Abstract
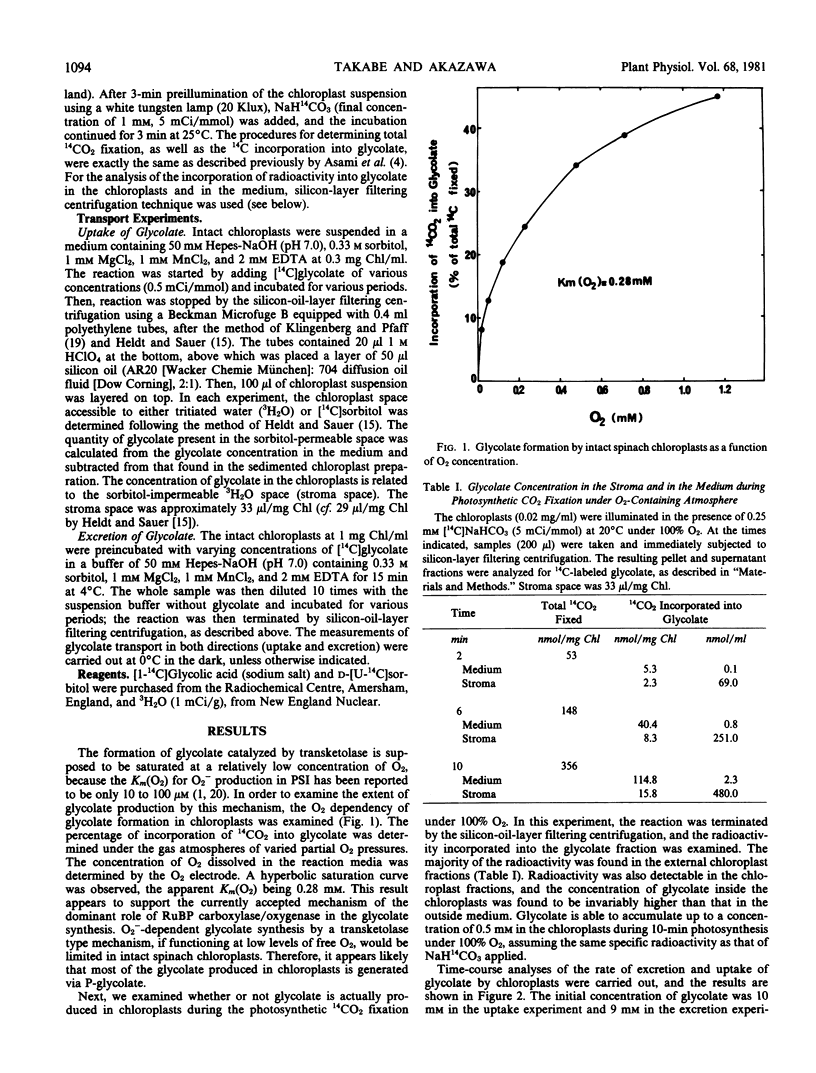
The incorporation of 14CO2 into glycolate by intact spinach leaf (Spinacia oleracea L. var. Kyoho) chloroplasts exposed to 14CO2 (NaH14CO3, 1 millimolar) in the light was determined as a function of O2 concentrations in the reaction media. A hyperbolic saturation curve was obtained, apparent Km (O2) of 0.28 millimolar, indicating that glycolate is produced predominantly by ribulose-1,5-bisphosphate carboxylase/oxygenase. A concentration gradient of glycolate was invariably observed between chloroplast stroma and the outside media surrounding chloroplasts during photosynthetic 14CO2 fixation under an O2 atmosphere.
