Abstract
While the neurobiology of post-traumatic stress disorder has been extensively researched, much less attention has been paid to the neural mechanisms underlying more covert but pervasive types of trauma (e.g., those involving disrupted relationships and insecure attachment). Here, we report on a neurobiological study documenting that mothers’ attachment-related trauma, when unresolved, undermines her optimal brain response to her infant’s distress. We examined the amygdala blood oxygenation level-dependent response in 42 first-time mothers as they underwent functional magnetic resonance imaging scanning, viewing happy and sad face images of their own infant, along with those of a matched unknown infant. Whereas mothers with no trauma demonstrated greater amygdala responses to the sad faces of their own infant as compared to their happy faces, mothers who were classified as having unresolved trauma in the Adult Attachment Interview (Dynamic Maturational Model) displayed blunted amygdala responses when cued by their own infants’ sadness as compared to happiness. Unknown infant faces did not elicit differential amygdala responses between the mother groups. The blunting of the amygdala response in traumatized mothers is discussed as a neural indication of mothers’ possible disengagement from infant distress, which may be part of a process linking maternal unresolved trauma and disrupted maternal caregiving.
Keywords: unresolved trauma, amygdala, attachment, mother-infant relationship
Neurobiological studies of trauma (Rauch et al., 2006; Mahan and Ressler, 2012) to date have primarily focused on the sequelae of severe threat or injury (e.g., assaults, violence, disasters), which are often associated with post-traumatic stress disorder (PTSD). However, more covert but pervasive types of trauma, such as those involving disrupted relationships and insecure attachment (Lyons-Ruth and Jacobvitz, 2008; Allen, 2013), have been relatively neglected in the neurobiology literature. These dysregulating experiences in attachment relationships are relatively subtle, but they are repetitive and sustained (e.g., chronic misattunement, prolonged separation). Their effects, though not often dramatic as in PTSD, are nonetheless profound, sometimes lifelong, and even transgenerational (Lyons-Ruth and Jacobvitz, 2008; Baradon, 2010; Solomon and George, 2011).
The construct of unresolved trauma has provided a unique conceptual framework from which to understand the disruptive transgenerational impact of attachment-related trauma. The classification of unresolved trauma, as derived from the Adult Attachment Interview (AAI; George et al., 1985), relies on relatively subtle and transient signs of absorption in past trauma (Hesse and van Ijzendoorn, 1999), and taps into the degree to which past trauma exerts an ongoing influence on the present socioemotional experiences (Fearon and Mansell, 2001; Crittenden and Landini, 2011). Two decades of longitudinal and cross-sectional research (van IJzendoorn, 1995; Hesse and Main, 1999; Schuengel et al., 1999; Lyons-Ruth et al., 2005) has shown that mothers with unresolved trauma are more likely to have infants who display profoundly disorganized attachment. These infants tend to show striking difficulties using the mother for comfort when distressed, and appear frightened and alarmed (e.g., showing immobilized behavior and dazed appearance) in the presence of their traumatized mothers. Despite the intense empirical scrutiny to which the topic has been subjected and the behavioral correlates identified (see Madigan et al., 2006 for review), the transgenerational mechanisms of unresolved trauma remain poorly understood. In the study reported here, we examine a neurobiological mechanism by which maternal unresolved trauma modifies maternal caregiving so as to disrupt the normative development of the offspring.
Ultimately, offspring survival and growth hinges upon the mother’s inborn neuroanatomy and naturally occurring endocrine changes during the pre- and postpartum period (Rosenblatt, 1994). However, the maternal brain critical to ensuring this vital process is also subject to modification by a number of maternal factors, most notably the mother’s own history of adversity (e.g., trauma) and her current mood disturbance (Barrett and Fleming, 2011). As seen in animal models (Rosvold et al., 1954; Dicks et al., 1968), the human amygdala has long been associated with social behavior (Adolphs et al., 1998), and a host of social dysfunctions have implicated abnormalities in this neural structure (Baron-Cohen et al., 2000; Rosenfeld et al., 2011). Consistent with the long-held focus on fear recognition in the amygdala literature (Adolphs et al., 1994; Vermetten and Lanius, 2012), extant research has emphasized the role of the amygdala in the generation of alerting signals that evoke avoidance behaviors. In recent years, however, the functions associated with the amygdala have been extended well beyond that of threat appraisal (Adolphs, 2010; Morrison and Salzman, 2010) to encompass the amygdala’s central contribution to the appraisal of personal and social salience (Sander et al., 2003; Ewbank et al., 2009; Adolphs, 2010; Markowitsch and Staniloiu, 2011; Strathearn and Kim, 2013). The amygdala, now seen as integral to the so-called “maternal circuitry,” is considered a key neural substrate supporting maternal responsive attunement (Barrett and Fleming, 2011). Drawing from nearly two decades of animal research as well as a steadily growing body of human neuroimaging studies, current view holds that the amygdala, spontaneously recruited at the mother’s sight of her child (Leibenluft et al., 2004; Ranote et al., 2004; Strathearn et al., 2008), interacts with the reward regions of the brain (e.g., nucleus accumbens; Ambroggi et al., 2008) to enhance the perceived appetitive value of infant stimuli (Morrison and Salzman, 2010), ultimately functioning to upregulate the mother’s attention and responsiveness to her infant (Numan et al., 2010).
