Abstract
Background
Concern exists regarding the potential electromagnetic interaction between pacemakers, implantable cardioverter-defibrillators (ICDs) and digital music players (DMPs). A preliminary study reported interference in 50% of patients whose devices were interrogated near Apple iPods.
Objective
Given the high prevalence of DMP use among young patients, we sought to define the nature of interference from iPods and evaluate other DMPs.
Methods
Four DMPs (Apple Nano, Apple Video, SanDisk Sansa and Microsoft Zune) were evaluated against pacemakers and ICDs (PM/ICD). Along with continuous monitoring, we recorded a baseline ECG strip, sensing parameters and lead impedance at baseline and for each device.
Results
Among 51 patients evaluated (age 6 to 60 years, median 22), there was no interference with intrinsic device function. Interference with the programmer occurred in 41% of the patients. All four DMPs caused programmer interference, including disabled communication between the PM/ICD and programmer, noise in the ECG channel, and lost marker channel indicators. Sensing parameters and lead impedance exhibited no more than baseline variability. When the DMPs were removed six inches, there were no further programmer telemetry interactions.
Conclusions
Contrary to a prior report, we did not identify any evidence for electromagnetic interference between a selection of DMPs and intrinsic function of PM/ICDs. The DMPs did sometimes interfere with device-programmer communication, but not in a way that compromised device function. Therefore, we recommend that DMPs not be used during device interrogation, but suggest that there is reassuring counterevidence to mitigate the current high level of concern for interactions between DMPs and implantable cardiac rhythm devices.
Keywords: pacemaker, implantable cardioverter defibrillator, electromagnetic interference, pediatrics, congenital heart disease
Introduction
Pacemakers and implantable cardioverter-defibrillators (ICDs) are intended to be life-saving devices; proper functionality is of paramount importance. Therefore, physicians and patients have frequently questioned whether external sources of electromagnetic interference could potentially alter the pacing, sensing, or defibrillating function or the diagnostic event recording properties of pacemakers and ICDs.
There are studies on both in-hospital and out-of-hospital interference with these cardiac rhythm disease management devices. Magnetic resonance imaging and electrocautery produce electrical interference, which may affect pacemaker/ICD functionality (1–3). Consumer electronics have also been investigated, including microwave ovens, metal detector gates, and electronic article surveillance systems (4–6). In 1997, a randomized, prospective trial concluded that cellular telephones impacted the functioning of pacemakers, but there was no adverse effect at any significant distance (7). In that study, a phone held to the ear in a normal position was sufficiently distant to prevent a significant health risk or device interaction.
Digital music players (DMPs) are a new format for portable music. Several digital compression technologies, including MP3 and AAC, are available, but the devices have been commonly called “MP3 players”, regardless of compression technology. Unlike phones, DMPs can be held almost anywhere. A case report and recent article concluded that Apple iPods cause pacemaker interference in 50% of patients, with over-sensing in 20% of patients, telemetry interference in 29% of patients and pacemaker inhibition in 1.2% of patients (8,9). The mean age for this sample was 77 years +/− 8 years. These preliminary reports prompted our study.
Although children and young adults account for only a small proportion of cardiac rhythm disease management devices, these patients likely have a high prevalence of DMP use. Therefore, the purpose of the present study was to describe the nature of the interference from four popular DMPs in common use. In addition, we specifically sought to include a younger age group, who may be more likely to use DMPs on a daily basis.
Methods
Patients and Procedures
This was a prospective, crossover study conducted at a single tertiary care pediatric hospital. The protocol was approved by our institution’s Committee on Clinical Investigation. The trial was registered at clinicaltrials.gov and opened for enrollment during a 4-month recruitment window. We obtained a convenience sample of patients who were included if they met the following criteria: (1) consent for participation or assent for participation with parental/guardian consent, (2) age over 4-years-old, (3) capacity to communicate uncomfortable symptoms, and (4) an active pacemaker/ICD, which was functioning appropriately at the time of interrogation based on internal diagnostics and the judgment of a pediatric electrophysiologist.