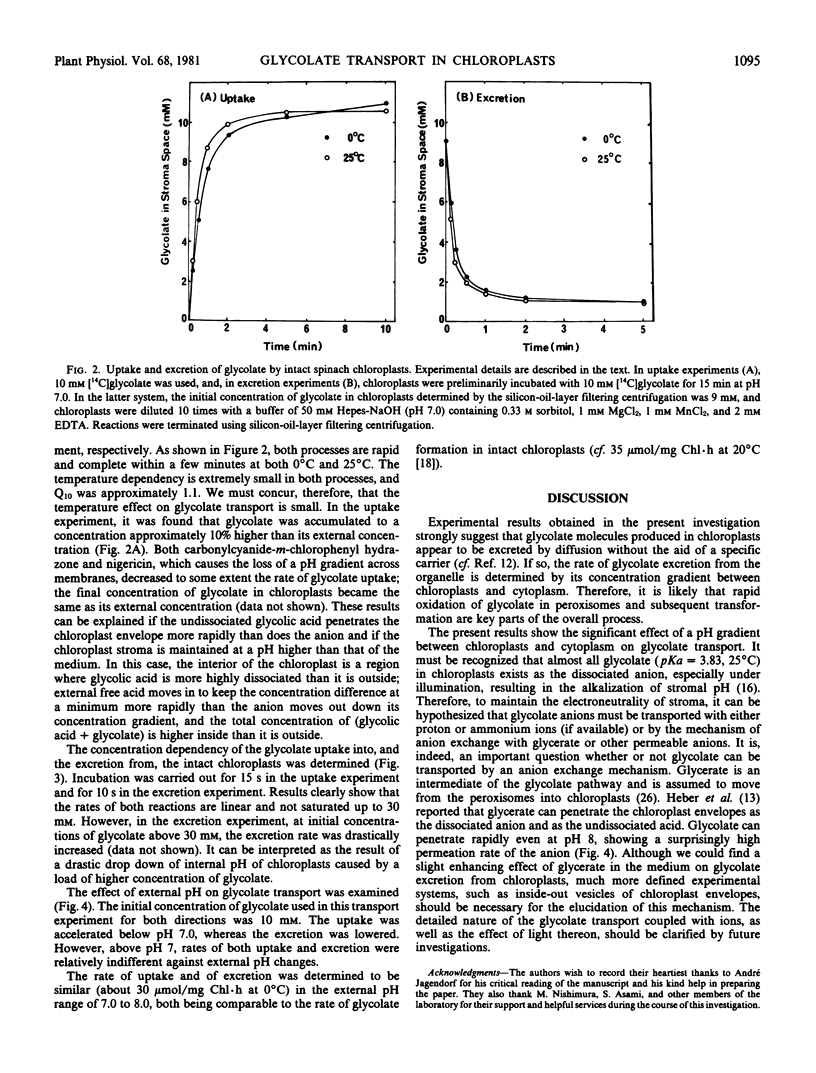
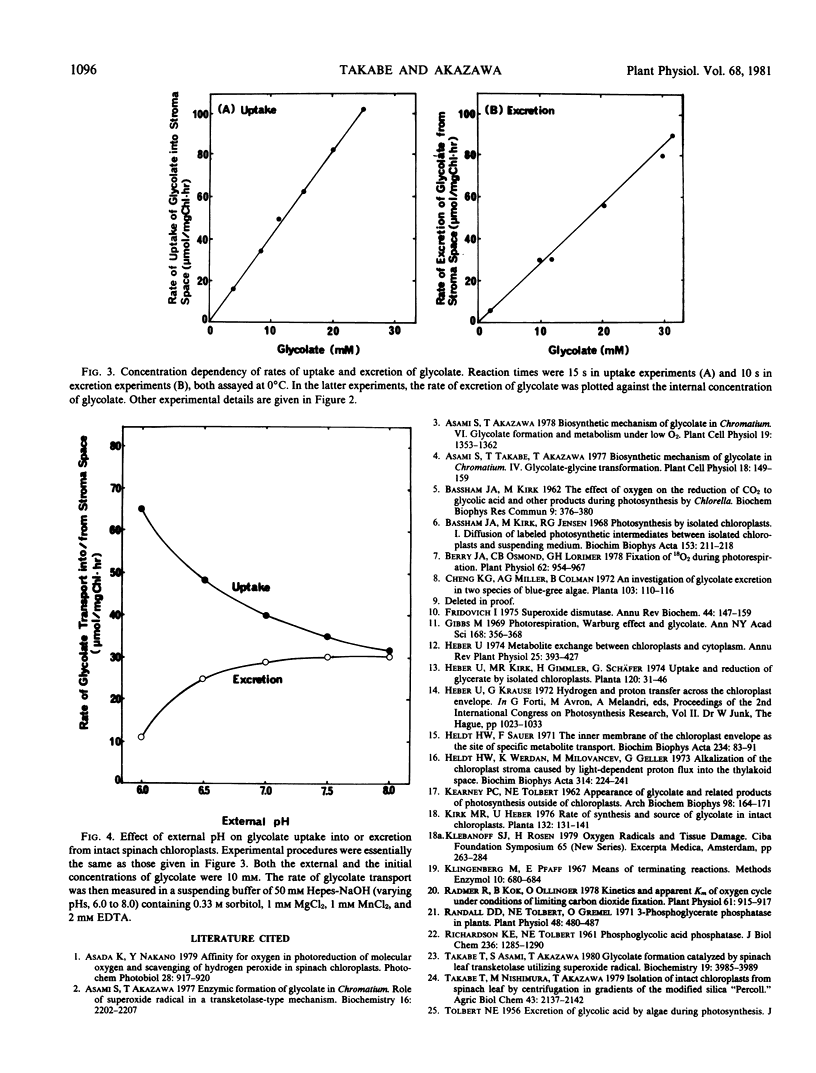
Glycolate transport into and out of chloroplasts was studied using the silicon oil centrifugation method. Both uptake and loss of glycolate were found to be rapid, with small temperature dependencies between 0°C and 25°C (Q10 = 1.1). The reaction rate as a function of the concentration of glycolate up to 30 millimolar was linear in both directions.
The effect of external pH on the reaction rate in both directions was also examined. Glycolate penetrates rapidly, even at pH 8, showing a surprisingly high permeation of the glycolate anion. This rate was about 30 micromoles per milligram Chl per hour at 0°C, and the initial concentration of glycolate of 10 millimolar with a pH range of 7 to 8. The observed rate is comparable to the reported value for glycolate synthesis in chloroplasts under photorespiratory conditions. The uptake of glycolate into chloroplasts was accelerated below pH 7, while the rate of excretion was considerably lowered. It is, thus, suggested that undissociated glycolic acid penetrates the chloroplast envelopes more rapidly than does the anion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami S., Akazawa T. Enzymic formation of glycolate in Chromatium. Role of superoxide radical in a transketolase-type mechanism. Biochemistry. 1977 May 17;16(10):2202–2207. doi: 10.1021/bi00629a025. [DOI] [PubMed] [Google Scholar]
