S-03] |
| Mixed Conifer Forest (MTN) | 2551m/8369ft NE (38°) | 32.41360 N, 110.72114 W | Near large Aspen grove, Mt. Bigelow. [CAT-MC-S-04] |
| Mixed Conifer Forest (MTN) | 2530m/8301ft NE (50°) | 32.41807 N, 110.72465 W | Near observatory on Mt. Bigelow. [CAT-MC-S-05] |
| Mixed Conifer Forest (MTN) | 2777m/9111 ft N (360°) | 32.44134 N, 110.78623 W | Sky Center parking lot area, Mt. Lemmon. [CAT-MC-02] |
| Mixed Conifer Forest (MTN) | 2691m/8829ft N (355°) | 32.44480 N, 110.78666 W | Near top of ski run area (“Heidi's Meadow”), Mt. Lemmon. [CAT-MC-03] |
| Mixed Conifer Forest (MTN) | 2587m/8488ft WNW (285°) | 32.45196 N, 110.78396 W | Ski slope area, Mt. Lemmon. [CAT-MC-04] |
| Mixed Conifer Forest (MTN) | 2541m/8337ft NE (35°) | 32.44643 N, 110.77675 W | Along Aspen Draw Trail, Mt. Lemmon. [CAT-MC-05) |
| Mixed Conifer Forest (MTN) | 2564m/8412ft NE (35°) | 32.44588 N, 110.77711 W | Along Aspen Draw Trail, Mt. Lemmon. [CAT-MC-06) |
| Grazing-Disturbed Grassland (N) | 1363m/4472ft NE (65°) | 32.5805 N, 110.7214 W | At Arizona Trail crossing of Control Rd., near old ranch house. [CAT-DS-N-03] |
| Grazing-Disturbed Grassland (N) | 1401m/4597ft W (265°) | 32.57300 N, 110.70277 W | At Arizona Trail crossing of Control Rd., next to old ranch house. [CAT-DS-N-04] |
| Grazing-Disturbed Grassland (N) | 1337m/4386ft SE (130°) | 32.5692 N, 110.7119 W | Near Sombrero Viejo Rd. (5 mi. from start of Control Rd. at Oracle). [CAT-DS-N-05] |
| Grazing-Disturbed Grassland (N) | 1341m/4340ft SW (220°) | 32.56757 N, 110.71040 W | Near Sombrero Viejo Rd. (5 mi. from start of Control Rd. at Oracle). [CAT-DS-N-06] |
| Grazing-Disturbed Grassland (N) | 1451m/4760ft NNW (340°) | 32.54188 N, 110.7139 W | Near Peppersauce Canyon Trail crossing (8 mi. from start of Control Rd. at Oracle). Limestone outcrop with many ocotillo. [CAT-DS-N-07] |
| Grazing-Disturbed Grassland (N) | 1440m/4624ft NW (315°) | 32.54196 N, 110.71013 W | Near Peppersauce Canyon Trail crossing, but not on limestone outcropping.[CAT-DS-N-08] |
| Grazing-Disturbed Grassland (N) | 1380m/4528ft NNW (345°) | 32.51539 N, 110.68622 W | [CAT-GL-N-06] |
| Desert Grassland (N) | 1488m/4882ft SE (135°) | 32.53323 N, 110.71042 W | 90% grass ground cover; some livestock disturbance evident. [CAT-GL-N-01] |
| Desert Grassland (N) | 1330m/4364ft NNE (20°) | 32.49794 N, 110.68372 W | 40% grass ground cover; on limestone outcrop. [CAT-GL-N-02] |
| Desert Grassland (N) | 1492m/4895ft SE (145°) | 32.48673 N, 110.70566 W | 90% grass ground cover, but mostly Lehmann's lovegrass. [CAT-GL-N-03] |
| Desert Grassland (N) | 1504m/4934ft NE (40°) | 32.47964 N, 110.71066 W | 70% ground cover, almost entirely grass, [CAT-GL-N-04] |
| Desert Grassland (N) | 1645m/5397ft | 32.47811 N, 110.72174 W | [CAT-GL-N-05] |
| Chaparral (N) | 1845m/6053ft W (260°) | 32.46355 N, 110.73198 W | 50% ground cover, mostly grasses. [CAT-CH-N-01] |
| Chaparral (N) | 1865m/6119ft SE (120°) | 32.46395 N, 110.73276 W | 65% ground cover, mostly grasses. [CAT-CH-N-02] |
| Chaparral (N) | 1925m/6316ft NE (35°) | 32.46138 N, 110.73971 W | [CAT-CH-N-05] |
| Chaparral (N) | 1962m/6437ft E (90°) | 32.45885 N, 110.74123 W | [CAT-CH-N-06] |
| Chaparral (N) | 1971m/6467ft NE (50°) | 32.45707 N, 110.73774 W | [CAT-CH-N-07] |
| Oak Woodland (N) | 1939m/6362ft SE (150°) | 32.46448 N, 110.74242 W | [CAT-OW-N-01] |
| Oak Woodland (N) | 2000m/6562ft ESE (109°) | 32.45633 N, 110.73994 W | 55% ground cover, much of it bullgrass (Muhlenbergia emersleyi). [CAT-OW-N-02] |
| Pine-Oak Woodland (N) | 2032m/6667ft NE (40°) | 32.45313 N, 110.73906 W | 30-40% ground cover, much of it bullgrass (Muhlenbergia emersleyi). [CAT-PO-N-01] |
| Pine-Oak Woodland (N) | 2080m/6824ft S (170°) | 32.44946 N, 110.73728 W | [CAT-PO-N-02] |
| Pine-Oak Woodland (N) | 2142m/7028ft N (360°) | 32.45270 N, 110.74248 W | Thick Fendler buckbrush; evidence of pine beetles. [CAT-PO-N-03] |
| Pine-Oak Woodland (N) | 2149m/7051ft NE (65°) | 32.45264 N, 110.74379 W | Thick Fendler buckbrush. [CAT-PO-N-04] |
| Pine-Oak Woodland (N) | 2313m/7589ft SSE (165°) | 32.45171 N, 110.75072 W | This is a Pine-Oak/Pine Forest transitional site. ∼50% live plant ground cover. [CAT-P-N-05] |
| Pine Forest (N) | 2218m/7277ft S (175°) | 32.44029 N, 110.74741 W | ∼5% live plant ground cover. [CAT-P-N-01] |
| Pine Forest (N) | 2228m/7310ft E (80°) | 32.45215 N, 110.74773 W | ∼5% live plant ground cover. [CAT-P-N-02] |
| Pine Forest (N) | 2280m/7480ft SE (130°) | 32.45063 N, 110.75037 W | ∼5% live plant ground cover. [CAT-P-N-03] |
| Pine Forest (N) | 2305m/7562ft NE (45°) | 32.44910 N, 110.75069 W | ∼5% live plant ground cover. [CAT-P-N-04] |
N = Northern slope transects (along Control Road); S = Southern slope transects (along Mt. Lemmon Highway); MTN = High elevation transects, on Mt. Lemmon and Mt. Bigelow.
Aspect is that of prevailing slope of transect (with compass heading).
Starting location of transect
In each biome, 100-m long transects were placed farther than 0.25 km from the road, to minimize possible road effects. On the south side of the range we established five transects in Desertscrub (at elevations of 1045–1172 m), six in Oak-Grassland (at elevations of 1384–1433 m), seven in Pine-Oak Woodland (at elevations of 1803–2422 m), two in Chaparral (at elevations of 1923–2052 m), and eight in Pine Forest (at elevations of 2224–2463 m). On the north side of the range we established seven transects in disturbed Desert Grassland (historically grazed areas at elevations of 1323–1451 m), six in relatively undisturbed Desert Grassland (at elevations of 1330–1645 m), two in Oak Woodland (at elevations of 1939–2000 m), five in Pine-Oak Woodland (2032–2149 m), five inChaparral (at elevations of 1845–1971 m), and four in Pine Forest (at elevations of 2218–2305 m). Ten transects in mixed conifer habitat were established in the Mt. Lemmon and Mt. Bigelow areas (at elevations of 2442–2777 m).
The southern slope elevation gradient along the Mt. Lemmon Highway does not have good Oak Woodland or Grassland; all grass habitats on the south side were deemed to be Oak-Grassland, a fact also observed by Whittaker and Niering (1965), although good Desert Grassland can be found by hiking west from Molino Basin a few miles on the Arizona Trail. The northern slope elevation gradient along the Control Road has no Desertscrub, it begins near the town of Oracle at 1220 m in elevation, a fact observed by Forrest Shreve and all subsequent works since the 1920s. However, the northern slope does have highly disturbed, overgrazed Grassland that has converted to scrubland (which we sampled) that resembles Desertscrub as overgrazed Grassland does throughout southern Arizona. The reduction of grasses by livestock and fire suppression, and the subsequent invasion by woody and shrubby desert plants in the Southwest, has been known since Aldo Leopold (1924) and a large literature exists on this subject. The north side also had no Oak-Grassland; it did, however, have relatively undisturbed Desert Grassland and some patches of Oak Woodland. Of course, it has long been recognized that these biomes, or plant communities, intergrade continuously (Whittaker and others 1968; Brown and Lowe 1980), and local variations in vegetation are due to gradients of slope, soil type, slope aspect, etc. This was a major focus of Whittaker and his colleagues' work in the Sky Islands for many years. A detailed series of papers (Whittaker and Niering 1964, 1965, 1968a,b, 1975; Whittaker and others 1968) explored relationships between plant species and a variety of environmental factors. It is not our goal to repeat those analyses but rather to accurately describe and classify the botany of our arthropod transect sites.
Transect sites for our 2011 fieldwork in the Catalina Mountains were categorized according to six environmental variables: elevation, slope, aspect, biome, as well as ground temperature and humidity. A Log-Tag HAXO-8 temperature and humidity recorder was placed 2 cm above the soil/litter in the center of each of the 66 transects. Log-Tags were placed in the field on May 6, 2011 and they recorded data every 30 min. Average temperature data for each site along the elevation gradients from May 7, 2011, through September 14, 2011, is plotted according to elevation and biome (fig. 3). Although we did not statistically analyze the temperature data, it is apparent that north- and south-side temperatures (per elevation) were not markedly different, and this is probably partly due to the fact that this was the hottest time of the year, although differences in slope aspect and other factors probably also contributed to evening out these averages. Nonetheless, the overall negative correlation between temperature vs. elevation and biome is clear.
Figure 3.