Trauma-induced neurobiological changes have been widely documented within the amygdala (Mitra et al., 2005; Sabatini et al., 2007; Tottenham et al., 2010; McCrory et al., 2011). The amygdala has been recognized as a primary locus of change in the aftermath of trauma (Caldji et al., 2000; Rauch et al., 2006; Lupien et al., 2009) and, as such, it is thought to be a crucial mediator of the long-term socio-emotional sequelae of trauma. In animals these changes (Fleming et al., 2002; Caldji et al., 2003) have been specifically linked to compromised maternal caregiving behavior (Troisi and D’Amato, 1991; Maestripieri et al., 2005), but the research on the corresponding mechanisms in humans is still in its nascent stage. Neuroimaging studies have widely documented amygdala hyperactivity in individuals with acute trauma exposure (Rauch et al., 2000; Protopopescu et al., 2005; Williams et al., 2006), although growing evidence now suggests that distinctive clinical and neurobiological features emerge following prolonged or chronic trauma, as in attachment-related trauma. Contrary to the heightened emotional responses (e.g., hyperarousal, re-experiencing) commonly observed in single, acute, adult-onset trauma, blunted emotional responses (e.g., emotional numbing, dissociation) have been reported in cases of prolonged, recurrent, early-onset trauma (Frewen and Lanius, 2006b; Lanius et al., 2010). Similarly, a contrasting pattern of amygdala reactivity is also seen in the brain, with hyperactive responses characterizing the former and hypoactive responses seen in the latter. A series of studies (Nijenhuis et al., 1998; Frewen and Lanius, 2006a) have conceptualized this hypoarousal as being akin to the defensive numbing (Bowlby, 1988) that develops upon continued traumatization. The excessive emotion modulation (or suppression) continues to be seen in the trauma’s aftermath in the presence of trauma-reminiscent cues, with rapid habituation buffering against the dysregulating content of trauma-related memories (Frewen and Lanius, 2006b).
It is well established that maternal responsiveness to infant distress is central to the formation of infant attachment security (Ainsworth et al., 1978; McElwain and Booth-LaForce, 2006; Beebe et al., 2010). A considerable consensus also exists on the challenge that maternal attachment trauma, particularly when unresolved, presents to the mother’s attunement to and management of her infant’s distress (Solomon and George, 2011). Although this has not yet been the subject of direct empirical scrutiny, attachment researchers have long speculated that infants’ distress signals may activate unresolved traumatic memories in their respective mothers, thereby initiating a cascade of compromised maternal responses (Fonagy et al., 1995; Lyons-Ruth et al., 1999; Schuengel et al., 1999). Given the longstanding, repetitive, and early-onset nature of attachment-related traumas (Frewen and Lanius, 2006b), we predicted that mothers with such trauma would demonstrate blunted emotional responsiveness when cued by their own infants’ distress, linked to abnormal patterns of rapid habituation. Preliminary support for this view comes from behavioral studies of mother-infant dyads, which document these mothers’ emotional withdrawal from and seeming denial of their infants’ distress (Lyons-Ruth et al., 1999; Goldberg et al., 2003; Beebe et al., 2010).
In the present study of mothers with unresolved trauma, we pursue a neurobiological investigation testing this hypothesis regarding blunted responsiveness. We employed a well-validated fMRI face paradigm (Strathearn et al., 2008) in which mothers viewed images of their own infants in the scanner, interspersed with those of unknown infants. Using happy face images as an affective control, we charted patterns of the mother’s amygdala response to sad infant face images—comparing those of her own with those of an unknown infant. Unlike mothers with no trauma, in whom the salient signal of infant distress would elicit robust amygdala activation, we predicted that mothers with unresolved trauma would demonstrate blunted amygdala responses to sad faces of their own infant, manifesting with prolonged exposure to the target stimulus.
Methods
Participants
Participants consisted of 42 first-time mothers aged 19 to 41 (M = 28.2 ± 0.7) years, recruited through local prenatal clinics and community advertisements as part of a larger study. Of 61 participants initially enrolled during the third trimester of pregnancy, 44 completed the scanning visit; the scans of two participants were additionally discarded due to poor quality and technical problems. All participants were right-handed, were free of past or present alcohol or substance abuse, were clear of nicotine use during pregnancy, and were not on psychotropic medications at the time of the study. Each participant provided written informed consent in accordance with the protocol approved by the institutional review board at Baylor College of Medicine.
Study Design
We adopted a longitudinal design: assessment of mothers’ unresolved trauma occurred during the third trimester of pregnancy, images of the infants were taken at 7 months postpartum, and mothers’ amygdala responses to those images were measured via fMRI at 11 months postpartum.
Instruments and Evaluation
The presence of unresolved trauma or loss was evaluated using the modified version of the AAI (George et al., 1985; Crittenden and Landini, 2011) during the prenatal visit in the third trimester of pregnancy. The AAI is a semi-structured interview consisting of a series of probes designed to elicit attachment-related autobiographical memories. Unresolved trauma or loss is indicated in this assessment by linguistic breakdowns (e.g. sudden slips or lapses of grammar) during discussions of trauma or loss. These disturbances in speech are thought to reflect brief collapses in discourse strategy due to momentary activation of and interference from avoided, segregated, and unassimilated traumatic information (Hesse and Main, 1999; Fearon and Mansell, 2001; Crittenden and Landini, 2011). Following the conceptualization of Bowlby (1982) and Crittenden (2011), loss is treated as a subcategory of trauma and hereafter encompassed under “unresolved trauma.” The AAIs were audio-recorded, transcribed, and blindly coded by reliable raters in accordance with the Dynamic Maturational Model (DMM) of Attachment and Adaptation (Crittenden and Landini, 2011; Landa and Duschinsky, 2013). The DMM method was chosen because of its focus on clinical phenomena, with expanded questions relating to past trauma (Shah and Strathearn, 2014), and validation from previously published neuroimaging data (Strathearn et al., 2009). The intraclass correlation for the unresolved trauma classification was .86.