- BASSHAM J. A., KIRK M. The effect of oxygen on the reduction of CO2 to glycolic acid and other products during photosynthesis by Chlorella. Biochem Biophys Res Commun. 1962 Nov 27;9:376–380. doi: 10.1016/0006-291x(62)90019-0. [DOI] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gibbs M. Photorespiration, Warburg effect and glycolate. Ann N Y Acad Sci. 1969 Dec 19;168(2):356–368. doi: 10.1111/j.1749-6632.1969.tb43123.x. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- KEARNEY P. C., TOLBERT N. E. Appearance of glycolate and related products of photosynthesis outside of chloroplasts. Arch Biochem Biophys. 1962 Jul;98:164–171. doi: 10.1016/0003-9861(62)90162-5. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. E., TOLBERT N. E. Phosphoglycolic acid phosphatase. J Biol Chem. 1961 May;236:1285–1290. [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E., Gremel D. 3-Phosphoglycerate Phosphatase in Plants: II. Distribution, Physiological Considerations, and Comparison with P-Glycolate Phosphatase. Plant Physiol. 1971 Oct;48(4):480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T., Asami S., Akazawa T. Glycolate formation catalyzed by spinach leaf transketolase utilizing the superoxide radical. Biochemistry. 1980 Aug 19;19(17):3985–3989. doi: 10.1021/bi00558a015. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]