Four types of digital music players were tested on each patient: an Apple 4 GB iPod Nano AAC/MP3 Player (2nd Generation), an Apple 30 GB iPod Video AAC/MP3 Player (5.5 Generation), a SanDisk Sansa M230 512 MB MP3 Player, and a Microsoft Zune 30 GB Digital Media Player. DMPs were operated with music playing and the headphones plugged into the headphone jack. The proprietary device programmer associated with the patient’s pacemaker/ICD was used for all interrogations. Patients were tested in the supine or near-supine position, with their pacemaker/ICD programmed to the patient’s usual settings. The device programmer head was placed over the patient’s pacemaker or ICD and a connection was established with the device programmer. A continuous electronic electrocardiogram (ECG) output was monitored for aberrations. A baseline ECG strip, telemetered intracardiac electrogram (IEGM) and marker channels, sensing parameters and lead impedances were recorded. In random order, each DMP was powered on and placed immediately adjacent to the patient’s pacemaker/ICD and the device programmer head. The same parameters and measured data were re-interrogated and representative ECG and IEGM strips were printed with each DMP tested. If interference was noted, the DMP was moved six inches away from the implanted pacemaker/ICD. Device parameters were then re-interrogated and representative ECG tracings, IEGM and marker channel recordings were printed.
Electromagnetic interference with the pacemaker or ICD device was defined as any aberration that caused a change in the cardiac rhythm as seen on the surface electrocardiogram or any clinical symptom, including discomfort, pain, dizziness, presyncope, syncope, headache, or palpitations that correlated with an electrocardiographic abnormality. Interference with the device programmer included any electronic aberration in the atrial or ventricular ECG channels when compared to baseline, loss of markers in the marker channels, or loss of telemetry communication between the programmer and the pacemaker or ICD.
At baseline and with each music player, we also obtained quantitative records of telemetered measured data including intrinsic p-wave sensing, r-wave sensing, atrial lead impedance, ventricular lead impedance, and (ICD lead) shock impedance. Appropriate data were obtained from each device as determined by the number and placement of leads in the pacemaker or ICD system. The prior pacemaker/ICD reports in medical records for a sub-sample of patients were examined to tabulate recent values for each measurement in order to control for routine variation in precision of measurement.
Data Collection and Statistical Analysis
Standardized data collection forms were used to collect patient demographics, weight, height, pacemaker or ICD model, primary indication for implantation, and position of the device. Printouts from the device programmer were obtained for quantitative data and to document surface ECG recordings, atrial and ventricular IEGM channels and marker channels. During the study design phase, we calculated a minimum number of 38 enrolled subjects in order to achieve sufficient statistical power to adequately detect a 10% rate of electromagnetic interference.
The presence or absence of interference is nominal data with a binary outcome. Contingency tables were used to compute a chi squared test statistic. P-wave sensing, r-wave sensing, atrial, ventricular and shock impedances are continuous variables. Previous p-wave sensing, r-wave sensing, atrial, ventricular and shock impedances were tabulated for a 20% subsample of patients. For each patient, a mean and standard deviation was calculated for each of these parameters. The retrospective standard deviation was compared to the prospective standard deviation from this trial using a two-tailed Student t test.
Results
We evaluated 51 patients (34 with a pacemaker, 17 with an ICD) who participated in five tests each: one at baseline and with each of four digital music players, tested in random order for a total of 255 tests (Table 1). Our sample contained 29 unique pacemaker/ICD models. These devices were manufactured by Medtronic, Inc., Minneapolis, MN (15 different models, 27 devices); Boston Scientific Corporation/Guidant, Natick, MA (7 different models, 13 devices); and St. Jude Medical, Inc., St. Paul, MN (7 different models, 11 devices). All leads and generators were confirmed to have normal behavior and acceptable performance parameters at baseline (prior to DMP exposure).
Table 1.