Several maternal characteristics were additionally assessed as potential confounds. Mothers were screened for symptoms of depression and personality disorders using the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) and Personality Disorder Questionnaire 4+ (PDQ-4+; Hyler et al., 1992), respectively. Maternal parenting stress was assessed using the Parenting Stress Index (PSI; Abidin, 1995), and maternal temperament was measured via the Adult Temperament Questionnaire-Short Form (ATQ; Rothbart et al., 2000). For details on the psychometric properties of these instruments, see Shah et al. (2010).
Stimuli and fMRI Paradigm
Experimental stimuli consisted of 40 infant-face images from four experimental conditions (Figure 1): 10 own happy (OH) infant faces, 10 own sad (OS) infant faces, 10 unknown happy (UH) infant faces, and 10 unknown sad (US) infant faces. For each mother, still face images were captured from the videos recorded of her own infant and of a single unknown infant matched on age and race (and sex if distinguishable). The video-recording was undertaken at the laboratory approximately 7 months postpartum, while eliciting happy and sad expressions from each infant. The adequacy of classification and matching was assessed by three independent female raters, who confirmed that own and unknown infant face images did not differ in terms of valence (MOH = 1.31 ± 0.06, MUH = 1.41 ± 0.05, p = .20; MOS = 6.60 ± 0.07, MUS = 6.62 ± 0.08, p = .79) or gaze direction (percentage direct: MOH = 71.5 ± 3.0, MUH = 74.9 ± 2.7, p = .43; MOS = 47.1 ± 3.7, MUS = 47.7 ± 4.1, p = .27; percentage averted: MOH = 27.4 ± 2.9, MUH = 22.7 ± 2.7, p = .93; MOS = 30.6 ± 3.5, MUS = 30.2 ± 3.2, p = .93). Additionally, happy and sad face images did not differ in intensity (MOH = 2.69 ± 0.06, MOS = 2.60 ± 0.07, p = .31; MUH = 2.59 ± 0.05, MUS = 2.62 ± 0.08, p = .72). The images were projected onto an overhead mirror display and presented to mothers during scanning at approximately 11 months postpartum. All 40 images appeared in a pseudorandom sequence with a stimulus duration of 2 seconds and the random inter-stimulus interval of 2, 4, or 6 seconds. In order to explore our hypothesis concerning blunted responses, the 40 images were presented in two consecutive functional runs. The first functional run (“early phase”) and the second run (“late phase”) of the experiment were analyzed both together and separately.
Figure 1.
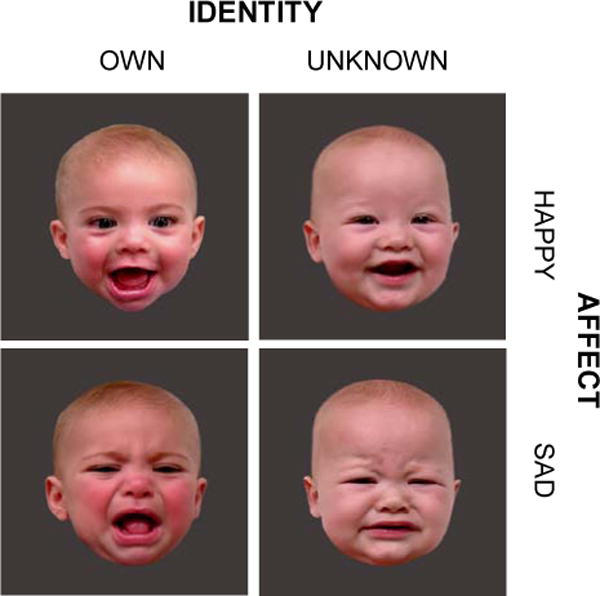
Four infant face categories: own happy (OH), own sad (OS), unknown happy (UH), and unknown sad (US) face images. The pictures were taken approximately 7 months postpartum (age of infant: 6.6 ± 0.3 months) and shown to mothers in the scanner 11 months postpartum. The mean interval between the stimulus acquisition and scanning was 4.9 ± 0.5 months. For each 6-minute functional run, 10 unique images from each face category were pseudorandomly presented with a stimulus duration of 2 seconds, each followed by a black screen for a variable interstimulus interval of 2 to 6 seconds.
fMRI Data Acquisition
Imaging was performed on a 3-Tesla Siemens Allegra scanner. High-resolution T1-weighted anatomical images were acquired (192 slices; in plane resolution, 256 × 256; field of view, 245 mm; slice thickness, 1 mm), followed by two whole-brain blood oxygenation level-dependent (BOLD) functional runs, each comprising 185 images, using a gradient recalled echo planar imaging sequence (37 slices; repetition time, 2000 msec; echo time, 25 msec; flip angle, 90°; matrix, 64 × 64; field of view, 220 mm; slice thickness, 3 mm). Axial slices were positioned at 30° to the line connecting the anterior and posterior commissures.
fMRI Data Analysis
Preprocessing and analyses of the fMRI data were performed using the BrainVoyager QX, version 2.3.1 (Brain Innovation, Maastricht, The Netherlands). Prior to analysis, BOLD images were corrected for slice timing and realigned to the first volume for head motion correction. Functional data were then coregistered with the anatomical data, transformed into 3 × 3 × 3 mm isotropic voxels, and normalized into the Talairach space. Spatial smoothing was not performed. Analyses were performed at two levels: first within the predefined region of interest (ROI) to test our a priori hypothesis, followed by the whole-brain voxel-wise analysis to evaluate the ROI findings in the context of the whole brain.
