Significance
Specifying which cells in the body will become neural precursor (NP) cells is a core process in nervous system development. Here, a computational method is used to identify NP-specific regulatory sequences near NP-expressed genes. The recovered enhancers proved to be distinct from others previously identified, indicating that the associated genes are served by two separate NP-specific modules. For the neuralized gene, it is shown that each individual NP enhancer directs sufficient expression to permit normal development, while deletion of both enhancers causes a major failure of neuralized function. Finally, this study experimentally validates a proposed regulatory “code” (combination of transcription factor binding sites) for NP-specific gene expression. These findings provide new insights into the mechanistic basis of NP fate specification.
Keywords: neural precursors, dual enhancer modules, cis-regulatory code, proneural proteins, bHLH repressors
Abstract
Transcriptional cis-regulatory modules (CRMs), or enhancers, are responsible for directing gene expression in specific territories and cell types during development. In some instances, the same gene may be served by two or more enhancers with similar specificities. Here we show that the utilization of dual, or “shadow”, enhancers is a common feature of genes that are active specifically in neural precursor (NP) cells in Drosophila. By genome-wide computational discovery of statistically significant clusters of binding motifs for both proneural activator (P) proteins and basic helix–loop–helix (bHLH) repressor (R) factors (a “P+R” regulatory code), we have identified NP-specific enhancer modules associated with multiple genes expressed in this cell type. These CRMs are distinct from those previously identified for the corresponding gene, establishing the existence of a dual-enhancer arrangement in which both modules reside close to the gene they serve. Using wild-type and mutant reporter gene constructs in vivo, we show that P sites in these modules mediate activation by proneural factors in “proneural cluster” territories, whereas R sites mediate repression by bHLH repressors, which serves to restrict expression specifically to NP cells. To our knowledge, our results identify the first direct targets of these bHLH repressors. Finally, using genomic rescue constructs for neuralized (neur), we demonstrate that each of the gene's two NP-specific enhancers is sufficient to rescue neur function in the lateral inhibition process by which adult sensory organ precursor (SOP) cells are specified, but that deletion of both enhancers results in failure of this event.
Specification of neural precursor (NP) cell fates is a core step in the process of neural development, and there has long been intense interest in its mechanistic basis. Among the most heavily investigated questions in this arena is how NP-specific expression of key regulatory factors associated with the NP fate is achieved. For example, multiple prior studies have used computational methods to identify NP-specific cis-regulatory modules (CRMs) genome-wide, in an attempt to define common transcription factor inputs that might underlie NP-specific gene expression (1–3).
Proneural transcriptional activators of the basic helix–loop–helix (bHLH) family are the top-level regulators of NP specification. They confer on cells in ectodermal tissue the potential to adopt the NP fate; loss of proneural gene function results in loss of all NPs and thus loss of expression of all NP-specific regulatory factors. However, proneural factors are not expressed only in NPs; rather, they are initially expressed in small groups of cells called proneural clusters (PNCs). At this stage of the process, most or all cells in the cluster have the potential to become an NP. This is prevented by “lateral inhibition”, in which the single NP that will ultimately arise from the PNC inhibits all other cells in the cluster from adopting this fate by signaling to them via the Notch pathway. This signaling event transcriptionally activates, specifically in the “non-NP” cells, genes that encode inhibitors of the NP fate.
Among the key targets of Notch pathway activation in the inhibited (“non-NP”) cells of the PNC are genes that encode bHLH transcriptional repressors of the Hairy/Enhancer of split (Hes) class (4–6). The Hes factors are required for successful lateral inhibition, and are ideal candidates for a role in direct repression of regulatory genes associated with the NP fate. Thus, a plausible scheme for achieving NP-specific expression of a gene is as follows: The gene would be directly activated by the proneural proteins, and directly repressed by the Hes factors (3, 7, 8). Although proneural activation of the gene would likely occur throughout the PNC (9), the Hes repressors would selectively inhibit expression in the “non-NP” cells (10, 11), with the result that the gene would be functionally active only in the NP.
Here we have used this scenario as the basis of a computational approach to identifying, in the Drosophila genome, NP-specific enhancer modules associated with NP-expressed genes (12). We searched for statistically significant motif clusters that include predicted binding sites for both proneural proteins (P) and Hes repressors (R), and successfully detected “P+R” enhancers in the vicinity of multiple genes with NP-specific expression. Significantly, these modules are distinct from others previously identified near the same genes, indicating that NP genes are often served by at least two enhancers with overlapping specificity; i.e., “shadow enhancers” (13, 14). In the case of the neuralized (neur) gene, which is expressed specifically in NPs and is required for the lateral inhibition process, we show that each of its two NP enhancers drives sufficient expression in sensory organ precursor cells to support normal development. However, deletion of both modules leads to a major failure of lateral inhibition. Finally, by mutational analysis with reporter transgenes, we have validated the in vivo functionality of the proposed “P+R” cis-regulatory code, thus defining, to our knowledge, the first direct targets of the Hes repressors in PNCs. Our results provide new insights into the mechanistic basis of NP fate specification.