Patient Demographics and Device Characteristics
| Characteristic | Value |
|---|---|
| Number of patients by gender (%) | |
| Male | 25 (49) |
| Female | 26 (51) |
| Age – years | |
| Median | 22 |
| Range | 6 – 60 |
| Height – cm | |
| Mean | 162 |
| Range | 111 – 187 |
| Weight – kg | |
| Mean | 66 |
| Range | 18.6 – 121 |
| Primary indication for device– number of patients (%) | |
| Sinus node dysfunction | 17 (33) |
| AV node dysfunction | 14 (33) |
| Long QT syndrome | 2 (4) |
| Ventricular tachycardia | 15 (30) |
| Device type – number of patients (%) | |
| Pacemaker | 34 (67) |
| ICD | 17 (33) |
| Pacemaker mode – number of patients (%) | |
| AAI | 14 (28) |
| VVI | 24 (47) |
| VDD | 1 (2) |
| DDD | 12 (24) |
| Device location – number of patients (%) | |
| Left chest | 34 (67) |
| Right chest | 7 (14) |
| Abdomen | 8 (16) |
| Other | 2 (4) |
Study patient characteristics.
AAI = atrial pacing, atrial sensing, inhibition response; VVI = ventricular pacing, ventricular sensing, inhibition response; VDD = ventricular pacing, atrial and ventricular sensing, inhibition and tracking response; DDD = atrial and ventricular pacing, atrial and ventricular sensing, inhibition and tracking response.