A random-effects ROI analysis was carried out in two steps. First, a general linear model (GLM) was specified for each functional run, which modeled the expected BOLD signal change for each experimental condition (OH, OS, UH, and US) using a hemodynamic response function. The resulting regression weights (β) were then z-transformed and extracted from our a priori ROI, bilateral amygdala, for second-level random-effects analyses. The extracted β values for run 1 and 2 represented z-normalized BOLD signal change values in voxels of the bilateral amygdala for early and late phases of the experiment, respectively. We utilized the amygdala mask (Figure 2) derived from the SPM Anatomy Toolbox (Eickhoff et al., 2005), converted for use within BrainVoyager. Its anatomical specificity was cross-checked using a published atlas (Mai et al., 2004).
Figure 2.
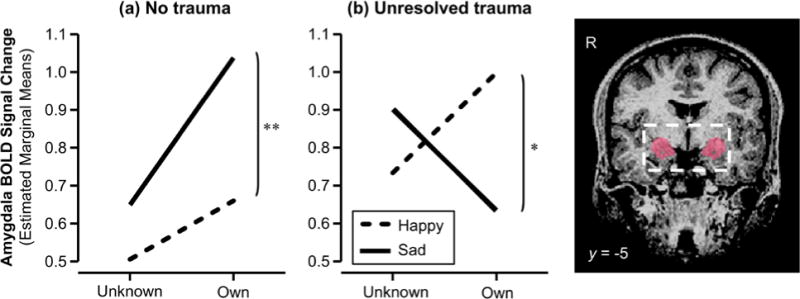
The interactive effect of infant identity and facial affect on the bilateral amygdala BOLD response illustrated for the (a) no trauma group and (b) unresolved trauma group. The anatomical mask used to define bilateral amygdala is shown on the right. * p < .05, ** p < .01
The BOLD signal change data extracted for early and late phases of the experiment were submitted to mixed-effects linear regression analysis with the STATA/SE, version 11.2 XTMIXED procedure. Mixed-effects models allow for explicit modeling and analysis of within- and between-subject heterogeneity, provide greater flexibility in specifying the best-fitting parsimonious structure over traditional models of repeated-measures analysis, and thus generally enable statistically more powerful comparisons of the contrasts of interest. BOLD signal data were inspected for normality via quantile-quantile plots of residuals against fitted values. Missing data were imputed using linear interpolation and mean substitution for the four participants who did not complete part of the late phase of the study. Model building was carried out as follows: (a) the initial model consisted of fixed main effects of phase (early vs. late), laterality (left vs. right), trauma status (unresolved vs. no trauma), infant face identity (own vs. unknown), and face affect (happy vs. sad); (b) systematic inter-individual variability was then modeled by adding subject-level random intercept and slope; (c) interaction terms were added sequentially and retained in the model if shown to improve model fit. The model was fitted by maximum likelihood estimation, and nested models were contrasted using likelihood-ratio chi-squares.
Whole-brain analysis was carried out using random-effects GLM with subject as a random factor. Between-group differences (unresolved trauma vs. no trauma) in BOLD signals were evaluated in the 2 (trauma) × 2 (identity) × 2 (affect) ANOVAs, separately for early and late phases. A statistical threshold of FDR corrected q < .05 and a cluster threshold of ≥ 100 mm3 were used to determine clusters of significant activation.
Results
Participant Characteristics
Participant characteristics are shown in Table 1 for the no trauma (n = 25) and unresolved trauma (n =17) groups. Participants were a non-clinical sample of mothers who were generally of middle to high socioeconomic status, with 75 percent holding a college or postgraduate degree. All mothers scored negative for symptoms of depression as assessed by the BDI-II, except one mother in the no trauma group who scored in the mildly depressed range. All scored below the clinical range for personality disorders and parenting stress as assessed by PDQ-4+ and PSI, respectively. No significant differences were observed between the two groups for any of the measured sociodemographic or behavioral variables.
Table 1.
Sociodemographic and behavioral characteristics of mothers by trauma status (N = 42)
| Characteristic | No trauma (n = 25) |
Unresolved trauma (n = 17) |
p |
|---|---|---|---|
| Maternal age, mean ± SD | 27.3 ± 4.3 | 29.5 ± 4.9 | .13 |
| Maternal race, n (%) | .11 | ||
| White | 13 (52.0) | 13 (76.5) | |
| Non-White | 12 (48.0) | 4 (23.5) | |
| Marital status, n (%) | .86 | ||
| Married | 17 (68.0) | 12 (70.6) | |
| Not married | 8 (32.0) | 5 (29.4) | |
| Maternal education, n (%) | .94 | ||
| College incomplete | 6 (24.0) | 4 (23.5) | |
| College/university degree | 10 (40.0) | 6 (35.3) | |
| Postgraduate degree | 9 (36.0) | 7 (41.2) | |
| Socioeconomic status, mean ± SDa | 49.9 ± 10.4 | 51.9 ± 9.8 | .58 |
| Maternal IQb | 109 ± 9.1 | 109.1 ± 7.2 | .86 |
| Maternal depression (BDI), mean ± SDc | 4.9 ± 4.4 | 3.5 ± 2.3 | .40 |
| Maternal personality pathology (PDQ) | |||
| Total score, mean ± SDd | 23.4 ± 12.0 | 19.4 ± 9.8 | .27 |
| Maternal parenting stress (PSI) | |||
| Child Domain Total Score, mean ± SD | 93.7 ± 15.0 | 91.4 ± 13.6 | .65 |
| Parent Domain Total Score, mean ± SD | 116.0 ± 24.2 | 112.4 ± 19.9 | .65 |
| Total Stress Score, mean ± SDe | 209.6 ± 31.8 | 196.0 ± 38.4 | .25 |
| Maternal temperament (ATQ) | |||
| Effortful Control, subscale score, mean ± SD | 4.5 ± 0.6 | 4.6 ± 0.9 | .80 |
| Negative Affect, subscale score, mean ± SD | 4.1 ± 0.7 | 3.8 ± 0.8 | .28 |
| Extraversion/Surgency, subscale score, mean ± SD | 4.8 ± 0.8 | 4.9 ± 0.7 | .72 |
| Orienting Sensitivity, subscale score, mean ± SD | 5.1 ± 0.7 | 4.9 ± 0.7 | .35 |
Note. The p values represent those from the t and χ2 tests for continuous and dichotomous variables, respectively. BDI = Beck Depression Inventory-II; PDQ = Personality Disorder Questionnaire-4+; PSI = Parenting Stress Index; ATQ = Adult Temperament Questionnaire – Short Form.