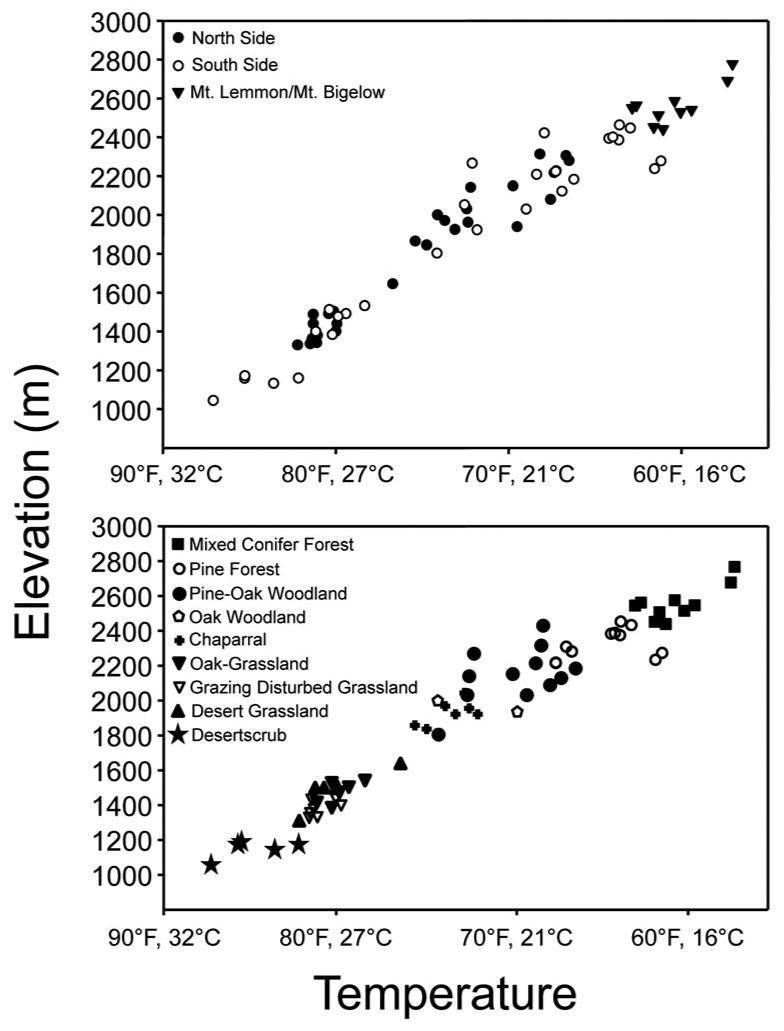
Average summer temperatures collected 7-May-2011 to 14-Sept-2011 along the transect elevation gradients in the Santa Catalina Mountains. Top: A comparison of average temperatures at sites on the South and North sides of the mountain, as well as those found at the highest elevations on Mt. Lemmon and Mt. Bigelow. Bottom: A comparison of average temperatures at sites in different plant biomes.
Ground Dwelling Arthropod Surveys
We set 10 pitfall traps arranged 10 m apart along each 100-m transect line. Pitfall trap design was adopted from Higgins (2010). Each trap consists of a heavy Pyrex glass “test tube” (3.2 cm diameter, 25 cm deep) inserted in a PVC sleeve (3.8 cm in diameter, 28 cm long) that had been buried in the ground. The opening of the trap is flush with the soil surface, and the glass sampling tubes can be exchanged while leaving the PVC sleeve in place. Traps were charged with propylene glycol (50% full). A PVC rain shield covers the opening of the trap, 3–4 cm above the ground. When the glass tubes are not in place, the PVC sleeves are capped to prevent them from filling with dirt or inadvertently capturing any animals.
In 2011 we sampled for 2 weeks in the spring (pre-monsoon, May 1–15) and 2 weeks in the late summer (post-monsoon, September 1–15). This project represents the first effort to extensively and quantitatively sample the arthropod fauna of any of the Sky Islands of the Madrean Archipelago and, as such, will provide baseline data on abundance, diversity, and community structure of this exceptionally diverse group for future studies.
Here, we present preliminary species lists of the ants and carabid beetles based on our first round of pitfall-trap sampling. These data were supplemented from specimen-level data in the University of Arizona Insect Collection to develop working lists of the known species from the Santa Catalina Mountains. We expect these species lists to grow with future collecting and monitoring efforts.
Ants
The ants, family Formicidae, are possibly the numerically dominant family of insects. Ants represent 10–15% of the entire animal biomass in terrestrial ecosystems (Hölldobler and Wilson 1990). Ants have played a significant role in the evolution of modern terrestrial biotic communities for at least the last 40–50 million years. More species of ants occur in Arizona than in any other U.S. State (Johnson 1996). While there have been no extensive surveys of the ant fauna of the Santa Catalina Mountains, we expect their diversity to be similar to that of other Sky Islands of comparable area and elevation range. The most well-known Sky Island ant fauna is that of the Chiricahua Mountains where 187 species have been documented, representing 59% of the total known ant fauna of Arizona (Stephan Cover, personal communication). To date, in the Santa Catalinas, 88 species have been identified through our pitfall traps and the University of Arizona Insect Collection (table 4).
Table 4.
Ant species known from the Santa Catalina Mountains.
| Dolichoderinae |
| Dorymyrmex bicolor Wheeler, 1906 |
| Forelius mccooki (McCook, 1880) |
| Forelius pruinosus (Roger, 1863) |
| Liometopum apiculatum Mayr, 1870 |
| Liometopum luctuosum Wheeler, 1905 |
| Tapinoma sessile (Say, 1836) |
| Ecitoninae |
| Neivamyrmex andrei (Emery, 1901) |
| Neivamyrmex harrisii (Haldeman, 1852) |
| Neivamyrmex nigrescens (Cresson, 1872) |
| Neivamyrmex opacithorax (Emery, 1894) |
| Neivamyrmex texanus Watkins, 1972 |
| Formicinae |
| Brachymyrmex depilis Emery, 1893 |
| Camponotus festinatus (Buckley, 1866) |
| Camponotus fragilis Pergande, 1893 |
| Camponotus laevigatus (F.Smith, 1858) |
| Camponotus modoc Wheeler, 1910 |
| Camponotus pudorosus Emery, 1925 |
| Camponotus sansabeanus (Buckley, 1866) |
| Camponotus schaefferi Wheeler, 1909 |
| Camponotus vicinus Mayr, 1870 |
| Formica aserva Forel, 1901 |
| Formica densiventris Viereck, 1903 |
| Formica fusca Linnaeus, 1758 |
| Formica gnava Buckley, 1866 |
| Formica neogagates Viereck, 1903 |
| Formica moki Wheeler, 1906 |
| Formica occulta Francoeur, 1973 |
| Formica wheeleri Creighton, 1935 |
| Lasius alienus (Foerster, 1850) |
| Lasius colei (Wing, 1968) |
| Lasius flavus (Fabricus, 1781) |
| Lasius occidentalis (Wheeler, 1909) |
| Lasius pallitarsus (Provancher, 1881) |
| Lasius sitiens Wilson, 1955 |
| Lasius subumbratus (Viereck, 1903) |
| Myrmecocystus flaviceps Wheeler, 1912 |
| Myrmecocystus mendax Wheeler, 1908 |
| Myrmecocystus mexicanus Wesmael, 1838 |
| Myrmecocystus mimicus Wheeler, 1908 |
| Myrmecocystus navajo Wheeler, 1908 |
| Polyergus breviceps Emery, 1893 |
| Prenolepis imparis (Say, 1836) |
| Myrmicinae |
| Aphaenogaster albisetosa Mayr, 1886 |
| Aphaenogaster huachucana Creighton, 1934 |
| Aphaenogaster texana Wheeler, 1915 |
| Cephalotes rohweri Wheeler, 1916 |
| Crematogaster browni Buren, 1968 |
| Crematogaster depilis Wheeler, 1919 |
| Crematogaster emeryana Creighton, 1950 |
| Crematogaster lineolata (Say, 1836) |
| Crematogaster opuntiae Buren, 1968 |
| Crematogaster vermiculata Emery, 1895 |
| Messor pergandei (Mayr, 1886) |
| Myrmica tahoensis Weber, 1948 |
| Myrmica wheeleri Weber, 1939 |
| Pheidole cerebrosior Wheeler, 1915 |
| Pheidole ceres Wheeler, 1904 |
| Pheidole desertorum Wheeler, 1906 |
| Pheidole diversipilosa Wheeler, 1908 |
| Pheidole hyatti Emery, 1895 |
| Pheidole obtusospinosa Pergande, 1896 |
| Pheidole rhea Wheeler, 1908 |
| Pheidole sciopbila Wheeler, 1908 |
| Pheidole spadonia Wheeler, 1915 |
| Pheidole titanis Wheeler, 1903 |
| Pheidole xerophila Wheeler, 1908 |
| Pogonomyrmex barbatus (F.Smith, 1858) |
| Pogonomyrmex californicus (Buckley, 1867) |
| Pogonomyrmex desertorum Wheeler, 1902 |
| Pogonomyrmex imberbiculus Wheeler, 1902 |
| Pogonomyrmex maricopa Wheeler, 1914 |
| Pogonomyrmex occidentalis (Cresson, 1865) |
| Solenopsis aurea Wheeler, 1906 |
| Solenopsis xyloni McCook, 1879 |
| Stenamma californicum Snelling, 1973 |
| Stenamma chiricahua Snelling, 1973 |
| Stenamma snellingi Bolton, 1957 |
| Temnothorax andrei (Emery, 1895) |
| Temnothorax neomexicanus (Wheeler, 1903) |
| Temnothorax nitens (Emery, 1895) |
| Temnothorax rugatulus (Emery, 1895) |
| Temnothorax whitfordi (Mackay, 2000) |
| Tetramorium hispidum (Wheeler, 1915) |
| Tetramorium spinosum (Pergande, 1896) |
| Trachymyrmex arizonensis (Wheeler, 1907) |
| Ponerinae |
| Odontomachus clarus Roger, 1861 |
| Pseudomyrmecinae |
| Pseudomyrmex apache Creighton, 1953 |
| Pseudomyrmex pallidus (F.Smith, 1855) |
Ground Beetles
The ground beetles, family Carabidae, comprise one of the largest beetle families with at least 40,000 described species worldwide. Most ground beetles represent apex predators of most soil arthropod communities and, thus, play an important ecological role in almost every terrestrial habitat. Because of this, they have been important subjects in ecological and climate change studies such as the long-term climate change study presently being conducted by the National Ecological Observatory Network (NEON). There are over 2500 described species of Carabidae known from North America. Based on the holdings of the UAIC, over 300 species occur in the Sky Island Region of Arizona. Transect samples and UAIC collection records document 69 species of Carabidae occurring in the Santa Catalina Mountains (table 5).
Table 5.