Potential interference events were classified into three types: (1) effect on the pacemaker or ICD itself, (2) effect on the device programmer and (3) effect on the communication between the pacemaker/ICD and the device programmer. There were no changes in the surface ECG during any baseline tests or during DMP tests (0/255 tests). There was no oversensing, undersensing, mode reversion, asynchronous pacing or abnormal functioning. There were no inappropriate ventricular tachycardia sensing events, nor inappropriate shocks. We did not observe any interaction between the DMPs and the routine function of the pacemakers or ICDs. Evaluation of ICD behavior during detection and defibrillation of an induced tachycardia was not considered justified in this volunteer study.
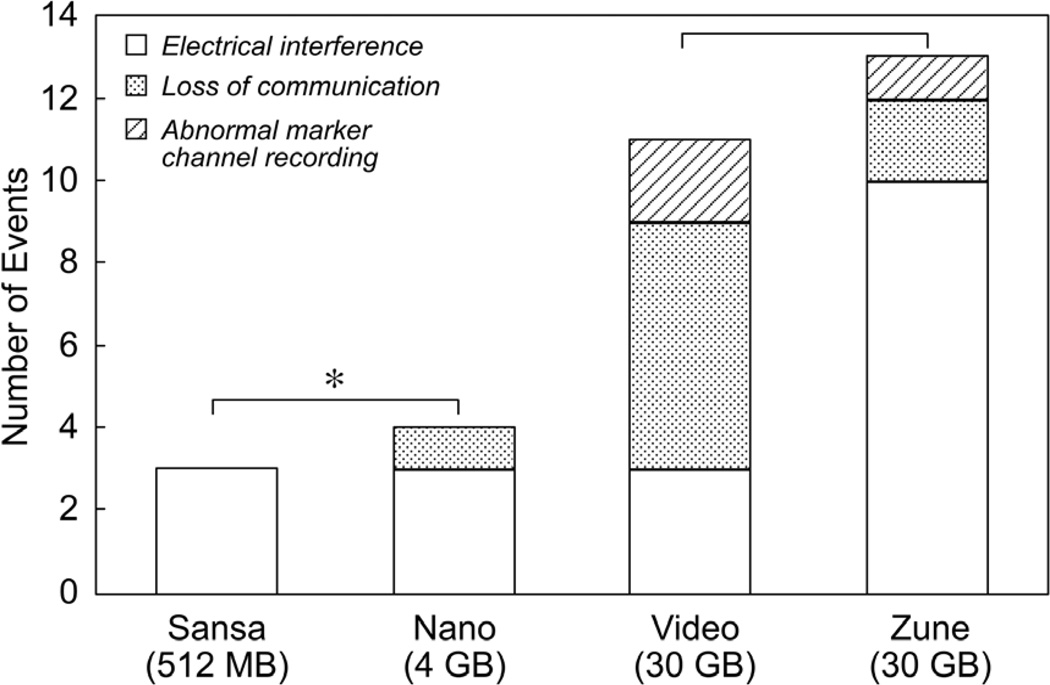
There was a high incidence of interference with telemetry programmer communication when the DMPs were placed directly against the patient (p<0.01). This interference was recorded in 29 of 204 total DMP tests (14%), accounting for 21 of 51 patients (41%). Among patients with interference, 11 had St. Jude Medical devices, 13 had Boston Scientific/Guidant devices, and 26 had Medtronic devices. These accounted for 21%, 26% and 53% of the devices, respectively. There was insufficient power to determine whether there was any difference in frequency between brands of programmer communication interference as a secondary outcome; however, larger DMPs (30 GB) had significantly more interference when compared to smaller DMPs (≤4 GB) (Figure 1). This may be due to the larger size of hard drive, larger batteries, or other factors that distinguish the larger memory devices from the smaller memory DMPs.
