Abstract
The succulent, cylindrical leaves of the C4 dicot Portulaca grandiflora possess three distinct green cell types: bundle sheath cells (BSC) in radial arrangement around the vascular bundles; mesophyll cells (MC) in an outer layer adjacent to the BSC; and water storage cells (WSC) in the leaf center. Unlike typical Kranz leaf anatomy, the MC do not surround the bundle sheath tissue but occur only in the area between the bundle sheath and the epidermis. Intercellular localization of photosynthetic enzymes was characterized using protoplasts isolated enzymatically from all three green cell types.
Like other C4 plants, P. grandiflora has ribulose 1,5-bisphosphate carboxylase and the decarboxylating enzyme, NADP+-malic enzyme, in the BSC. Unlike other C4 plants, however, phosphoenolpyruvate carboxylase, pyruvate, Pi dikinase, and NADP+-malate dehydrogenase of the C4 pathway were present in all three green cell types, indicating that all are capable of fixing CO2 via phosphoenolpyruvate carboxylase and regenerating phosphoenolpyruvate. Other enzymes were about equally distributed between MC and BSC similar to other C4 plants. The enzyme profile of the WSC was similar to that of the MC but with reduced activity in most enzymes, except mitochondrion-associated enzymes.
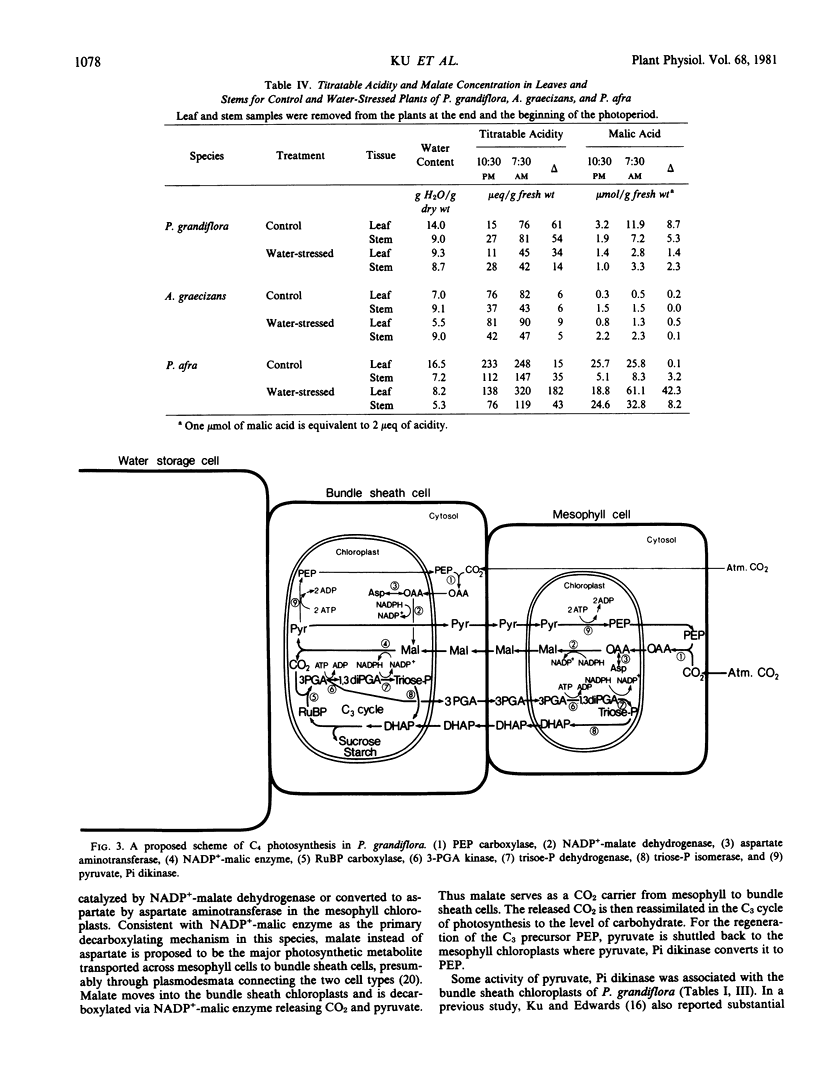
Intracellular localization of enzymes was studied in organelles partitioned by differential centrifugation using mechanically ruptured mesophyll and bundle sheath protoplasts. Phosphoenolpyruvate carboxylase was a cytosolic enzyme in both cells; whereas, ribulose 1,5-bisphosphate carboxylase and NADP+-malic enzyme were exclusively compartmentalized in the bundle sheath chloroplasts. NADP+-malate dehydrogenase, pyruvate, Pi dikinase, aspartate aminotransferase, 3-phosphoglycerate kinase, and NADP+-triose-P dehydrogenase were predominantly localized in the chloroplasts while alanine aminotransferase and NAD+-malate dehydrogenase were mainly present in the cytosol of both cell types. Based on enzyme localization, a scheme of C4 photosynthesis in P. grandiflora is proposed.