Results
Identification of an Intronic SOP Enhancer in neur.
neur encodes an E3 ubiquitin ligase that monoubiquitinates the intracellular domain of the Notch ligand Delta (Dl), a step required to activate Dl's capacity to send an inhibitory signal from the NP (15). Thus, loss of neur function results in a failure of lateral inhibition.
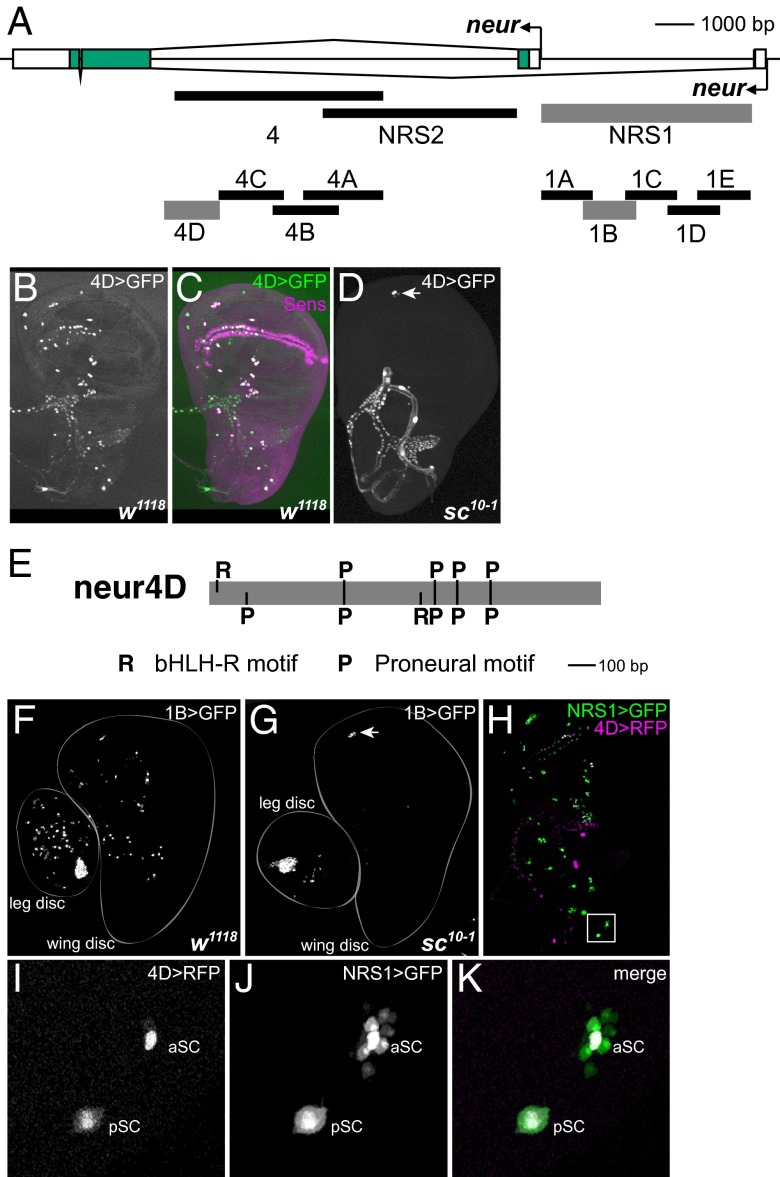
In preliminary studies, we found that a 5.4-kb EcoRI fragment located at the 3′ end of neur’s large first intron (“4”; Fig. 1A) drives reporter transgene expression in the SOPs that give rise to adult mechanosensory bristle organs. Subsequent experiments using smaller intron segments in the region identified a 1.4-kb fragment (“4D”; Fig. 1A) that fully recapitulates this SOP enhancer activity (Fig. 1 B and C). As expected, reporter gene expression driven by the 4D fragment is lost in the sc10-1 genetic background, which eliminates the function of the achaete (ac) and scute (sc) proneural genes, leading to SOP loss (Fig. 1D).