Socioeconomic status was estimated using Hollingshead (1975)’s Four-Factor Index of Social Status, and represents joint information with partner, when applicable.
Maternal Full Scale IQ was estimated from the Wechsler Test of Adult Reading (WTAR).
BDI-II score of ≤ 9 indicate minimal depression.
PDQ-4+ total score of ≥ 50 is highly suggestive of DSM-IV personality disorder.
PSI Total Stress Score of < 260 is considered normal range.
fMRI Findings
Means and standard errors of early- and late-phase amygdala BOLD responses are listed in Table 2 for trauma status by stimulus type. The optimal model (Wald χ2(16) = 116.49, p < .0001) was obtained with a random effects structure that included a subject-level random intercept (LR χ2(1) = 79.14, p < .0001) and a random coefficient for phase (LR χ2(1) = 96.31, p < .0001). Significant fixed effects included: main effect of phase (βphase = −.48, 95% CI = −.88 to −.08, z = −2.37, p = .018) and identity (βidentity = .92, 95% CI = .65 to 1.20, z = 6.61, p < .001), 2-way phase × identity (βphase × identity = −.77, 95% CI = −1.16 to −.38, z = −3.89, p < .001) and affect × identity (βaffect × identity = −.54, 95% CI = −.93 to −.15, z = −2.74, p = .006) interactions, which were qualified by the 4-way trauma × phase × identity × affect interaction (β trauma × phase × identity × affect = −1.11, 95% CI = −1.97 to −.24, z = −2.51, p = .012).
Table 2.
Early and late phase amygdala BOLD response for trauma status by stimulus type.
| (a) No trauma (n = 25)
|
||||||
|---|---|---|---|---|---|---|
| Early phase
|
Late phase
|
|||||
| Own | Unknown | Difference (z)b | Own | Unknown | Difference (z)b | |
|
|
|
|||||
| Happy | 1.91 ± 0.18 | 0.99 ± 0.15 | 5.23*** | 0.66 ± 0.23 | 0.51 ± 0.19 | 0.96 |
| Sad | 1.61 ± 0.19 | 1.22 ± 0.17 | 2.16* | 1.04 ± 0.18 | 0.65 ± 0.21 | 2.42* |
|
| ||||||
| Difference (z)a | −2.58** | 2.01* | 3.00** | 1.14 | ||
| (b) Unresolved trauma (n = 17)
|
||||||
|---|---|---|---|---|---|---|
| Early phase
|
Late phase
|
|||||
| Own | Unknown | Difference (z)b | Own | Unknown | Difference (z)b | |
|
|
|
|||||
| Happy | 1.48 ± 0.20 | 0.87 ± 0.26 | 2.86** | 1.00 ± 0.19 | 0.73 ± 0.16 | 1.36 |
| Sad | 1.44 ± 0.20 | 1.03 ± 0.21 | 1.92† | 0.63 ± 0.15 | 0.90 ± 0.12 | −1.37 |
|
| ||||||
| Difference (z)a | −0.25 | 1.15 | −2.38* | 1.10 | ||
Note. The values shown are z-normalized BOLD signal change values (M ± SE) extracted from the anatomically defined bilateral amygdala mask. There was no effect of laterality; data from the left and right amygdala are hence presented in conjunction.
z-statistic comparing sad and happy infant faces, at each level of infant face identity (own vs. unknown) x trauma status (no trauma vs. unresolved trauma), within the final mixed-effects linear regression model.
z-statistic comparing own and unknown infant faces, at each level of infant face affect (happy vs. sad) x trauma status (no trauma vs. unresolved trauma), within the final mixed-effects linear regression model.
< .10,
p < .05,
p < .01,
p < .001.
To decompose the nature of the 4-way interaction, mixed-effects models were fitted separately for early and late phase BOLD data. No effect of trauma emerged in the early phase, while a significant interaction effect of identity and affect was found, in line with the results reported in Strathearn and Kim (2013). However, trauma became a significant predictor in interaction with identity and affect in the late phase (β trauma × identity × affect = −.76, 95% CI = −1.31 to −.22, z = −2.73, p = .006), accounting for the significant 4-way interaction in the combined dataset. The optimal model for the late phase data included a subject-level random intercept (LR χ2(1) = 101.78, p < .0001) and a random coefficient for identity (LR χ2(1) = 17.09, p < .0001), providing a significant fit (Wald χ2(8) = 22.15, p = .005). Decomposition of the 3-way trauma × identity × affect interaction revealed a simple effect of affect for own infant faces in both no trauma and trauma groups (z = 3.00, p = .003 and z = −2.38, p = .017 respectively), with the direction of the effect reversed in the two groups: whereas mothers with no trauma showed a greater amygdala response to sad than happy faces of their own infant, mothers with trauma displayed a blunted amygdala response to their own infants’ sadness as compared to happiness (Table 2 and Figure 2). For unknown infant faces, the simple effect of affect was not observed in either the no trauma (z = 1.14, p = .254) or trauma groups (z = 1.10, p = .273).