Well-watered plants of P. grandiflora exhibit a diurnal fluctuation of total titratable acidity, with an amplitude of 61 and 54 microequivalent per gram fresh weight for the leaves and stems, respectively. These changes were in parallel with changes in malic acid concentration in these tissues. Under severe drought conditions, diurnal changes in both titratable acidity and malic acid concentration in both leaves and stems were much reduced. However, another C4 dicot Amaranthus graecizans (nonsucculent) did not show any diurnal acid fluctuation under the same conditions. These results confirm the suggestion made by Koch and Kennedy (Plant Physiol. 65: 193-197, 1980) that succulent C4 dicots can exhibit an acid metabolism similar to Crassulacean acid metabolism plants in certain environments.
Full text
PDF



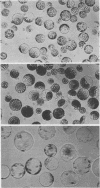
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Berry J. A., Hatch M. D. Photosynthetic metabolism in bundle sheath cells of the C4 species Zea mays: Sources of ATP and NADPH and the contribution of photosystem II. Arch Biochem Biophys. 1980 Jul;202(2):330–341. doi: 10.1016/0003-9861(80)90435-x. [DOI] [PubMed] [Google Scholar]
- Edwards G. E. Isolation of Intact and Functional Chloroplasts from Mesophyll and Bundle Sheath Protoplasts of the C(4) Plant Panicum miliaceum. Plant Physiol. 1979 May;63(5):821–827. doi: 10.1104/pp.63.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Kanai R., Black C. C. Phosphoenolpyruvate carboxykinase in leaves of certain plants whick fix CO 2 by the C 4 -dicarboxylic acid cycle of photosynthesis. Biochem Biophys Res Commun. 1971 Oct 15;45(2):278–285. doi: 10.1016/0006-291x(71)90814-x. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Kagawa T. NAD malic enzyme in leaves with C-pathway photosynthesis and its role in C4 acid decarboxylation. Arch Biochem Biophys. 1974 Jan;160(1):346–349. doi: 10.1016/s0003-9861(74)80043-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Kagawa T. Photosynthetic activities of isolated bundle sheath cells in relation to differing mechanisms of C-4 pathway photosynthesis. Arch Biochem Biophys. 1976 Jul;175(1):39–53. doi: 10.1016/0003-9861(76)90483-5. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K., Kennedy R. A. Characteristics of Crassulacean Acid Metabolism in the Succulent C(4) Dicot, Portulaca oleracea L. Plant Physiol. 1980 Feb;65(2):193–197. doi: 10.1104/pp.65.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Shomer-Ilan A., Beer S., Waisel Y. Suaeda monoica, a C(4) Plant without Typical Bundle Sheaths. Plant Physiol. 1975 Nov;56(5):676–679. doi: 10.1104/pp.56.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomer-Ilan A., Neumann-Ganmore R., Waisel Y. Biochemical Specialization of Photosynthetic Cell Layers and Carbon Flow Paths in Suaeda monoica. Plant Physiol. 1979 Dec;64(6):963–965. doi: 10.1104/pp.64.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]