Carabid beetle species known from the Santa Catalina Mountains.
| Anthiinae: Helluonini |
| Helluomorphoides latitarsis (Casey, 1913) |
| Brachininae: Brachinini |
| Brachinus costipennis Motschulsky, 1859 |
| Brachinus elongatulus Chaudoir, 1876 |
| Brachinus favicollis Erwin, 1965 |
| Brachinus gebhardis Erwin, 1965 |
| Brachinus hirsulus Bates, 1884 |
| Brachinus lateralis Dejean, 1831 |
| Brachinus mexicanus Dejean, 1831 |
| Carabinae: Carabini |
| Calosoma peregrinator Guérin-Méneville, 1844 |
| Calosoma prominens LeConte, 1853 |
| Carabinae: Cychrini |
| Scaphinotus petersi catalinae Van Dyke, 1924 |
| Cicindelinae: Cicindelini |
| Amblycheila baroni Rivers, 1890 |
| Cicindela lemniscata LeConte, 1854 |
| Cicindela ocellata Klug, 1834 |
| Cicindela sedecimpunctata Klug, 1834 |
| Dryptinae: Galeritini |
| Galerita atripes LeConte, 1858 |
| Galerita mexicana Chaudoir, 1872 |
| Harpalinae: Anisodactylini |
| Anisodactylus anthracinus (Dejean, 1829) |
| Notiobia mexicana (Dejean, 1829) |
| Harpalinae: Harpalini |
| Discoderus congruens Casey, 1914 |
| Discoderus pinguis Casey, 1884 |
| Harpalus fraternus LeConte, 1852 |
| Harpalus laevipes Zetterstedt, 1828 |
| Selenophorus otiosus Casey, 1914 |
| Harpalinae: Stenolophini |
| Bradycellus rupestris (Say, 1823) |
| Lebiinae: Cyclosomini |
| Tetragonoderus fasciatus (Haldeman, 1843) |
| Lebiinae: Lebiini |
| Apristus tuckeri Casey, 1920 |
| Cymindis arizonensis Schaeffer, 1910 |
| Cymindis punctiger LeConte, 1851 |
| Lebia abdita Madge, 1967 |
| Lebia histrionica Bates, 1883 |
| Lebia pimalis (Casey, 1920) |
| Lebia pulchella Dejean, 1826 |
| Lebia tuckeri (Casey, 1920) |
| Lebia viridis Say, 1823 |
| Lebiinae: Odacanthini |
| Lachnophorus elegantulus Mannerheim, 1843 |
| Licininae: Chlaeniini |
| Chlaenius cumatilis LeConte, 1851 |
| Chlaenius leucoscelis monachus LeConte, 1851 |
| Chlaenius obsoletus LeConte, 1851 |
| Chlaenius ruficauda Chaudoir, 1856 |
| Nebriinae: Notiophilini |
| Notiophilus semiopacus Eschscholtz, 1833 |
| Paussinae: Ozaenini |
| Goniotropis kuntzeni Banninger, 1927 |
| Platyninae: Platynini |
| Colpodes longiceps (Schaeffer, 1910) |
| Platynus brunneomarginatus (Mannerheim, 1843) |
| Platynus cohni Liebherr and Will, 1996 |
| Platynus ovatulus (Bates, 1884) |
| Platynus lyratus (Chaudoir, 1878) |
| Platynus megalops (Bates, 1882) |
| Platynus rufiventris (Van Dyke, 1926) |
| Rhadine anthicoides Casey, 1913 |
| Sericoda bembidioides Kirby, 1837 |
| Synuchus dubius (LeConte, 1854) |
| Platyninae: Sphordrini |
| Calathus gregarius (Say, 1823) |
| Calathus peropacus Casey, 1920 |
| Pterostichinae: Pterostichini |
| Pterostichus adstrictus Eschscholtz, 1823 |
| Pterostichinae: Zabrini |
| Amara californica Dejean, 1828 |
| Scaritinae: Clivinini |
| Clivina ferrea LeConte, 1857 |
| Schizogenius falli Whitehead, 1972 |
| Schizogenius pygmaeus Van Dyke, 1925 |
| Scaritinae: Scaritini |
| Pasimachus californicus Chaudoir, 1850 |
| Pasimachus viridans LeConte, 1858 |
| Trechinae: Bembidiini |
| Bembidion flavopictum Casey, 1918 |
| Bembidion mexicanum Dejean, 1831 |
| Bembidion rapidum (LeConte, 1848) |
| Bembidion striola LeConte, 1852 |
| Bembidion subangustatum Hayward, 1897 |
| Elaphropus nebulosus (Chaudoir, 1868) |
| Pericompsus laetulus LeConte, 1852 |
| Tachyta nana inornata (Say, 1823) |
Initial analyses of patterns of species distribution of carabids and ants find patterns for the fauna of the Santa Catalina Mountains similar to those Halffter (1987) recognized in the mountains of Mexico. Species with temperate ancestral distributions are generally found at higher elevations (Pine Forest and Mixed Conifer Forest), while Neotropical species are generally found at lower elevations (Desertscrub, Desert Grassland, and Oak Woodland). This pattern is also similar to those reported in the studies of Ball (1968), Liebherr (1994), and Marshall and Liebherr (2000) for montane carabids in Mexico. For example, in the Catalinas, the temperate genus Scaphinotus is represented by one species, Scaphinotus petersi catalinae Van Dyke, which is restricted to Pine Forest and Mixed Conifer Forest and the tropical genus Goniotropis, represented by Goniotropis kuntzeni Bänninger, which is restricted to Oak-Grassland. The Madrean Archipelago and the neighboring northern Sierra Madre mountains are the only areas in the world where members of these two genera, with such disparate ancestral distributions, can be found within the same mountain range.
Plant Surveys
Plant surveys were conducted August 5-14, 2011. Surveys were made for each transect site in two ways. First, the transect line was walked and every plant recorded within one meter on either side of the line. This produced a 200-m2 belt transect record of plant species and abundance. Then, the transect was walked again, this time scanning the broader area outside the 200-m2 area, noting the presence but not abundances of any plants that might not have been in the belt transect itself. For practical reasons, Rocky Mountain ponderosa pine and Arizona pine were counted together, and no attempt was made to distinguish between these two species. In the Catalina Mountains, the Coronado National Forest manages these as a “single species,” and past workers have considered Arizona pine to be a variety of ponderosa (P. ponderosa var. arizonica), although this is not current opinion.
Surveys of plants in the 66 transect sites in the Catalina Mountains recorded a total of 316 species: 24 trees, 30 woody shrubs, 23 stem and rosette succulents (including one hybrid agave), 38 grasses, and 201 small shrubs, herbs and annuals. Data are summarized in table 6, and a complete list of plants-by-biome is given in table 7. For each biome, there are two species lists, one of “common species” that occurred in two or more transects and the other of “uncommon species” that occurred in only one transect. The complete plant database is available from W. Moore. Whittaker and Niering's (1964) benchmark study of the southern slopes of the Catalina Mountains listed 700 plant species (those above 2743 m being from the southern slopes of the Pinaleño Mountains), but they did not report these plant occurrences relative to specific biomes as we do in this preliminary study. Instead, they reported them relative to a large matrix of environmental variables and growth forms. Although we do not use that approach here (we are interested in documenting plant species associated with our arthropod transect sites), the ASAP project is collecting detailed environmental data and may undertake such an analysis in the future as relevant to arthropod species and communities. Also, none of the publications by Whittaker, Niering, or Lowe analyzed plant occurrences statistically in the Catalina Mountains relative to elevation or biome.
Table 6.
Biomes sampled in the Santa Catalinas, with numbers of plant species recorded from transects in each.
| Biome a | Common species b | Uncommon species c | Total species | Elevation range sampled |
|---|---|---|---|---|
| Desertscrub (S) 5 transect sites |
44 | 30 | 74 | 1045-1172 m (3428-3845 ft) |
| Desert Grassland (N) 5 transect sites |
61 | 70 | 131 | 1330-1645 m (4364-5397 ft) |
| Grazing-Disturbed Grassland (N) 7 transect sites |
51 | 48 | 99 | 1323-1451 m (4340-4760 ft) |
| Oak-Grassland (S) 6 transect sites |
52 | 36 | 88 | 1384-1433 m (4541-5030 ft) |
| Oak Woodland (N) 2 transect sites |
9 | 21 | 30 | 1939-2000 m (6362-6562 ft) |
| Chaparral (N) 5 transect sites |
32 | 22 | 54 | 1845-1971 m (6053-6467 ft) |
| Chaparral (S) 2 transect sites |
10 | 17 | 27 | 1923-2052 m (6309-6732 ft) |
| Pine-Oak Woodland (N) 5 transect sites |
29 | 17 | 46 | 2032-2149 m (6667-7051 ft) |
| Pine-Oak Woodland (S) 7 transect sites |
26 | 24 | 50 | 1803-2422 m (5915-7946 ft) |
| Pine Forest (N) 4 transect sites |
10 | 13 | 23 | 2218-2305 m (7277-7562 ft) |
| Pine Forest (S) 8 transect sites |
25 | 20 | 45 | 2224-2463 m (7297-8081 ft) |
| Mixed Conifer Forest (MTN) 10 transect sites |
24 | 19 | 43 | 2442-2777 m (8012-9111 ft) |
N = northern slopes of range (along Control Road). S = southern slopes of range (along Mt. Lemmon Highway). MTN = Mt. Lemmon and Mt. Bigelow sites.
“Common Species” are species found in two or more transects in the given biome.
“Uncommon Species” are species recorded from only a single transect in the given biome.
Table 7.
List of plant species found in each biome for Catalinas Transects 2011 arthropod survey. DS = Desertscrub. DG = Desert Grassland. DDG = Grazing Disturbed Desert Grassland. OG = Oak Grassland. OW=Oak Woodland. C = Chaparral. PO = Pine-Oak Woodland. P = Pine Forest. MC = Mixed Conifer Forest. (S) = South side transects. (N) = North side transects. (MTN) = Mt. Lemmon and Mt. Bigelow transects.