Fig. 1.
neur has two separate enhancer modules with SOP specificity. (A) Diagram of the neur locus, showing genomic DNA fragments tested for enhancer activity. Three fragments for which the activity patterns are shown in this figure (4D, 1B, NRS1) are represented by thicker gray lines. Scale is shown at upper right. (B and C) The 4D enhancer fragment drives GFP reporter expression (green) specifically in SOPs of the wing imaginal disc, marked by anti-Sens antibody (magenta); this fragment overlaps enhancer fragments described by Aerts et al. (1) and Rouault et al. (3). (D) SOP activity of the 4D enhancer is lost in the sc10-1 background, which is mutant for the proneural genes ac and sc. The exception is in the ventral radius (arrowhead), where SOPs for chordotonal organs are specified by the proneural gene atonal (ato). (E) The 4D enhancer fragment includes clustered binding sites for both proneural (P; RCAGSTG) and bHLH repressor (R; GGCACGYGHY, allowing a one-base mismatch at any of the four positions flanking the core hexamer) proteins (Fig. S1). Scale is shown at lower right. (F) The 1B enhancer fragment is active in SOPs of the leg and wing imaginal discs. (G) 1B reporter expression is abolished in the sc10-1 background, again with the exception of the ato-dependent positions (arrowhead). (H–K) The 4D (red) and 1B (green) enhancers (the latter is contained within the NRS1 fragment; A) have overlapping specificities (white in K). 4D is more strongly SOP-specific, whereas 1B (NRS1) also exhibits weaker expression in a subset of surrounding non-SOP cells. aSC, pSC: anterior scutellar and posterior scutellar macrochaete positions.
Analysis of transcription factor binding motifs within the 4D fragment revealed the presence of high-affinity sites for both proneural proteins (P) and bHLH repressor proteins (R) (Fig. 1E). The functionality of these motifs is suggested by their nearly complete conservation in 12 different Drosophila species (Fig. S1).
The neur 4D Enhancer Is Not Required for Lateral Inhibition During Sensory Bristle Development.
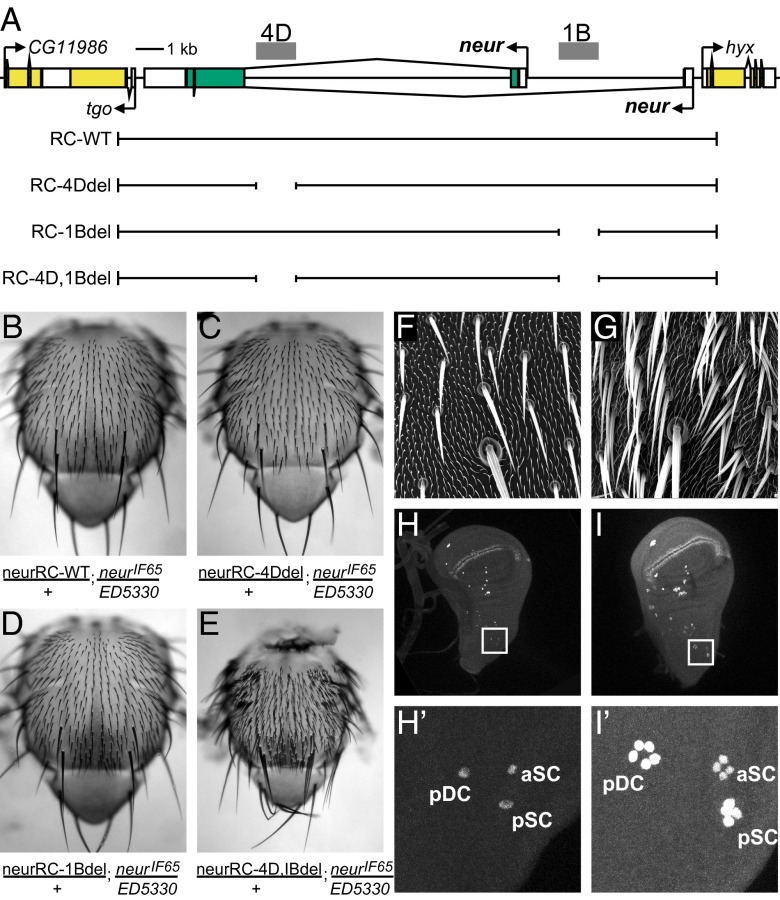
If the 4D enhancer is indeed responsible for the specific expression of neur in mechanosensory organ SOPs, then removing it should lead to loss of neur function during the lateral inhibition process by which individual SOPs are specified. To test this prediction, we compared the adult phenotypes of neur null flies bearing one copy of either a wild-type neur genomic DNA rescue construct, or a mutant construct in which the 4D enhancer had been deleted (Fig. 2 A–C). We saw no significant difference in the capacity of the two constructs to rescue the lethality associated with the neur mutant genotype. Surprisingly, both constructs also yielded adult flies with apparently normal patterns of adult mechanosensory bristles, indicating full rescue of neur function in lateral inhibition (Fig. 2 B and C). Thus, despite its capacity to direct robust reporter gene expression in bristle SOPs, the neur 4D enhancer is not strictly required for neur activity in these cells.