On whole-brain analysis, the 2 (trauma) × 2 (identity) × 2 (affect) ANOVAs yielded no significant findings at the stringent statistical threshold of FDR corrected q < .05. However, a 3-way trauma × identity × affect interaction emerged in the amygdala in the late phase when the threshold was dropped to p = .01 (uncorrected). Consistent with the ROI analysis, blunted activity was seen in the amygdala when mothers with unresolved trauma viewed sad faces of their own infants. The blunting of the amygdala response was not found in the early phase or in response to images of unknown infants.
Discussion
The present results are the first, to our knowledge, to show that mothers with unresolved trauma demonstrate blunted amygdala responsiveness to the distress cues of their own infants. As hypothesized, the blunting of the maternal amygdala response was observed only in attachment-related contexts, when these mothers viewed images of their own infants. Furthermore, the effect was specific to sad faces, as opposed to happy faces, of the mother’s own infant. This finding corroborates observations emerging from behavioral studies of mother-infant dyads (Beebe et al., 2010), underscoring that traumatized mothers may not evidence a global impairment of emotional engagement, but rather a remarkably specific failure of attunement during moments of infant distress. By contrast, the response profile obtained for non-traumatized mothers was consistent with that frequently reported in the amygdala literature, with heightened amygdala responsiveness observed for the negative affect in comparison to the positive affect displayed by their own infants.
To date, only a small body of research has examined attachment-related differences in maternal brain response to infant cues. The few existing studies, including our own previous work using a subset of the present sample (Strathearn et al., 2009), have elucidated the role of attachment security by comparing individuals who have secure attachment to those who have insecure attachment. Of these, two recent studies (Riem et al., 2012; Lenzi et al., 2013) documented heightened amygdala response by utilizing unknown infant cues to probe amygdala responsiveness in insecurely attached nulliparous women. Hence we extend the scant literature by going beyond mothers’ attachment insecurity to document the role of their unresolved trauma in modulating their amygdala response. The present work is also the first to contrast mothers’ own infant cues with unknown infant cues to demonstrate that the blunting of the amygdala in mothers with unresolved trauma is specific to attachment-related contexts. This is particularly noteworthy given recent reports demonstrating that amygdala (dys)functions manifest in a highly context-specific manner (Adolphs, 2010; Strathearn and Kim, 2013).
It is important to note that, as hypothesized, the blunted pattern of amygdala activation was found only in the late phase of the study. Little difference in BOLD responses between individuals with unresolved and resolved trauma could be detected in the first half of the experiment. The effect of trauma status was seen only in response to attachment activating stimuli (participant’s own distressed infant) in the second half of the study when the anomalous excessive blunting of emotional response manifested (Nijenhuis et al., 1998; Frewen and Lanius, 2006a). At least three explanations of the observed pattern are plausible. First, an examination of our data shows evidence of amygdala habituation (i.e., decrement in amygdala response magnitude in the late phase) in both groups of mothers. The function of habituation lies in reducing responses to inconsequential stimuli, while maintaining one’s responsiveness to salient, novel, unexpected stimuli (Groves and Thompson, 1970; Rankin et al., 2009). To non-traumatized mothers, distress cues of their own infants are relatively unexpected, novel, and accordingly salient (Beebe et al., 2010); sad images of their own infants may hence have diminished habituation and elicited increased amygdala response in these mothers. Second, contrary to the modest habituation displayed by non-traumatized mothers to the sad faces of their infants, we speculate that there may be emotional suppression at play in their traumatized counterparts. The blunting of their amygdala response observed in the late phase is beyond what would be expected from ordinary habituation. We understand this as a reflection of active suppression in these mothers at the continued sight of their infants’ distress. As has previously been suggested (Lyons-Ruth et al., 1999; Solomon and George, 2011), repeated cues of their infants’ distress may have been less tolerable for mothers with unresolved trauma, who themselves have experienced prolonged distress in attachment relationships. Third, a ceiling effect in the early phase could have obscured a subtle blunting. Infant faces have a unique salience to their respective mothers, and hence constitute powerful elicitors of amygdala activity, particularly at the time of initial presentation (Leibenluft et al., 2004; Ranote et al., 2004; Strathearn et al., 2008). More subtle inter- and intra-group differences could have been masked by the heightened salience of the own infant cues in the early phase.
While the observed data are consistent with all three of these possibilities, the results provide a neurobiological underpinning for how mothers with unresolved trauma appear to be less behaviorally responsive to their infants’ distress. Beebe and colleagues (2010) have lucidly described these mothers’ disrupted attunement to their infants’ distress. Smiling or showing surprise when their infant is sad, these mothers fail to indicate that they sense their infants’ distress. Rather than comforting or soothing their infant, the mothers look away and withhold affectionate touch. The blunted amygdala response that we have documented in this study suggests that these mothers’ misattunement to distress may be underpinned by a weakened limbic signal, permitting an atypical response to be triggered. This finding is worthy of attention, given the specific strong association between this type of maternal misattunement (i.e., disengagement from distress) and the development of profoundly disorganized attachment in infants (Lyons-Ruth et al., 1999; Madigan et al., 2006; Beebe et al., 2010).
The observation of blunted amygdala response could contribute to an account of the transgenerational transmission of trauma. We may speculate that the blunting of response is maintained because it serves the function of protecting the mother from re-experiencing dysregulating memories of her own trauma. Yet, as an inevitable corollary, the infant is left psychologically ‘alone,’ with no one to share, join in, or mitigate his emotional pain. On the basis of research on profoundly insecure mother-infant attachment, we theorize that this has traumatologic potential, as trauma results from the combination of extreme emotional distress experienced in psychological isolation, such that the distress is prolonged and unrelieved (Allen, 2013). Our findings offer a possible picture of how these mothers, who manifest indications of ongoing traumatization, are at an increased risk of leaving their child alone in unmitigated distress, due to the psychobiological vulnerability generated by their own trauma.