| DS (S) | DG (N) | DDG (N) | OG (S) | OW (N) | C (N) | C (S) | PO (N) | PO (S) | P (N) | P (S) | MC (MTN) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abies bifolia var. arizonica | corkbark fir | C | |||||||||||
| Abies concolor | white fir | C | C | ||||||||||
| Abutilon coahuilae | caliche Indian mallow | U | C | ||||||||||
| Abutilon incanum | Indian mallow | U | |||||||||||
| Acacia angustissima | fern acacia | U | U | C | |||||||||
| Acacia constricta | whitethorn acacia | C | C | U | |||||||||
| Acacia greggii | catclaw acacia | C | U | C | |||||||||
| Acalypha neomexicana | New Mexico copperleaf | C | U | C | U | ||||||||
| Acer glabrum | Rocky Mountain maple | U | |||||||||||
| Acer grandidentatum | bigtooth maple | C | |||||||||||
| Achillea millefolium | common yarrow | U | U | ||||||||||
| Acourtia wrightii | brownfoot | ||||||||||||
| Adenophyllum porophylloides | San Felipe fetid marigold | U | |||||||||||
| Agave chrysantha | golden-flower agave | U | C | C | C | C | C | ||||||
| Agave schottii | shindagger agave | C | C | ||||||||||
| Allionia incarnata | desert windmills | C | C | U | |||||||||
| Alnus oblongifolia | alder | U | |||||||||||
| Aloysia wrightii | lemon verbena | C | C | C | |||||||||
| Amaranthus nr. palmeri | amaranth | C | U | C | U | ||||||||
| Amaranthus fimbriatus | fringed amaranth | U | |||||||||||
| Ambrosia ambrosioides | canyon ragweed | C | |||||||||||
| Ambrosia confertiflora | slimleaf ragweed | C | C | C | |||||||||
| Amorpha fruticosa | false indigobush | U | |||||||||||
| Andropogon sp. | Poaceae | U | U | ||||||||||
| Anoda cristata | spurred anoda | U | |||||||||||
| Aquilegia chrysantha | golden columbine | U | C | ||||||||||
| Arbutus arizonica | Arizona madrone | C | U | U | |||||||||
| Arceuthobium vaginatum | pineland dwarf mistletoe | U | C | U | |||||||||
| Arctostaphylos pungens | pointleaf manzanita | C | U | C | U | C | C | C | |||||
| Arenaria lanuginosa ssp, saxosa | sandwort | U | C | ||||||||||
| Argemone pleiacantha | prickly poppy | U | U | ||||||||||
| Aristida adscencionis | six-weeks three awn | U | U | ||||||||||
| Aristida purpurea | purple three-awn | C | C | U | U | ||||||||
| Aristida ternipes var. ternipes | spidergrass | C | C | C | C | C | C | ||||||
| Aristolochia watsonii | southwestern pipevine | C | |||||||||||
| Artemisia ludoviciana | white sagebrush | U | U | U | |||||||||
| Artemisia sp. | wormwood; Asteraceae | U | |||||||||||
| Asclepias asperula/engelmanniana | milkweed | ||||||||||||
| Asclepias hypoleuca | mahogany milkweed | C | U | ||||||||||
| Astragalus sp. | locoweed; Fabaceae | U | |||||||||||
| Ayenia compacta | ayenia | C | |||||||||||
| Baccharis sarothroides | desert broom | C | C | ||||||||||
| Baccharis sp. | broom; Asteraceae | C | |||||||||||
| Bahia absinthifolia | hairy bahia | C | C | U | |||||||||
| Boerhavia sp. | spiderling; Nyctaginacea | U | C | C | C | ||||||||
| Bommeria hispida | copper fern | U | |||||||||||
| Bothriochloa barbinodis | cane beardgrass | C | U | C | |||||||||
| Bouteloua barbata var. rothrockii | Rothrock grama | U | |||||||||||
| Bouteloua chondrosioides | sprucetop grama | U | U | ||||||||||
| Bouteloua curtipenduta | side-oats grama | C | C | C | C | C | |||||||
| Bouteloua gracilis | blue grama | U | |||||||||||
| Bouteloua hirsuta | hairy grama | ||||||||||||
| Bouteloua repens | slender grama | U | |||||||||||
| Bouvardia ternifolia | scarlet houvardia | C | U | ||||||||||
| Brickellia baccbaridea | resin-leaf brickellbush | U | |||||||||||
| Brickellia californica | California brickellbush | C | C | C | U | U | |||||||
| Brickellia nr, diffusa | brickellbush | C | |||||||||||
| Brickellia coulteri | brickellbush | C | |||||||||||
| Brickellia sp. | brickellbush | C | U | C | |||||||||
| Bromus rubens | red brome | C | |||||||||||
| Bromus sp. | brome; Poaceae | U | C | ||||||||||
| Calliandra eriophylla | fairy duster | C | C | C | |||||||||
| Carnegiea gigantea | saguaro | C | |||||||||||
| Caryopbyllaceae | U | ||||||||||||
| Castelleja chromosa | Indian paintbrush | U | U | ||||||||||
| Ceanothus fendleri | Fendler buckbrush | C | C | U | C | C | C | C | |||||
| Celtis pallida | desert hackberry | C | |||||||||||
| Cercocarpus montanus | mountain mahogany | U | C | ||||||||||
| Chamaecrista nictitans | partridge pea | C | C | ||||||||||
| Chamaesyce arizonica | Arizona sandmat | U | |||||||||||
| Chamaesyce sp. C. revoluta, or C. gracillima | sandmat: Euphorbiaceae | U | |||||||||||
| Cheilanthes nr. yavapense | graceful lipfern | C | U | U | C | U | |||||||
| Cheilanthes lindheimeri | Lindheimer lipfern, fairy swords | C | |||||||||||
| Cheilanthes wrightii | Wright's lipfern | U | |||||||||||
| Cheilanthes yavapense | graceful lipfern | ||||||||||||
| Chenopodium sp. | Amaranthaceae | U | |||||||||||
| Cirsium arizonicum | Arizona thistle | U | U | U | |||||||||
| Cirsium neomexicanum | New Mexico thistle | U | C | U | |||||||||
| Cirsium sp. | thistle; Asteraceae | C | |||||||||||
| Cologania angustifolia | longleaf colognia | U | |||||||||||
| Commelina erecta | whitemouth dayflower | U | C | U | U | ||||||||
| Commicarpus scandens | bush spiderling | U | U | U | |||||||||
| Composite with licorice smell | Asteraceae | U | U | ||||||||||
| Composite with Mentzelia – shaped leaves | Asteraceae | U | U | ||||||||||
| Composite with white rays | Asteraceae | U | |||||||||||
| Condalia warnockii var. kearneyana | condalia | U | |||||||||||
| Conyza sp. | Asteraceae | U | |||||||||||
| Coryphantha vivipara | beehive cactus | C | C | ||||||||||
| Cupressus arizonica | Arizona cypress | U | U | ||||||||||
| Cuscuta indecora | dodder | U | |||||||||||
| Cuscuta sp. | dodder; Convolvulaceae | U | |||||||||||
| Cylindropuntia bigelovii | teddybear cholla | U | |||||||||||
| Cylindropuntia fulgida | jumping cholla | C | |||||||||||
| Cylindropuntia leptocaulis | desert Christmas cholla | U | U | ||||||||||
| Cylindropuntia spinosior | cane cholla | ||||||||||||
| Cylindropuntia versicolor | staghorn cholla | C | U | ||||||||||
| Cyperus sp. | Cyperaceae | U | U | U | C | C | U | ||||||
| Dalea albiflora | whiteflower prairie clover | U | C | C | |||||||||
| Dalea formosa | feather plume | U | |||||||||||
| Dalea pulchra | pea bush, Santa Catalina prairie clover, indigo bush | C | |||||||||||
| Dalea sp. | upright herb; Fabaceae | U | |||||||||||
| Dasylirion wheeleri | sotol, desert spoon | C | C | C | C | ||||||||
| Dasyochloa pulchella | fluff grass | U | |||||||||||
| Datura wrightii | sacred datura | U | |||||||||||
| Desmanthus nr. cooleyi | bundleflower | U | |||||||||||
| Desmodium batocaulon | bushy tick clover | C | C | U | C | ||||||||
| Desmodium procumbens | trailing lick clover | C | |||||||||||
| Dicliptera resupinata | Arizona foldwing | U | C | ||||||||||
| Digitaria californica | Arizona cottontop | U | U | ||||||||||
| Echeandia flavescens | Torrey's craglily | U | |||||||||||
| Echinocereus bonkerae | Bonker hedgehog cactus | ||||||||||||
| Echinocereus coccineus | claret-cup hedgehog cactus | U | U | C | |||||||||
| Echinocereus fasciculatus | robust hedgehog cactus | C | C | U | |||||||||
| Elymus elymoides | squirreltail | U | C | ||||||||||
| Encelia farinosa | brittlebush | C | |||||||||||
| Enneapogon mollis | soft feather pappus grass | U | |||||||||||
| Eragrostis cilianensis | stinkgrass | U | |||||||||||
| Eragrostis curvula | weeping lovegrass | U | U | ||||||||||
| Eragrostis intermedia | plains lovegrass | U | C | U | |||||||||
| Eragrostis lehmanniana | Lehmann lovegrass | C | C | C | C | C | |||||||
| Ericameria laricifolia | turpentine bush | U | C | C | U | ||||||||
| Erigeron arizonicus | Arizona fleabane | U | C | ||||||||||
| Erigeron divergens | fleabane | U | U | U | |||||||||
| Erigeron sp. | upright form; Asteraceae | C | |||||||||||
| Eriogonum nr. abertianum | Abert's buckwheat | U | U | U | U | ||||||||
| Eriogonum wrightii | buckwheat | U | C | C | C | ||||||||
| Erythrina flabelliformis | coral bean | C | |||||||||||
| Euphorbia florida | spurge | C | |||||||||||
| Euphorbia heterophylla | Mexican fireplant | C | U | C | |||||||||
| Euphorbia hyssopifolia | spurge | C | C | C | |||||||||
| Euphorbia melanadenia | spurge | C | |||||||||||
| Euphorbia polycarpa | spurge | C | |||||||||||
| Evolvulus alsinoides | Arizona blue eyes | C | C | U | |||||||||
| Fern; unknown genus | Pteridaceae | U | |||||||||||
| Ferocactus wislizeni | fishhook barrel cactus | C | C | C | U | ||||||||
| Fouquieria splendens | ocotillo | C | C | C | |||||||||
| Fragaria sp. | wild strawberry; Rosaceae | C | U | ||||||||||
| Frangula californica | California buckthorn, coffeeberry | C | |||||||||||
| Funastrum cynanchoides | =Sarcostemma cynanchoides; milkweed vine | U | |||||||||||
| Galactia wrightii | rock bean, Wright's milkpea | U | C | ||||||||||
| Galium aparine | bedstraw | U | U | ||||||||||
| Galium fendleri | bedstraw | C | C | C | |||||||||
| Galium sp. | bedstraw; Rubiaceae | U | C | U | U | ||||||||
| Garrya wrightii | silktassle bush | C | U | C | U | C | |||||||
| Geranium caespitosum | pineywoods geranium | U | C | C | |||||||||
| Glandularia bipinnatifida | small-flowered verbena, Dakota mock vervain | U | C | ||||||||||
| Gomphrena caespitosa | tufted globe amaranth | C | |||||||||||
| Goodyera oblongifolia | rattlesnake plantain | U | U | U | |||||||||
| Guardiola platyphylla | Apache plant | U | |||||||||||
| Gutierrezia sp. | snakeweed; Asteraceae | U | U | ||||||||||
| Gymnosperma glutinosum | gumhead | U | U | ||||||||||
| Haplophyton cimicidum var. crooksii | cockroach plant | U | |||||||||||
| Hedeoma nr, hyssopifolia | mock pennyroyal | U | U | U | U | C | U | U | |||||
| Helianthella quinquenervis | nodding dwarf sunflower | U | U | C | U | ||||||||
| Helianthus sp. | wild sunflower; Asteraceae | U | |||||||||||
| Heliomeris longifolia ssp. annua | longleaf false goldeneye | U | C | ||||||||||
| Heliopsis annua | C | U | C | ||||||||||
| Hesperidanthus linearifolius | slimleaf plainsmustard | U | C | ||||||||||
| Heteropogon contortus | tanglehead | C | U | C | |||||||||
| Heterotheca psamophila | camphorweed | C | |||||||||||
| Heuchera sanguinea | coralbells | U | |||||||||||
| Hibiscus coulteri | Coulter hibiscus | C | |||||||||||
| Hilaria belangeri | curly mesquite grass | U | C | ||||||||||
| Hymenopappus filifolius | fineleaf woolly white | C | U | ||||||||||
| Hymenopappus sp. | Asteraceae | U | |||||||||||
| Hymenothrix wislizeni | Trans-Pecos thimblehead | U | |||||||||||