Figure 1.
DMP interference, classified by type. *More interference was noted with larger players (30 GB) than with smaller DMPs (≤4 GB) by chi-square analysis (p<0.01).
Out of 51 patients, only one patient complained of symptoms. That patient complained of mild tingling and burning at the site of the pacemaker throughout the process. The recordings did not show interference with the pacemaker and the sensation remained present when the music players were moved away. It seemed to be related to the programmer head being placed on the patient’s chest, not to the presence of DMPs. There were no complaints of dizziness, pre-syncope, headache or any hemodynamic changes in any patients.
There were 31 episodes of interference with programmer-device communication over 29 positive tests; two tests had more than one type of interference. We observed abnormal electronic signals (“noise”) in the atrial and ventricular recording channels (Figures 2 & 3), abnormal assignment of the marker channel or loss of marker assignment (Figure 4), and loss of communication between the pacemaker/ICD and the device programmer. When the music players were moved six inches away from the patient, connection was reestablished in cases where it had been lost and the tracings on the device programmer all reverted to normal. The electrical noise has no effects on pacemaker/ICD functionality. Specifically, there was no inappropriate sensing, inhibition of pacing, or ICD shock delivery due to electromagnetic interference.
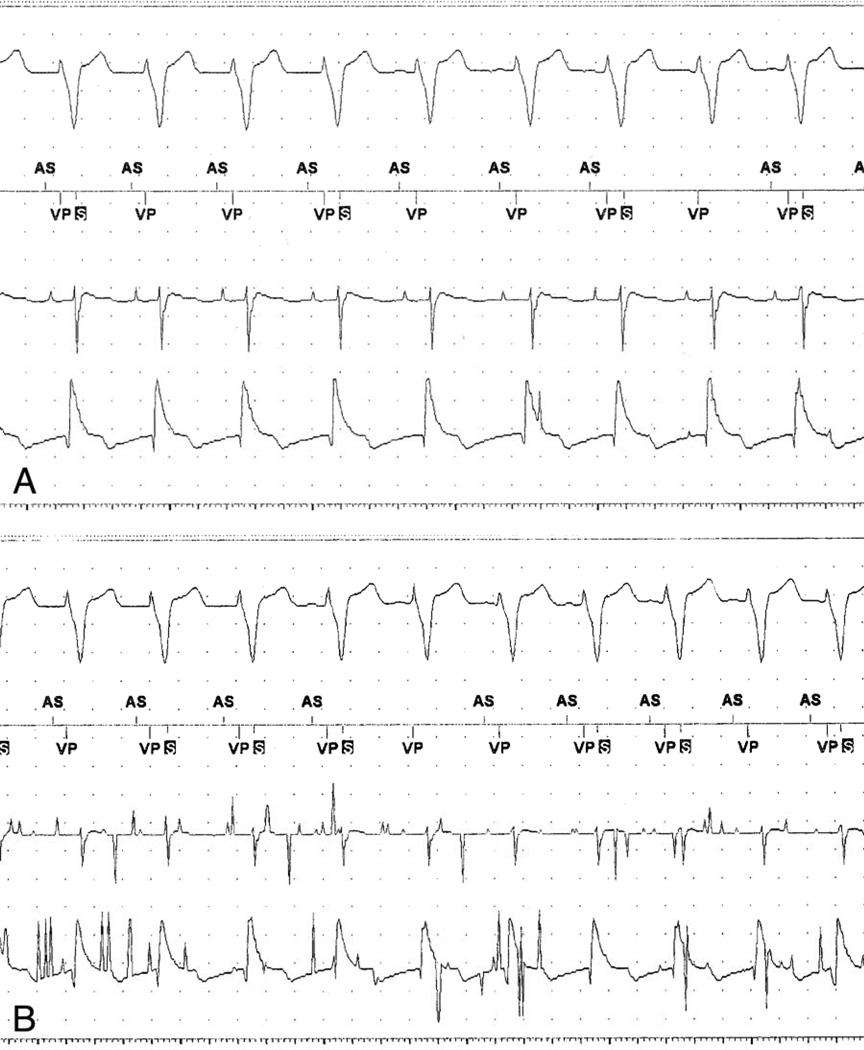
Figure 2.
A. Baseline rhythm strip showing surface ECG, marker channel, atrial and ventricular intracardiac electrogram leads. There is intermittent ventricular T-wave oversensing observed during the post-ventricular refractory period (“S” in black box) at baseline. B. Rhythm strip after placement of DMP causing interference with the atrial channel, the ventricular channel, an absent atrial sensing marker on the marker channel, but no change in the ventricular channel and no change in intervals on the surface ECG. The electrical noise detected by the programmer is not interfering with the actual pacemaker function.
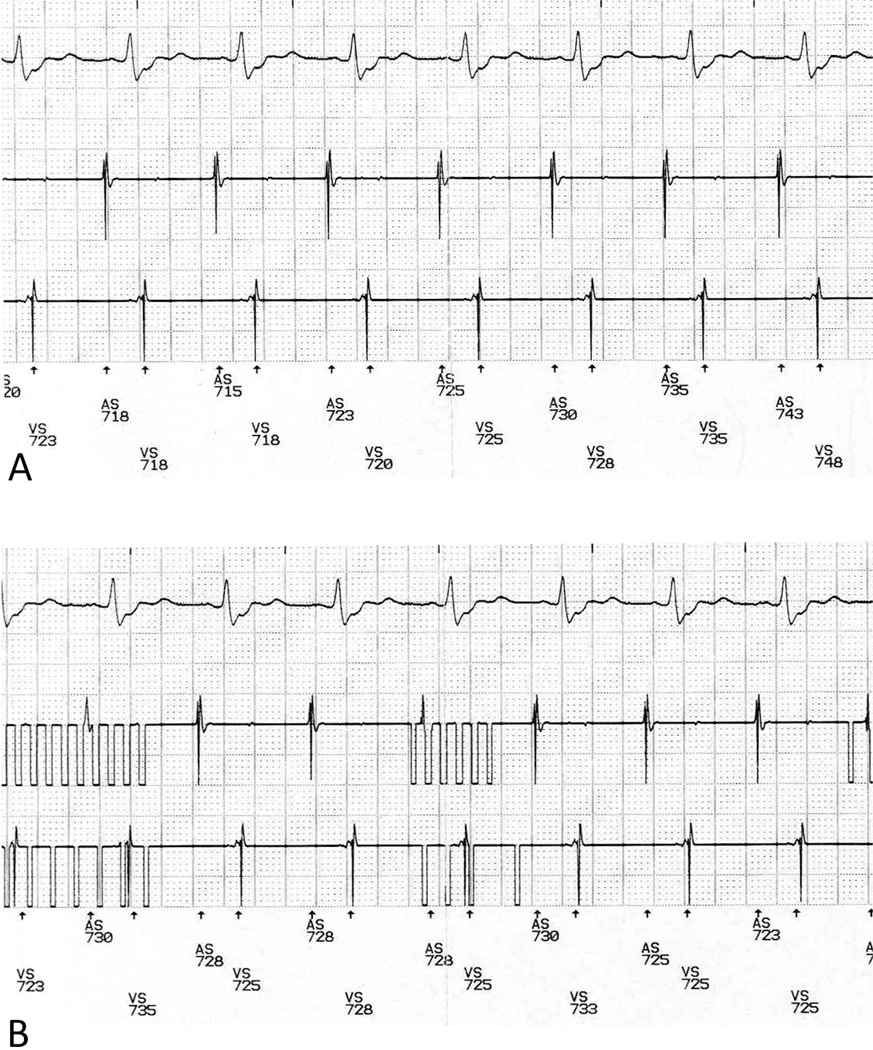
Figure 3.
A. Baseline rhythm strip showing surface ECG, atrial intracardiac electrogram lead, ventricular intracardiac electrogram lead, and marker channel. B. Rhythm strip after placement of DMP causing programmer telemetry interference with the atrial and ventricular intracardiac electrogram recordings, but no change in the intervals on the surface ECG.
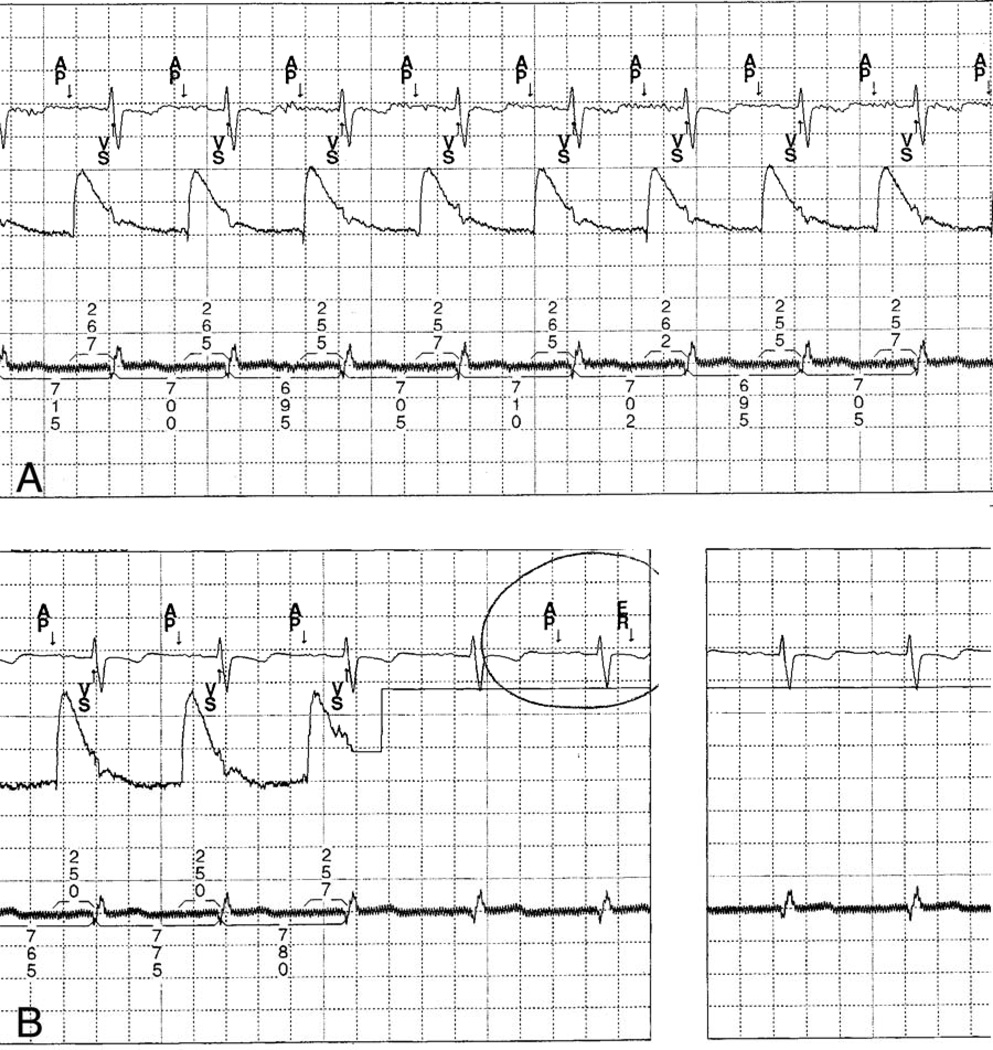
Figure 4.