Several limitations of the study should be recognized. First, although we have conceptually distinguished unresolved trauma from PTSD (Harari et al., 2009), we did not conduct a formal screening for PTSD in our sample. However, all measures utilized in the study yielded no evidence of psychopathology in our sample. Second, our sample consisted largely of middle to upper class mothers of average to above-average intelligence, and hence may not have been representative of the general population. We also note, given the nature of the study, that mothers with unresolved trauma, especially those with concerns relating to upcoming motherhood, may have been more likely to volunteer. This may have contributed to the relatively high prevalence of unresolved trauma in our sample. Third, we coded the AAI using Crittenden’s DMM method which has been validated less thoroughly than the Berkeley AAI system (Main et al., 2003), despite the usefulness of the DMM in the study of clinical phenomena (Fonagy, 2013; Landa and Duschinsky, 2013).
The present study marks the first attempt to delineate neurobiological correlates of at-risk mothering in mothers with unresolved trauma. We have found evidence consistent with the assumption that maternal unresolved trauma, even in the absence of blatant symptoms (e.g., PTSD), can undermine optimal maternal response to infant distress. Neural indications of possible maternal disengagement from infant distress may be part of a process linking maternal unresolved trauma and disrupted maternal caregiving. These results point us toward a strategy for intervening in mother-infant dyads where maternal trauma presents a challenge. Helping mothers maintain their engagement during moments of infant distress may be a particularly promising path toward restoring and bolstering attachment security during the crucial period of infant development.
Acknowledgments
We would like to thank Udita Iyengar and Sheila Martinez for assistance with data management and analysis, and the technical staff of the Center for Advanced Magnetic Resonance Imaging (CAMRI) at Baylor College of Medicine for assistance with conducting the experiments.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [K23 HD43097, R01HD065819]; General Clinical Research Center [MO1 RR00188]; the Baylor Child Health Research Center: Pediatrics Mentored Research Program [K12 HD41648]; and the National Institute on Drug Abuse [R01DA026437]. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes or the National Institutes of Health.
Footnotes
The authors declare no competing financial interests.
Contributor Information
Sohye Kim, Email: sohyek@bcm.edu.
Peter Fonagy, Email: p.fonagy@ucl.ac.uk.
Jon Allen, Email: jallen@menninger.edu.
Lane Strathearn, Email: lanes@bcm.edu.
References
- Abidin R. Parenting Stress Index, 3rd edition, Professional manual. Lutz, FL: Psychological Assessments Resources; 1995. [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–103. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio A. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: a psychological study of the Strange Situation. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Allen JG. Mentalizing in the development and treatment of attachment trauma. London, UK: Karnac; 2013. [Google Scholar]
- Ambroggi F, Ishikawa A, Fields H, Nicola S. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–709. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradon T. Relational trauma in infancy: Psychoanalytic, attachment, and neuropsychological contributions to parent-infant psychotherapy. New York, NY: Routledge; 2010. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Bullmore E, Wheelwright S, Ashwin C, Williams S. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–419. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming A. Annual research review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beebe B, Jaffe J, Markese S, Buck K, Chen H, Cohen P, et al. The origins of 12-month attachment: a microanalysis of 4-month mother-infant interaction. Attach Hum Dev. 2010;12:3–141. doi: 10.1080/14616730903338985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Loss: sadness and depression. New York, NY: Basic Books; 1982. [Google Scholar]
- Bowlby J. A secure base : parent-child attachment and healthy human development. New York: Basic Books; 1988. [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Crittenden P, Landini A. Assessing adult attachment: A dynamic-maturational approach to disourse analysis. New York, NY: W. W. Norton & Company; 2011. [Google Scholar]
- Dicks D, Myers R, Kling A. Uncus and amygdala lesions: effects on social behavior in the free-ranging rhesus monkey. Science. 1968;165:69–140. [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ewbank M, Barnard P, Croucher C, Ramponi C, Calder A. The amygdala response to images with impact. Soc Cogn Affect Neurosci. 2009;4:127–160. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon RM, Mansell W. Cognitive perspectives on unresolved loss: insights from the study of PTSD. Bulletin of the Menninger Clinic. 2001;65:380–396. doi: 10.1521/bumc.65.3.380.19845. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacology, Biochemistry and Behavior. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Fonagy P. Commentary on “letters from ainsworth: contesting the ‘organization’ of attachment”. J Can Acad Child Adolesc Psychiatry. 2013;22:178–179. [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Steele H, Steele M, Leigh T, Kennedy R, Mattoon G, et al. Attachment, the reflective self, and borderline states: The predictive specificity of the Adult Attachment Interview and pathological emotional development. In: Goldberg S, Muir R, Kerr J, editors. Attachment theory: Social, developmental, and clinical perspectives. Hillsdale, NJ: The Analytic Press; 1995. pp. 217–233. [Google Scholar]
- Frewen PA, Lanius RA. Neurobiology of dissociation: unity and disunity in mind-body-brain. Psychiatric Clinics of North America. 2006a;29:113–128. doi: 10.1016/j.psc.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences. 2006b;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- George C, Kaplan N, Main M. Adult Attachment Interview. Department of Psychology, University of California at Berkeley; 1985. [Google Scholar]
- Goldberg S, Benoit D, Blokland K, Madigan S. Atypical maternal behavior, maternal representations, and infant disorganized attachment. Development and Psychopathology. 2003;15:239–257. doi: 10.1017/s0954579403000130. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Harari D, Bakermans-Kranenburg MJ, de Kloet CS, Geuze E, Vermetten E, Westenberg HG, et al. Attachment representations in Dutch veterans with and without deployment-related PTSD. Attach Hum Dev. 2009;11:515–536. doi: 10.1080/14616730903282480. [DOI] [PubMed] [Google Scholar]