| Ipomoea barbatisepala | canyon morning glory | U | U | ||||||||||
| Ipomoea cristulata | scarlet creeper | C | C | ||||||||||
| Ipomoea leptotoma | triple-leaf morning glory | U | |||||||||||
| Ipomoea sp. | morning glory vine; Convolvulaceae | C | C | C | U | ||||||||
| Isocoma tenuisecta | burroweed | U | C | U | |||||||||
| jamesia americana | cliff bush | C | |||||||||||
| janusia gracilis | desert vine | C | |||||||||||
| jatropha cardiophylla | limberbush | C | |||||||||||
| juniperus arizonica | red-berry juniper | U | |||||||||||
| juniperus deppeana var. deppeana | alligator juniper | U | U | C | C | C | C | U | |||||
| justicia longii | white needle flower | C | |||||||||||
| Kallstroemia californica | caltrop | C | |||||||||||
| Kallstroemia californica or parviflora | |||||||||||||
| Kallstroemia grandiflora | Arizona poppy | U | U | ||||||||||
| Koeleria macrantha | junegrass | U | |||||||||||
| Krameria erecta | range ratany | U | U | ||||||||||
| Krameria lanceolata | trailing ratany | C | |||||||||||
| Laennecia schiedeana | Asteraceae | C | C | ||||||||||
| Lathyrus sp. | pea; Fabaceae | C | C | ||||||||||
| Lepidium sp. | Brassicaceae | U | |||||||||||
| Leptochloa dubia | green sprangletop | ||||||||||||
| Leptochloa fiscal ssp. uninervia | |||||||||||||
| Linum neomexicanum | New Mexico yellow flax | U | C | U | |||||||||
| Lotus sp. | fine-leaf form; Fabaceae | U | U | C | U | U | |||||||
| Lotus greenei | Greene's lotus | C | C | C | C | ||||||||
| Lotus sp. | Fabaceae | C | U | C | C | C | |||||||
| Lupinus sp. | lupine; Fabaceae | U | |||||||||||
| Lycium berlandieri | wolfberry | C | |||||||||||
| Lycium fremontii | woIfberry | C | |||||||||||
| Lycium pallidum | woIfberry | U | U | ||||||||||
| Lycium sp. | woIfberry; Solanaceae | U | U | ||||||||||
| Machaeranthera tortifolia | Asteraceae | C | |||||||||||
| Maianthemum racemosum | false Solomon's seal | U | |||||||||||
| Malaxis tenuis | adder's tongue | C | C | ||||||||||
| Mammillaria grahamii | fishhook pincushion cactus | C | |||||||||||
| Mammillaria heyderi | cream cactus, pincushion cactus | U | |||||||||||
| Marina nr. diffusa | spreading false prairie clover | C | C | C | C | ||||||||
| Marrubium vulgare | horehound | U | |||||||||||
| Menodora scabra | twinfruit | U | |||||||||||
| Mentzelia sp. | Loasaceae | U | |||||||||||
| Mimosa aculeaticarpa | wait-a-minute bush | C | C | C | C | U | U | ||||||
| Mirabilis laevis | desert four o'clock | C | |||||||||||
| Mirabilis sp. | Nyctaginaceae | U | C | C | |||||||||
| Mirabilis sp. Oxybaphus-type | Nyctaginaceae | U | |||||||||||
| Monarda sp. | Lamiaceae | U | |||||||||||
| Monotropa hypopitys | pinesap | U | |||||||||||
| Muhlenbergia alopecuroides | wolftail | U | U | C | |||||||||
| Muhlenbergia emersleyi | bullgrass | C | C | C | C | C | C | C | C | U | |||
| Muhlenbergia fragilis | delicate muhly | U | |||||||||||
| Muhlenbergia porteri | bush muhly | C | C | ||||||||||
| Muhlenbergia sp. 1 | Poaceae | U | U | ||||||||||
| Muhlenbergia sp. 2 | Poaceae | U | |||||||||||
| Nolina microcarpa | beargrass | U | U | C | U | C | C | U | |||||
| Oenothera sp. | evening primrose; Onagraceae | U | |||||||||||
| Opuntia engelmannii | Engelmann prickly pear cactus | C | C | C | C | ||||||||
| Opuntia engelmannii X O. phaeacantha | hybrid | U | |||||||||||
| Opuntia phaeacantha var. major | sprawling prickly pear cactus | C | C | U | |||||||||
| Oxalis sp. | Oxalidaceae | U | |||||||||||
| Packera neomexicana | New Mexico groundsel | U | C | C | U | C | U | ||||||
| Panicum hirticaule | Mexican panic grass | C | U | C | |||||||||
| Parkinsonia florida | blue palo verde | C | |||||||||||
| Parkinsonia microphylla | foothills palo verde | C | |||||||||||
| Pellaea sp. | Pteridaceae | U | C | U | |||||||||
| Penstemon barbatus | scarlet bugler | C | C | C | |||||||||
| Penstemon eatonii | firecracker penstemon | U | |||||||||||
| Penstemon pseudospectabilis | desert penstemon | C | |||||||||||
| Phaseolus maculatus ssp. ritensis | spotted bean | U | |||||||||||
| Phaseolus parvulus | wild bean | U | C | C | |||||||||
| Phaseolus sp. | bean; Fabaceae | C | U | ||||||||||
| Phemeranthus aurantiacus | flame flower | U | C | C | U | ||||||||
| Phoradendron californicum | desert mistletoe | U | |||||||||||
| Physalis crassifolia | ground cherry | U | |||||||||||
| Physalis hederifolia vat. fendleri | Fendler's ground cherry | U | C | U | |||||||||
| Physalis sp. | tiny flowers; Solanaceae | U | |||||||||||
| Pinus chihuahuana | Chihuahua pine | C | C | C | |||||||||
| Pinus discolor | = Pinus cembroides var. bicolor; border pinyon pine | C | U | ||||||||||
| Pinus ponderosa var. scopulorum/P. arizonica | Rocky Mountain ponderosa pine/Arizona Pine | C | C | C | C | C | |||||||
| Pinus strobiformis | southwestern white pine | U | C | C | C | C | |||||||
| Polemoniaceae | phlox family | U | U | ||||||||||
| Polygala sp. | milkwort; Polygalaceae | U | U | ||||||||||
| Populus tremuloides | quaking aspen | U | C | ||||||||||
| Porophyllum gracile | odora, yerba de venado | U | |||||||||||
| Porophyllum ruderale | yerba porosa | C | U | C | |||||||||
| Portulaca oleracea | common purslane | U | U | C | C | ||||||||
| Portulaca pilosa | kiss me quick | U | C | C | C | ||||||||
| Portulaca umbraticola | Chinese hat | C | U | C | |||||||||
| Potentilla thurberi | scarlet cinquefoil | U | |||||||||||
| Prosopis velutina | =Prosopis juliflora var. velutina; velvet mesquite | ||||||||||||
| Prunus virginiana | chokecherry | U | U | ||||||||||
| Pseudocymopterus montanus | mountain parsley | C | C | ||||||||||
| Pseudognaphalium sp. | cudweed; Asteraceae | C | C | C | C | C | U | U | |||||
| Pseudotsuga menziesii var. glauca | Rocky Mountain Douglas-fir | C | C | C | C | C | |||||||
| Psilostrophe cooperi | whitestem paperflower | U | |||||||||||
| Pteridium aquilinum | bracken fern | U | U | C | C | ||||||||
| Quercus arizonica | Arizona white oak | C | U | C | C | C | C | C | |||||
| Quercus emoryi | Emory oak | C | C | U | C | U | U | ||||||
| Quercus gambelii | Gambel oak | C | C | ||||||||||
| Quercus hypoleucoides | silverleaf oak | C | C | C | C | C | C | C | U | ||||
| Quercus oblongifolia | Mexican blue oak | U | |||||||||||
| Quercus turbinella | scrub oak | U | |||||||||||
| Rhamnus crocea | hollyleaf buckthorn | U | U | U | |||||||||
| Rhus aromatica | skunkbush | U | U | ||||||||||
| Robinia neomexicana | New Mexico locust | U | U | U | C | C | |||||||
| Rubus neomexicanus | New Mexico raspberry | U | C | ||||||||||
| Rubus sp. | smaller; Rosaceae | C | |||||||||||
| Rubus sp. | raspberry-like; Rosaceae | U | |||||||||||
| Rumex sp. | dock: Polygonaceae | U | |||||||||||
| Salvia sp. | Lamiaceae | U | |||||||||||
| Sarcostemma crispum | climbing milkweed | U | |||||||||||
| Scrophularia parviflora | pineland figwort | U | U | ||||||||||
| Selaginella arizonica | spike moss | U | |||||||||||
| Selaginella rupincola | rockloving spike moss | U | |||||||||||
| Senna bauhiniodes | twinleaf senna | C | C | ||||||||||
| Senna covesii | desert senna | C | |||||||||||
| Setaria leucopila | bristlegrass | U | |||||||||||
| Sida abutifolia | spreading fanpetals | U | |||||||||||
| Solanum eleagnifolium | horse nettle | C | |||||||||||
| Solanum sp. | Solanceae | U | |||||||||||
| Solidago wrightii | golden rod | U | U | C | C | C | C | U | |||||
| Sphaeralcea fendleri | Fendler's globe mallow | U | |||||||||||
| Sphaeralcea laxa | caliche globe mallow | C | U | C | U | ||||||||
| Sphinctospermum constrictum | hourglass peaseed | U | |||||||||||
| Sporobolus airoides | alkali sacaton | U | |||||||||||
| Stemodia durantifolia | whitewooly twintip | U | U | U | |||||||||
| Stephanomeria pauciflora | wire lettuce | ||||||||||||
| Stevia sp. | Asteraceae | U | |||||||||||
| Symphphoricarpos oreophilus | mountain snowberry | C | C | ||||||||||
| Thalictrum fendleri | meadow rue | C | U | C | |||||||||
| Thamnosoma sp. | Rutaceae | U | |||||||||||
| Thymophylla pentachaeta | dogweed | U | U | ||||||||||
| Tradescantia pinetorum | pinewoods spiderwort | U | |||||||||||
| Tragia nepetifolia | catnip noseburn | U | |||||||||||
| Trichostema arizonicum | Arizona blue curls | C | |||||||||||
| Tridens muticus | slim tridens | C | U | U | |||||||||
| Unknown composite | fernlike leaves | ||||||||||||
| Urochloa arizonica | Arizona panic grass | C | C | U | C | ||||||||
| Verbascum thapsus | common mullein | U | U | ||||||||||
| Verbena neomexicana | hillside vervain | U | U | ||||||||||
| Verbesina encelioides | U | ||||||||||||
| Vicia sp. | vetch; Fabaceae | U | U | U | |||||||||
| Viola sp. | violet; Violaceae | U | C | C | |||||||||
| white-backed fern | Pteridaceae | U | |||||||||||
| Xanthisma gracilis | slender goldenweed | U | U | ||||||||||
| Xanthisma spinulusa | spiny goldenweed | U | |||||||||||
| Xylorhiza tortifolia | Mojave woodyaster | C | |||||||||||
| Yucca elata | soaptree yucca | C | |||||||||||
| Yucca madrensis | mountain yucca | U | C | C | C | U | C | C | U | ||||
| Zinnia acerosa | desert zinnia | U | U | U | |||||||||
| Ziziphus obtusiflora | graythorn | U | C | ||||||||||
| Zornia latifolia | U |
After classifying sites according to biome using the categorical system previously described, we used a series of analysis of similarity (ANOSIM) tests as implemented in PRIMER 5.2.9 (Clarke and Gorley 2001) to ask if the plant communities in these biomes significantly differ from one another. We calculated the Bray-Curtis distance among all possible pairs of sites based on species composition and abundance of the 77 species of trees, perennial woody shrubs, and succulents found in our sites. We then used ANOSIM to establish overall differences among plant biomes, followed by a series of pairwise biome comparisons. To visualize the relationships among sites we provide the multidimensional scaling (MDS) ordination plot (fig. 4). ANOSIM revealed significant overall differences in plant community composition among biomes (R = 0.858, P < 0.001). Furthermore, pairwise comparisons indicate that all plant biomes significantly differ from one another, which suggests that our classification system does identify sites that are unique with regard to their plant assemblages. Oak-Woodland sites were excluded from these analyses because of the low sample size (two sites). To the best of our knowledge, the ANOSIM analysis of our 66 sites based on plant abundance and species composition is the first statistical analysis of plant distributions in this range.