A. Baseline rhythm strip showing surface electrocardiogram, atrial intracardiac electrogram, and ventricular intracardiac (top, middle, bottom), with atrial pacing (Ap) and ventricular sensing (Vs) noted in the marker channel, and intervals noted in milliseconds. B. Rhythm strip with placement of DMP causing electromagnetic interference (ER) with the atrial intracardiac channel and the marker channel, but no change in the ventricular channel and no change in intervals on the surface ECG. There is no effect of the digital music player on the actual rhythm.
P-wave amplitude sensing and/or R-wave amplitude sensing were tested, as appropriate for the specific pacemaker/ICD configuration. The lead impedance(s) and shock impedance (for ICD leads) were tested for atrial leads, ventricular leads, or both, as appropriate. There was some variation in sensing and impedance measurements during the 5 consecutive tests for each patient; however, when compared to a retrospective sample of previous measurements from a subset of these patients, these measurements exhibited no more than typical baseline variability (p=NS).
Discussion
Given the remarkable growth in the prevalence of portable DMPs over the last 5 years, it has become quite common for clinicians to field questions about interference between DMPs and pacemakers or ICDs. We examined a cohort of patients who are very likely to be using digital music players, with a median age in our study of 22 years old. We presume that this is a relatively high-use group for digital music players, including a sample of children and adults.
We did not record any evidence of interactions between pacemakers or ICDs and DMPs. Amplitude sensing and impedance measurements demonstrate no more than their usual degree of variability when exposed to DMPs. There was interference between the DMPs in our study and telemetry communication between the device programmers that are used to interrogate pacemakers and ICDs. When the music players were moved six inches away from the pacemaker/ICDs and the device programmers, there was no further electromagnetic interference.
Very recently, Thaker and colleagues reported a similar prospective study of Apple iPods in adult pacemaker patients, with a mean age of 77 ± 8 years (10). Similar to our findings, they identified frequent telemetry interference in 51% of patients after exposure to an iPod, and also discovered inappropriate detection of high rate episodes on the pacemaker stored electrograms, but no pacemaker inhibition or mode-switching events (10). They did not evaluate other manufacturers’ music player devices.
There are four primary limitations to our study. First, although adequately powered for primary outcome analysis, there were a relatively small number of patients enrolled, limiting our ability to evaluate secondary outcomes such as the impact of different devices or device manufacturers on the frequency of interference. Secondly, we tested only four DMPs out of an already-vast industry that continues to innovate and market new DMP models. Thirdly, we only tested for acute interactions and did not evaluate the potential effect on arrhythmia sensing, mode-switching, and/or anti-tachycardia therapy delivery during either spontaneous or induced arrhythmias. Finally, the design of our study required us to take measurements while the DMP was adjacent to both the implanted device and the device programmer head, allowing for two simultaneous potential sources of interference
Conclusion
No evidence for electromagnetic interference between a selection of DMPs and pacemakers or ICDs was observed in the present study. The DMPs did sometimes interfere with device programmer communication, but never in a way that compromised device function. Therefore, we recommend that DMPs not be used in immediate proximity to the pacemaker/ICD during device interrogation, but suggest that there is reassuring counterevidence to mitigate the current high level of concern for interactions between DMPs and implantable cardiac rhythm devices. These digital music players appear safe for use by patients with implanted pacemakers or ICDs. As a precaution, patients might be advised to keep their DMP at least 6 inches away from their device pocket, and potential electromagnetic interference should always be considered when assessing pacemaker/ICD stored episodes.