- Hesse E, van Ijzendoorn MH. Propensities towards absorption are related to lapses in the monitoring of reasoning or discourse during the adult attachment interview. A preliminary investigation. Attach Hum Dev. 1999;1:67–91. doi: 10.1080/14616739900134031. [DOI] [PubMed] [Google Scholar]
- Hesse E, Main M. Second-generation effects of unresolved trauma as observed in non-maltreating parents: Dissociated, frightened, and threatening parental behavior. Psychoanalytic Inquiry. 1999;19:481–540. [Google Scholar]
- Hyler SE, Skodol AE, Oldham JM, Kellman HD, Doidge N. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Comprehensive Psychiatry. 1992;33:73–77. doi: 10.1016/0010-440x(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Landa S, Duschinsky R. Crittenden’s Dynamic-Maturational Model of Attachment and Adaptation. Review of General Psychology. 2013;17:326–338. [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini M, Harrison T, Haxby J. Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56:225–257. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Lenzi GL, Ammaniti M. Attachment models affect brain responses in areas related to emotions and empathy in nulliparous women. Human Brain Mapping. 2013;34:1399–1414. doi: 10.1002/hbm.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Jacobvitz D. Attachment disorganization: genetic factors, parenting contexts, and developmental transformation from infancy to adulthood. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. 2. New York, NY: Guilford Press; 2008. pp. 666–697. [Google Scholar]
- Lyons-Ruth K, Bronfman E, Parsons E. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monographs of the Society for Research in Child Development. 1999;64:67–96. doi: 10.1111/1540-5834.00034. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Yellin C, Melnick S, Atwood G. Expanding the concept of unresolved mental states: hostile/helpless states of mind on the Adult Attachment Interview are associated with disrupted mother-infant communication and infant disorganization. Development and Psychopathology. 2005;17:1–23. doi: 10.1017/s0954579405050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Moran G, Pederson DR, Benoit D. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: a review and meta-analysis of a transmission gap. Attach Hum Dev. 2006;8:89–111. doi: 10.1080/14616730600774458. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neuroscience and Biobehavioral Reviews. 2005;29:51–57. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neurosciences. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. 2. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- Main M, Goldwyn R, Hesse E. Adult attachment scoring and classification systems. Department of Psychology, University of California at Berkeley; 2003. [Google Scholar]
- Markowitsch H, Staniloiu A. Amygdala in action: relaying biological and social significance to autobiographical memory. Neuropsychologia. 2011;49:718–751. doi: 10.1016/j.neuropsychologia.2010.10.007. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain NL, Booth-LaForce C. Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. Journal of Family Psychology. 2006;20:247–255. doi: 10.1037/0893-3200.20.2.247. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. Re-valuing the amygdala. Current Opinion in Neurobiology. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis ER, Vanderlinden J, Spinhoven P. Animal defensive reactions as a model for trauma-induced dissociative reactions. Journal of Traumatic Stress. 1998;11:243–260. doi: 10.1023/A:1024447003022. [DOI] [PubMed] [Google Scholar]
- Numan M, Bress J, Ranker L, Gary A, Denicola A, Bettis J, et al. The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behavioural Brain Research. 2010;214:368–444. doi: 10.1016/j.bbr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel K, Mitchell R, Deakin J, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–1834. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, van IMH, Out D, Rombouts SA. Attachment in the brain: adult attachment representations predict amygdala and behavioral responses to infant crying. Attach Hum Dev. 2012;14:533–551. doi: 10.1080/14616734.2012.727252. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatrica Supplement. 1994;397:3–11. doi: 10.1111/j.1651-2227.1994.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld A, Lieberman J, Jarskog L. Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophrenia Bulletin. 2011;37:1077–1164. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. Journal of Comparative and Physiological Psychology. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–319. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schuengel C, Bakermans-Kranenburg MJ, Van IMH. Frightening maternal behavior linking unresolved loss and disorganized infant attachment. Journal of Consulting and Clinical Psychology. 1999;67:54–63. doi: 10.1037//0022-006x.67.1.54. [DOI] [PubMed] [Google Scholar]
- Shah PE, Strathearn L. Similarities and differences of the ABCD model and the DMM classification systems for attachment: A practitioner’s guide. In: Holmes P, Farnfield S, editors. The guidebook to attachment theory and interventions. London, UK: Routledge; 2014. [Google Scholar]
- Shah PE, Fonagy P, Strathearn L. Is attachment transmitted across generations? The plot thickens. Clin Child Psychol Psychiatry. 2010;15:329–345. doi: 10.1177/1359104510365449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J, George C. Disorganization of maternal caregiving across two generations: The origins of caregiving helplessness. In: Solomon J, George C, editors. Disorganized attachment and caregiving. New York, NY: Guilford Press; 2011. pp. 25–51. [Google Scholar]
- Strathearn L, Kim S. Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Front Neurosci. 2013;7:176. doi: 10.3389/fnins.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, D’Amato FR. Anxiety in the pathogenesis of primate infant abuse: a pharmacological study. Psychopharmacology. 1991;103:571–572. doi: 10.1007/BF02244261. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychological Bulletin. 1995;117:387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Lanius RA. Biological and clinical framework for posttraumatic stress disorder. Handb Clin Neurol. 2012;106:291–342. doi: 10.1016/B978-0-444-52002-9.00018-8. [DOI] [PubMed] [Google Scholar]
- Williams L, Kemp A, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–404. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]