Figure 4.
MDS ordination of sites based on Bray-Curtis similarity values. Sites were classified according to the composition and abundance of 77 species of trees, perennial woody shrubs, and succulents found in the transect sites. Plant biome designations for each site were determined a-priori using the criteria outlined in this paper. Note that the MDS ordination shown in this figure is a 2-dimensional compression of a multi-dimensional clustering of the sites, so graphical representation is not perfect. However, the stress value of 0.07 indicates that information in the 2-dimensional figure does a good job of describing patterns in the multi-dimensional space.
Plant diversity data are summarized in tables 6 and 7. The highest plant diversity occurred in our Desert Grassland sites on the north side of the Catalina Mountains, where the relatively undisturbed transect sites had a total of 131 species and the grazing-disturbed sites had a total of 99 species. This was followed by the southern slope Oak-Grassland sites with 88 species, and the Desertscrub sites with 74 species. Pine-Oak Woodland sites had 46 plant species (northern slopes) and 50 species (southern slopes). The lowest diversity sites were Pine Forest of the northern slopes (23 species) and Chaparral on the southern slopes (27 species). This is the same biome diversity trend noted by Whittaker and Niering (1965) and other workers over the years. A number of our plant records are new species to the Catalina Mountains, or significant elevation extensions, and these will be discussed in future papers.
Desertscrub
Because the base of the Catalina Mountains on its northern side is ∼1220 m, there is no Desertscrub. Instead, the biome at the base (the start of the Control Road, in the town of Oracle) is Grassland. However, a well-developed Arizona Upland Desertscrub is at the base of the mountain on the south side, and it contains all of the signature plants of this Sonoran Desert subprovince (table 7). Whittaker and Niering (1965) called this the “Sonoran Desert of Mountain Slopes” biome, even though Forrest Shreve had coined the well-accepted name Arizona Upland in 1951 for this subprovince of the Sonoran Desert. Three of our Desertscrub sites are near Babad Do'ag Viewpoint, along the Babad Do'ag Trail. Because of its elevation (1133–1160 m) and proximity to the opening of Molino Canyon, the Babad Do'ag sites are wetter than the other Desertscrub site (1045 m), and thus velvet mesquite (Prosopis velutina) and desert hackberry (Celtis pallida) are present (and palo verde/Parkinsonia sp. and saguaro/Carnegiea gigantea are fewer in number); also shindagger agave (Agave schottii), turpentinebush (Ericameria laricifolia), limberbush (Jatropha cardiophylla), sprawling prickly pear (Opuntia phaeacantha var. major), and many wildflowers. For example, purple ground cherry (Physalis crassifolia), desert windmills (Allionia incarnata) and spike moss (Selaginella arizonica) occur at this wetter site, indicative of its proximity to the transition into Grassland habitat. Although none of our transects had invasive buffelgrass (Pennisetum ciliare), large patches of it occur across the Desertscrub landscape on the southern slopes of the Catalina Mountains and in the Babad Do'ag Trail area large patches of invasive Lehmann lovegrass (Eragrostis lehmanniana) also are found. Desertscrub habitat extends to ∼1177 m on the southern slopes of the Catalina Mountains along the Mt. Lemmon Highway.
Undisturbed Savanna-like Habitats: Desert Grassland and Oak-Grassland
About half the plant species found in these two savannalike biomes are shared, so we discuss them together. There are no large swaths of Desert Grassland along the Mt. Lemmon Highway on the south side of range, and there Desertscrub transitions quickly into Oak-Grassland dominated by Emory oak, Arizona white oak, and four grasses - side-oats grama (Bouteloua curtipendula), cane beardgrass (Bothriochloa barbinodis), Arizona panic grass (Urochloa arizonica), and the elevationally wide-ranging bullgrass (Muhlenbergia emersleyi) (a total of 14 grasses were recorded in our Oak-Grassland transects). Other abundant plants of our Oak-Grassland sites are pointleaf man-zanita (Arctostaphylos pungens), shindagger agave, sotol (Dasylirion wheeleri), beargrass (Nolina microcarpa), mountain yucca (Yucca madrensis), scarlet creeper (Ipomoea cristulata), Amaranth (Amaranthus nr. palmeri), and three species of Portulaca. Although our Grassland and Oak-Grassland sites share many species, each also has many unique components (table 7). The transition between Grassland and Oak-Grassland biomes is most easily seen in the turnover of grass species along elevational gradients and the increasing abundance of oaks with higher elevations. Although Arizona white oak (Quercus arizonica) and manzanita make their first appearance in these grassy habitats, these are elevationally broad-ranging plants that are also found in Chaparral, Oak Woodland, and Pine-Oak Woodland. In contrast to the southern slopes, the north side of the Catalina Mountains has accessible, expansive, rolling hills of Desert Grassland but no well-developed Oak-Grassland (along the Control Road). These north-side Desert Grassland sites harbored 131 plant species, making this the most botanically diverse biome in our study, including 22 grass species, although 4 species dominated in abundance: side-oats grama, Arizona panic grass, Mexican panic grass (Panicum hirticaule), and the invasive Lehmann lovegrass. In addition to these grasses, velvet mesquite, whitethorn acacia (Acacia constricta), wait-a-minute bush (Mimosa aculeaticarpa), fairy duster (Calliandra eriophylla) were all present in high numbers, indicative of some history of grazing disturbance. On Grassland sites with strong limestone presence, the most abundant plants included a half-dozen typical Desertscrub species, with ocotillo (Fouquieria splendens) dominating. Ground cover by grasses was high in all Desert Grassland transects, often 90%, and sometimes dominated by the invasive Lehmann's lovegrass.
Grazing-Disturbed Grassland
On the north side of the Catalina Mountains, most Grassland habitats have been converted to “scrubland” by over a century of grazing, giving the landscape a desert-like appearance. Seven transects were established in this highly disturbed Desert Grassland near the base of the mountain along the Control Road, from 1337 to 1451 m. All of these sites showed evidence of decades of heavy livestock traffic, with a great deal of disturbed, bare dirt and disrupted soil and, in some areas, erosion down to bedrock. These transects had 99 plant species that were a mix of Desert Grassland and Desertscrub species characteristic of long-overgrazed Grasslands of southeastern Arizona. Typical invasive native Desertscrub plants included velvet mesquite, whitethorn acacia, catclaw acacia (Acacia greggii), fishhook barrel cactus (Ferocactus wislizeni), slim ragweed (Ambrosia confertiflora), and fairy duster, and the abundance and biomass of these species generally exceeded that of the Grassland species. The large number of grass species (19) in these disturbed sites might be due, in part, to the history of livestock grazing and imported forage. This was the only site where we found red brome (Bromus rubens), an exotic invasive grass.
Oak Woodland
Oak Woodland is generally characterized by stands of oaks, with junipers and pinyons. This biome reaches its maximum development in the Sierra Madre of Mexico, and in general, more northern Sky Islands have less Oak Woodland than more southern ranges. In the Catalina Mountains, well-developed Oak Woodland does not occur along the Mt. Lemmon Highway, on the south side of the mountains. However, on the north side of the Catalina Mountains, patches of Oak Woodland do occur along the Control Road, and in our two transect sites in this habitat there was a total of 30 plant species (tables 6 and 7). Seven of the nine signature species of this biome listed by Whittaker and Niering (1968b) occurred in our site transects. These woodlands are dense with foliage, the large dominants being Arizona white oak, silverleaf oak, Chihuahua pine (Pinus chihuahuana), alligator juniper (Juniperus deppeana), and Fendler buckbrush (Ceonothus fendleri). Bullgrass covers much of ground between the trees. In the Catalinas, the elevationally broadly occurring alligator juniper, Fendler buckbrush, and adder's tongue make their first appearances in Oak Woodlands and are then found all the way into Pine Forest habitat and in the case of adder's tongue into the Mixed Conifer Forest.
Chaparral
Interior Chaparral occurs on both the north and south sides of the Catalina Mountains, but it is more extensively developed along the Control Road on the northern slopes. Our Chaparral sites on the south side of the range had 27 plant species, whereas the northern sites had 54. Even though the plant diversity on the north-side sites was greater, neither border pinyon nor sotol were found in those transect sites. The north-side Chaparral transects had abundant alligator juniper, Arizona white oak, Emory oak (Quercus emoryi), Fendler buckbrush, mountain mahogany (Cercocarpus montanus), silktassle bush (Garrya wrightii), and mountain yucca—all missing from the more depauperate south-side sites (tables 6 and 7). This is not to say these typical Chaparral plants do not occur on the southern slopes of the Catalina Mountains; they do, but just not in our transect sites. Virtually all of the Chaparral along the Mt. Lemmon Highway was burned in the 2003 Aspen fire, including our two south-side transect sites (near Manzanita Vista and Windy Point Vista), and it is likely this was at least partly the reason these sites were depauperate relative to those on the north side of the mountains (none of our five north-side Chaparral sites burned in the Aspen Fire). These south-side plant communities are only now beginning to show strong recovery. Chaparral transects on both sides of the range were dominated by pointleaf manzanita, silverleaf oak, golden-flower agave (Agave chrysantha), beargrass, and spidergrass (Aristida ternipes var. ternipes). Higher plant diversity in the north-slope sites is likely also due to the greater variety of soil types there, including limestone soils. Whittaker and Niering (1965) and others have shown that limestone soils shift plant biomes upward on Sky Island mountains, such that at elevations where Grassland would normally occur one finds Desertscrub plants (with a strong presence of limestone-loving species such as ocotillo). This shift presumably occurs because limestone is porous and does not retain moisture, creating a more xeric soil condition that Desertscrub plants are adapted to. Several of our Chaparral sites were on limestone soils and these showed the expected up-elevation shift in Desertscrub plants.
Pine-Oak Woodlands
Our Pine-Oak Woodland transects had a total of 50 species (south slopes) and 46 species (north slopes). Abundant on both slopes were alligator juniper, Chihuahua pine, Rocky Mountain ponderosapine/Arizona pine, Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca), silverleaf oak, Fendler buckbrush, mountain yucca, cudweed (Pseudognaphalium sp.), New Mexico groundsel (Packera neomexicana), goldenrod (Solidago wrightii), and bullgrass. It is here that Rocky Mountain Douglas-fir and Rocky Mountain ponderosa pine/Arizona pine make their first appearance in the Catalina Mountains, to continue all the way into Mixed Conifer Forest. Silverleaf oak, with its broad elevational range, occurs from Pine-Oak Woodland and Chaparral well into Pine Forest habitat. All six of Whittaker and Niering's (1968b) signature plant species of Pine-Oak Woodland occurred in our transect sites.