Acknowledgments
Funding Sources
Supported by the Boston Children’s Heart Foundation.
This work was supported in part by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
Dr. Cecchin is a consultant for St. Jude Medical. Dr. Triedman is a consultant for Biosense Webster. Dr. Berul received prior research funding for an unrelated project from Medtronic and Guidant, and is a consultant for Medtronic.
Abbreviations
- AAC
Advanced Audio Coding
- DMP
Digital music player
- ECG
Electrocardiogram
- GB
Gigabyte
- ICD
Implantable cardioverter-defibrillator
- MB
Megabyte
- MP3
Moving Picture Experts Group 1, Audio Layer 3
References
- 1.Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–2891. doi: 10.1161/CIRCULATIONAHA.107.187256. [DOI] [PubMed] [Google Scholar]
- 2.Wong DT, Middleton W. Electrocautery-induced tachycardia in a rate-responsive pacemaker. Anesthesiology. 2001;94:710–711. doi: 10.1097/00000542-200104000-00028. [DOI] [PubMed] [Google Scholar]
- 3.El-Gamal HM, Dufresne RG, Saddler K. Electrosurgery, pacemakers and ICDs: a survey of precautions and complications experienced by cutaneous surgeons. Dermatol Surg. 2001;27:385–390. doi: 10.1046/j.1524-4725.2001.00287.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonney CH, Rustan PL, Jr, Ford GE. Evaluation of effects of the microwave oven (915 and 2450 MHz) and radar (2810 and 3050 MHz) electromagnetic radiation on noncompetitive cardiac pacemakers. IEEE Trans Biomed Eng. 1973;20:357–364. doi: 10.1109/TBME.1973.324288. [DOI] [PubMed] [Google Scholar]
- 5.Copperman Y, Zarfati D, Laniado S. The effect of metal detector gates on implanted permanent pacemakers. Pacing Clin Electrophysiol. 1988;11:1386–1387. doi: 10.1111/j.1540-8159.1988.tb04985.x. [DOI] [PubMed] [Google Scholar]
- 6.Groh WJ, Boschee SA, Engelstein ED, et al. Interactions between electronic article surveillance systems and implantable cardioverter-defibrillators. Circulation. 1999;100:387–392. doi: 10.1161/01.cir.100.4.387. [DOI] [PubMed] [Google Scholar]
- 7.Hayes DL, Wang PJ, Reynolds DW, et al. Interference with cardiac pacemakers by cellular telephones. N Engl J Med. 1997;336:1473–1479. doi: 10.1056/NEJM199705223362101. [DOI] [PubMed] [Google Scholar]
- 8.Patel MB, Thaker JP, Punnam S, Jongnarangsin K. Pacemaker interference with an iPod. Heart Rhythm. 2007;4:781–784. doi: 10.1016/j.hrthm.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Thaker JP, Patel MB, Li H, et al. Pacemaker interference with iPod MP3 players. Heart Rhythm. 2007;4(5):S32. doi: 10.1016/j.hrthm.2007.02.018. (abst). [DOI] [PubMed] [Google Scholar]
- 10.Thaker JP, Patel MB, Jongnarangsin K, et al. Electromagnetic interference with pacemakers caused by portable media players. Heart Rhythm. 2008 doi: 10.1016/j.hrthm.2008.01.028. online early. [DOI] [PubMed] [Google Scholar]