Pine Forest
Our Pine Forest transect sites had a total of 45 species (south slopes) and 23 species (north slopes). Abundant on both slopes were Rocky Mountain ponderosa pine/Arizona pine, southwestern white pine (Pinus strobiformis), Rocky Mountain Douglas-fir, silverleaf oak, Fendler buckbrush, brickellbush (Brickellia sp.), butterweed, goldenrod, pineland dwarf mistletoe (Arceuthobium vaginatum), cudweed, bracken fern (Pteridum aquilinum), and bullgrass. Although white fir occurred in some of the south-side Pine Forest transects, this tree is more typical of the Mixed Conifer Forest and did not occur below 2255 m in our transects. Rocky Mountain ponderosa pine/Arizona pine was dominant conifers in both the Pine Forest and Mixed Conifer biomes. Whittaker and Niering (1968b) noted five signature plant species of Pine Forest, although two were lower-elevation transitionals to Pine-Oak Forest/Woodland, and all but one of these (their transitional netleaf oak/Quercus rugosa) also occurred in our Pine Forest transects. It is in the upper Pine-Oak Woodland and Pine Forest biomes that ponderosa Pine Forests dominate the visual landscape of the Catalina Mountains and most of Arizona's other high Sky Islands.
Mixed Conifer Forest
Transect sites in our Mixed Conifer Forest sites, on Mt. Lemmon and Mt. Bigelow, at 2442–2777 m had 43 species (tables 6 and 7). Dominants, in terms of numbers of individuals, were Rocky Mountain ponderosa pine/Arizona pine, white fir (Abies concolor), southwestern white pine, Rocky Mountain Douglas-fir, pineywoods geranium (Geranium caespitosum), Oxalis sp. (an unidentified Oxalidaceae), mountain parsley (Pseudocymopterus montanus), braken fern, meadow rue (Thalictrum fendleri), and Viola spp (an unidentified violet). Corkbark fir, which is rare south of the Mogollon Rim, occurred in only 2 of our 10 Mixed Conifer Forest transects. There are no naturally occurring spruces (Picea) in the Santa Catalina Mountains, and probably also no limber pine (Pinus flexilis), a more northern species, despite some old (dubious) records from the Catalina Mountains. Whittaker and Niering (1964) and Whittaker and others (1968) subdivided the Mixed Conifer Forest into two elevational zones, “montane fir forest” and “subalPine Forest” (above 2440 m/8000 ft). They distinguished their “montane fir forest” biome by six species that occurred on our sampling transects.
Summary
The Arizona Sky Island Arthropod Project (ASAP) was launched in 2011. Parameters and over-arching goals of the project, and preliminary results of year one, are presented here. We define the boundaries of the Madrean Sky Island Region somewhat more broadly than they have been in the past, with less of an emphasis on the Madrean flora components and more of an emphasis on their location as isolated Cordilleran Gap ranges. The Mexican Sky Islands are still not well known and adjustments regarding the range and extent of the Sky Island Region will no doubt be fine tuned in the future. We point out the important difference between “mountain islands” vs. “habitat islands.” We present the first statistical analysis of plant species and biomes in the Santa Catalina Mountains, and use this to preliminarily establish the biome names and boundaries that we will use in our arthropod analyses. Year one ant and ground beetle (Carabidae) data for the Catalinas are presented; both groups are high in diversity (88 ant species, 69 ground beetle species) and show a trend of species affiliation to plant biomes.
Acknowledgments
This paper would not have been possible without the assistance of many people, who generously helped in the field and with the research, and who put up with our endless badgering with questions. Grateful thanks go to David Bertelsen, Mark Dimmitt, George Ferguson, George Montgomery, and Julie Wiens for their botanical tutorials. Thanks to Aaron Flesch for information on Sonoran Sky Islands and for his review of the maps. Our thanks are also extended to Steven Cover for his help identifying the ants in this study. This work would not have been possible without the assistance of many undergraduate students including Rebecca Compoy, Kayla Jones, Hee Ju, Chris McGinnis, Gabe Oropeza, Chelsea Powers, Shawna Rodgers, Carol Tepper, and Eryn Wouri. Special thanks go to Emmanuel Bernal, Reilly McManus, Jeff Henkel, Ryan McInroy, Garrett Hughes, Paul Marek, Jason Schaller, and James Robertson for their help setting and harvesting pitfall traps and sorting pitfall trap samples. Our work in the Catalinas is facilitated by U.S. Forest Service Special Use Permit # SAN0287. Thanks to Linda Brewer and Goggy Davidowitz for their careful reviews of this manuscript that greatly improved the quality of this paper. The University of Arizona's Department of Entomology and Center for Insect Science, as well as National Geographic Society (8964-11) and the National Science Foundation (EF - 1206382) provided funding for this project.
Footnotes
Authors Note: This paper represents the combination of four presentations made by the authors in a Session on Biodiversity of Arthropods at the conference. The titles of these presentations were “The Arizona Sky Island Arthropod Project (ASAP): Systematics, Biogeography, Ecology, and Population Genetics of Ground-Dwelling Arthropods,” “Do Plant Biomes Along the Elevation Gradient in the Santa Catalina Mountains Harbor Unique Arthropod Communities?” “Diversity and Species Composition of Ant Assemblages in the Santa Catalina Mountains,” and “Hidden Biodiversity of Sky Island Arthropods.”
The content of this paper reflects the views of the authors, who are responsible for the facts and accuracy of the information presented herein.
Contributor Information
Wendy Moore, Department of Entomology, University of Arizona, Tucson, Arizona.
Wallace M. Meyer, III, Department of Entomology, University of Arizona, Tucson, Arizona.
Jeffrey A. Eble, Department of Entomology, University of Arizona, Tucson, Arizona
Kimberly Franklin, Department of Entomology, University of Arizona, Tucson, Arizona.
John F. Wiens, Arizona-Sonora Desert Museum, Tucson, Arizona
Richard C. Brusca, Arizona-Sonora Desert Museum, Tucson, Arizona
References
- Ball GE. Barriers and southward dispersal of the Holarctic boreomontane element of the family Carabidae in the mountains of Mexico. Anales de la Escuela Nacional de Ciencias Biologicas. 1968;17:91–112. [Google Scholar]
- Barber PH. Patterns of gene flow and population genetic structure in thecanyontreefrog, Hyla arenicolor (Cope) Molecular Ecology. 1999a;8:563–576. doi: 10.1046/j.1365-294x.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- Barber PH. Phylogeography of the canyon treefrog, Hyla arenicolor (Cope) based on mitochondrial DNA sequence data. Molecular Ecology. 1999b;8:547–562. doi: 10.1046/j.1365-294x.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Freeman C, Ostle NJ. Microbial contributions to climate change through carbon cycle feedbacks. ISME Journal. 2008;2:805–814. doi: 10.1038/ismej.2008.58. [DOI] [PubMed] [Google Scholar]
- Behan-Pelletier V, Newton G. Linking soil biodiversity and ecosystem function—the taxonomic dilemma. BioScience. 1999;49:149–153. [Google Scholar]
- Betancourt J, Van Devender TR, Martin PS, editors. Packrat Middens: The Last 40,000 Years of Biotic Change. University Arizona Press; Tucson, AZ: 1990. [DOI] [PubMed] [Google Scholar]
- Boyd AE. Morphological analysis of Sky Island populations of Macromeria viridiflora (Boraginaceae) Systematic Botany. 2002;27:116–126. [Google Scholar]
- Bowers JE, McLaughlin SP. Flora of the Huachuca Mountains, a botanically rich and historically significant Sky Island in Cochise County, Arizona. Journal of the Arizona-Nevada Academy of Sciences. 1996;29:66–107. [Google Scholar]
- Brown JH. Mammals on mountaintops: non-equilibrium insular biogeography. The American Naturalist. 1971;105:467–478. [Google Scholar]
- Brown DE, editor. Biotic Communities. Southwestern United States and Northwestern Mexico. University of Utah Press; Salt Lake City: 1994. [Google Scholar]
- Brown DE, Lowe CH. GTR-RM-78. U.S. Department of Agriculture, U.S Forest Service, Rocky Mountain Forest and Range Experiment Station; Fort Collins, CO: 1980. Biotic communities of the Southwest. [Google Scholar]
- Brown DE, Lowe CH, Pase CP. A digitized classification system for the biotic communities of North America, with community (series) and association examples for the Southwest. Journal of the Arizona-Nevada Academy of Science. 1979;14:1–16. [Google Scholar]
- Clarke R, Gorley RN. PRIMER v5: User manual/tutorial. Plymouth: PRIMER-E; 2001. [Google Scholar]
- University of Arizona; [accessed March 2012]. CLIMAS (Climate Assessment for the Southwest) Project. http://www.climas.arizona.edu/projects/southwest-climate-outlook. [Google Scholar]
- Coope GR. The effects of Quaternary climatic changes in insect populations: Lessons from the past. In: Harrington R, Stork NE, editors. Insects in a Changing Environment. Academic Press; London: 1995. pp. 30–49. [Google Scholar]
- Coviella CE, Trumble JT. Effects of elevated carbon dioxide on insect plant interactions. Conservation Biology. 1999;13:700–712. [Google Scholar]
- Davis R, Brown DE. Documentation of the transplanting of Abert's squirrels. Southwestern Naturalist. 1988;33:490–492. [Google Scholar]
- Dimmitt MA, Brusca RC, Krebbs K, McIntyre CL, Viers J, Filippone C, Berry A. Natural Resource Condition Assessment: Chiricahua National Monument, Coronado National Memorial, and Fort Bowie National Historic Site. Arizona-Sonora Desert Museum and Sonoran Institute; Tucson, AZ: 2011. [Google Scholar]
- Dominguez F, Canon J, Valdes J. IPCC-AR4 climate simulations for the Southwestern US: the importance of future ENSO projections. Climatic Change. 2010;99:499–514. [Google Scholar]
- Downie DA. Phylogeography in a galling insect, grape phylloxera, Daktulosphaira vitifoliae (Phylloxeridae) in the fragmented habitat of the Southwest USA. Journal of Biogeography. 2004;31:1759–1768. [Google Scholar]
- Flesch AD, Hahn LA. Distribution of birds and plants at the western and southern edges of the Madrean Sky Islands in Sonora, Mexico. In: Gottfried GJ, Gebow BS, Eskew LG, Edminster CB, editors. Connecting mountain islands and desert seas: biodiversity and management of the Madrean Archipelago II. Proceedings RMRS-P-36. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; 2005. pp. 80–87.pp. 631 [Google Scholar]
- Graham RW, Grimm EC. Effects of global climate change on the patterns of terrestrial biological communities. Trends in Ecology and Evolution. 1990;5:289–292. doi: 10.1016/0169-5347(90)90083-P. [DOI] [PubMed] [Google Scholar]
- Gutzler DS, Robbins TO. Climate variability and projected change in the western United States: regional downscaling and drought statistics. Climate Dynamics. 2010;34(7-8) doi: 10.1007/s00382-010-0838-7. [DOI] [Google Scholar]
- Halffter G. Biogeography of the montane entomofauna of Mexico and Central America. Annual Reviews of Entomology. 1987;32:95–114. [Google Scholar]
- Heald WF. Sky Islands of Arizona. Natural History. 1951;60:56–63. 95–96. [Google Scholar]
- Higgins JW. Master's thesis. Northern Arizona University; Flagstaff, AZ: 2010. Ground dwelling arthropod responses to successional endpoints: burned versus old growth pinyon-juniper woodlands. [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Harvard University Press; Cambridge, MA: 1990. [Google Scholar]
- Hughes L. Climate change and Australia: trends, projections and impacts. Australian Ecology. 2003;28:423–443. [Google Scholar]
- IPPC (Intergovernmental Panel on Climate Change) Climate change 2001 synthesis report: Summary for policy makers. Intergovernmental Panel on Climate Change; Geneva, Switzerland: 2001. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Climate change 2007: The Physical Science Basis. Cambridge University Press; Cambridge, UK: 2007. [Google Scholar]
- Jablonski D, Sepkoski JJ. Paleobiology, community ecology, and scales of ecological pattern. Ecology. 1996;77:1367–1378. [PubMed] [Google Scholar]
- Johnson RA. Arizona ants. Arizona Wildlife Views. 1996 Jun;:2–5. [Google Scholar]
- Kupfer JA, Balmat J, Smith JL. Shifts in the potential distribution of Sky Island plant communities in response to climate change. Proceedings RMRS-P-36. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Fort Collins, CO: 2005. [Google Scholar]
- Leopold AS. Grass, brush, timber, and fire in southern Arizona. Journal of Forestry. 1924;22:1–10. [Google Scholar]
- Liebherr JK. Biogeographic patterns of montane Mexican and Central American Carabidae (Coleoptera) The Canadian Entomologist. 1994;126:841–860. [Google Scholar]
- Linhart YB, Permoli AC. Genetic variation in central and disjunct populations of Lilium parryi. Canadian Journal of Botany. 1994;72:79–85. [Google Scholar]
- Lowe CH. Biotic communities in the sub-Mogollon region of the inland Southwest. Journal Arizona Academy Science. 1961;42(2):40–49. [Google Scholar]
- Maddison WP, McMahon MM. Divergence and reticulation among montane populations of the jumping spider Habronattus pugillis Griswold. Systematic Biology. 2000;49:400–421. doi: 10.1080/10635159950127312. [DOI] [PubMed] [Google Scholar]
- Marshall CJ, Liebherr JK. Cladistic biogeography of the Mexican transition zone. Journal of Biogeography. 2000;27:203–216. [Google Scholar]
- Marshall JT., Jr Birds of the Pine-Oak Woodland in southern Arizona and adjacent Mexico. Pacific Coast Avifauna. 1957;32:1–125. [Google Scholar]
- Martin PS. The Last 10,000 Years: a Fossil Pollen Record of the American Southwest. University of Arizona Press; Tucson: 1963. [Google Scholar]
- Masta S. Phylogeography of the jumping spider Habronatus pugillis (Araneae: Salticidae): recent vicariance of Sky Island populations? Evolution. 2000;54:1699–1711. doi: 10.1111/j.0014-3820.2000.tb00714.x. [DOI] [PubMed] [Google Scholar]
- McCord RD. Phylogeny and biogeography of the land snail, Sonorella, in the Madrean Archipelago. In: DeBano LF, Ffolliott PF, Ortega-Rubio A, Gottfried G, Hamre RH, Edminster CB, editors. Biodiversity and management of the Madrean Archipelago: the Sky Islands of Southwestern United States and Northwestern Mexico; 1994, Tucson, AZ, Gen Tech Rep RM-GTR-264. U.S. Department of Agriculture, U.S. Forest Service, Rocky Mountain Research Station; Fort Collins, CO: 1995. pp. 317–323. Tech. Coord. [Google Scholar]
- McCormack JE, Bowen BS, Smith TB. Integrating paleoecology and genetics of bird populations in two Sky Island archipelagos. BioMed Central Biology. 2008;6:28. doi: 10.1186/1741-7007-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SP. A floristic analysis of the southwestern United States. Great Basin Naturalist. 1986;46:46–65. [Google Scholar]
- McLaughlin SP. An overview of the flora of the Sky Islands, Southeastern Arizona: diversity, affinities and insularity. In: DeBano LF, Ffolliott PF, Ortega-Rubio A, Gottfried G, Hamre RH, Edminster CB, editors. Biodiversity and management of the Madrean Archipelago: the Sky Islands of Southwestern United States and Northwestern Mexico; 1994, Tucson, AZ. Gen Tech Rep RM-GTR-264. U.S. Department of Agriculture, U.S. Forest Service, Rocky Mountain Research Station; Fort Collins, CO: 1995. pp. 60–70. Tech. Coord. [Google Scholar]
- Moore W, Brusca RC. A Natural History of the Santa Catalina Mountains, Arizona, with an Introduction to the Madrean Sky Islands. Arizona-Sonora Desert Museum Press; Tucson, AZ: In press. [Google Scholar]
- Niering WA, Lowe CH. Vegetation of the Santa Catalina Mountains: community types and dynamics. In: Peet RK, editor. Plant Community Ecology: papers in Honor of Robert H. Whittaker. W. Junk Publishers/Kluwer-Academic Publishing; Boston, MA: 1985. pp. 159–184. [Google Scholar]
- Ober KA, Matthews B, Ferrieri A, Kuhn S. The evolution and age of populations of Scaphinotus petersi Roeschke on Arizona Sky Islands (Coleoptera, Carabidae, Cychrini) Zookeys. 2011;147:183–197. doi: 10.3897/zookeys.147.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overpeck JT, Udall B. Dry times ahead. Science. 2010;328:1642–1643. doi: 10.1126/science.1186591. [DOI] [PubMed] [Google Scholar]
- Powers JS, Montgomery RA, Adair EC, et al. Decomposition in tropical forests: a pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. Journal of Ecology. 2009;97:801–811. [Google Scholar]
- Shreve F. The vegetation of a desert mountain range as conditioned by climatic factors. Vol. 217. Carnegie Institute of Washington, Publication; 1915. p. 112. [Google Scholar]
- Shreve F. A comparison of the vegetational features of two desert mountain ranges. Plant World. 1919;22:291–307. [Google Scholar]
- Shreve F. Conditions indirectly affecting vertical distribution on desert mountains. Ecology. 1922;3:269–274. [Google Scholar]
- Shreve F. The transition from desert to Chaparral. Madroño. 1936;3:257–264. [Google Scholar]
- Shreve F. Vegetation of the Sonoran Desert. Vol. 591. Carnegie Institute of Washington, Pub; 1951. pp. 1–129. [Google Scholar]
- Slentz S, Boyd AE, McDade LA. Patterns of morphological differentiation among Madrean Sky Island populations of Castilleja austromontana (Scrophulariaceae) Madroño. 1999;46:100–111. [Google Scholar]
- Smith CI, Farrell BD. Phylogeography of the longhorn cactus beetle Moneilema appressum LeConte (Coleoptera: Cerambycidae): was the differentiation of the Madrean Sky Islands driven by Pleistocene climate changes? Molecular Ecology. 2005a;14:3049–3065. doi: 10.1111/j.1365-294X.2005.02647.x. [DOI] [PubMed] [Google Scholar]
- Smith CI, Farrell BD. Range expansions in the flightless longhorn cactus beetles, Moneilema gigas and Moneilema armatum, in response to Pleistocene climate changes. Molecular Ecology. 2005b;14:1025–1044. doi: 10.1111/j.1365-294X.2005.02472.x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Micro-evolutionary differentiation and biogeographic structure among coniferous forest populations of the Mexican woodrat (Neotoma mexicana) in the American Southwest: a test of the vicariance hypothesis. Journal of Biogeography. 1994;21:369–389. [Google Scholar]
- Tennessen JA, Zamudio KR. Genetic differentiation among mountain island populations of the striped plateau lizard, Sceloporus virgatus (Squamata: Phrynosomatidae) Copeia. 2008;3:558–564. [Google Scholar]
- Van Devender TR. Environmental history of the Sonoran Desert. In: Fleming TH, Valiente-Banuet A, editors. Columnar cacti and their mutualists: evolution, ecology, and conservation. University Arizona Press; Tucson, AZ: 2002. [Google Scholar]
- Van Devender TR, Spaulding WG. Development of vegetation and climate in southwestern United States. Science. 1990;204:701–710. doi: 10.1126/science.204.4394.701. [DOI] [PubMed] [Google Scholar]
- Voight W, Perner J, Davis AJ. Trophic levels are differentially sensitive to climate. Ecology. 2003;84:2444–2454. [Google Scholar]
- Warshall P. The Madrean Sky Island Archipelago: A planetary overview. In: DeBano LF, Ffolliott PF, Ortega-Rubio A, Gottfried G, Hamre RH, Edminster CB, editors. Biodiversity and management of the Madrean Archipelago: the Sky Islands of Southwestern United States and Northwestern Mexico; 1994, Tucson, AZ. Gen Tech Rep RM-GTR-264. U.S. Department of Agriculture, U.S. Forest Service, Rocky Mountain Research Station; Fort Collins, CO: 1995. pp. 6–18. Tech. Coord. [Google Scholar]
- Watling JI, Donnelly MA. Fragments as islands: a synthesis of faunal responses to habitat patchiness. Conservation Biology. 2006;20:1016–1025. doi: 10.1111/j.1523-1739.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- Whittaker RH, Buol SW, Niering WA, Havens YH. A soil and vegetation pattern in the Santa Catalina Mountains, Arizona. Soil Science. 1968;105:440–450. [Google Scholar]
- Whittaker RH, Niering WA. Vegetation of the Santa Catalina Mountains, Arizona. I. Ecological classification and distribution of species. Journal of the Arizona Academy of Science. 1964;3:9–34. [Google Scholar]
- Whittaker RH, Niering WA. Vegetation of the Santa Catalina Mountains, Arizona. A gradient analysis of the south slope. Ecology. 1965;46:429–452. [Google Scholar]
- Whittaker RH, Niering WA. Vegetation of the Santa Catalina Mountains, Arizona. III. Species distribution and floristic relations on the north slope. Journal of the Arizona Academy of Science. 1968a;5:3–21. [Google Scholar]
- Whittaker RH, Niering WA. Vegetation of the Santa Catalina Mountains, Arizona. IV. Limestone and acid soils. Journal of Ecology. 1968b;56:523–544. [Google Scholar]
- Whittaker RH, Niering WA. Vegetation of the Santa Catalina Mountains, Arizona. V. Biomass, production, and diversity along the elevation gradient. Ecology. 1975;56:771–790. [Google Scholar]
- Yang X, Chen J. Plant litter quality influences the contribution of soil fauna to litter decomposition in humid tropical forests, southwestern China. Soil Biology and Biochemistry. 2009;41:910–918. [Google Scholar]