Fig. 2.
The neur 4D and 1B SOP enhancers have overlapping functions. (A) Diagram of neur and flanking loci, showing genomic DNA fragments used in rescue transgene experiments. Scale is shown. (B–E) All four transgenes (A) can rescue the lethality and other mutant phenotypes associated with the neurIF65/Df(3R)ED5330 genotype, except the double-deletion construct neurRC-4D,1Bdel, which yields a strong “bristle tufting” phenotype characteristic of a failure of Notch-mediated lateral inhibition. (F and G) Scanning electron micrographs showing macrochaete and microchaete bristles on the thorax of neurIF65/Df(3R)ED5330 flies bearing the wild-type (neurRC-WT; F) or double-deletion (neurRC-4D,1Bdel; G) rescue transgenes. Note extensive “bristle tufting” phenotype in G. (H and I) The “bristle tufting” phenotype conferred by rescue with the double-deletion transgene (E and G) is due to ectopic SOPs (marked by anti-Sens antibody) arising within PNCs, indicating a failure of lateral inhibition. The number of SOPs per cluster observed here (H' and I') is comparable to that seen in clones homozygous for the neur1 allele (45), which bears a point mutation that abolishes Neur binding to Dl (46). pDC, aSC, pSC: posterior dorsocentral, anterior scutellar, and posterior scutellar macrochaete positions.
neur Has a Second SOP Enhancer.
The foregoing result suggested that the neur gene might be served by another SOP enhancer with overlapping function. Accordingly, we surveyed the remaining noncoding sequences within the neur locus for such an enhancer activity. We found that the fragment NRS1, which encompasses the region between the two neur promoters (Fig. 1A), does include sequences capable of driving SOP-specific expression (Fig. 1 F and H). As expected, this expression strictly requires proneural gene activity (Fig. 1G). That the specificities of the 4D and NRS1 enhancer fragments (Fig. 1A) overlap strongly in SOPs is directly demonstrated by comparing in the same animal the expression of 4D-RFP and NRS1-GFP reporter constructs (Fig. 1 H–K). Interestingly, whereas the 4D enhancer is quite tightly SOP-specific, the NRS1 fragment shows not only strong activity in SOPs but also weaker activity in a small number of surrounding non-SOP cells.
Functional Overlap Between the Two neur SOP Enhancers.
The successful identification of a second SOP enhancer associated with the neur gene prompted us to extend our phenotypic rescue experiments to compare the functional requirements for the two modules. Using additional reporter gene constructs containing smaller genomic DNA fragments, we first refined the localization of the NRS1 enhancer activity primarily to the “1B” region shown in Fig. 1A. We next observed that, as with the 4D enhancer, the 1B enhancer is not individually required for neur gene function in lateral inhibition, because a construct lacking this module can rescue a normal pattern of adult mechanosensory bristles (Fig. 2 A, B, and D). However, deletion of both the 4D and 1B enhancers results in a striking “bristle tufting” phenotype, in which multiple bristles appear at each position instead of the individual, well-spaced bristles that comprise the wild-type pattern (Fig. 2 B and E–G). As we have shown previously (16, 17), this is the classic manifestation of a failure of Notch-mediated lateral inhibition during adult sensory organ development: Multiple cells in the PNC, rather than only one, adopt the SOP fate and then execute the normal lineage divisions to give rise to multiple complete bristles that are immediately adjacent (Fig. 2 F and G). We verified this interpretation by staining wing imaginal discs from third-instar larvae bearing either the wild-type (neurRC-WT) or double enhancer deletion (neurRC-4D,1Bdel) rescue constructs with anti-Senseless (Sens) antibody as a specific marker for the SOP cell fate (Fig. 2 H and I). Whereas neurRC-WT discs display single SOPs at each macrochaete bristle position (Fig. 2H′), neurRC-4D,1Bdel discs show multiple adjacent Sens-positive cells at each position (Fig. 2I′), consistent with the respective adult phenotypes (Fig. 2 B and E–G). We conclude that the neur gene is served by two distinct enhancer modules with SOP specificity, and that each is substantially sufficient to drive functional levels of neur activity in imaginal disc SOPs.
Computational Identification of Motif Clusters Representing Binding Sites for Proneural Activator and Hes Repressor Proteins.
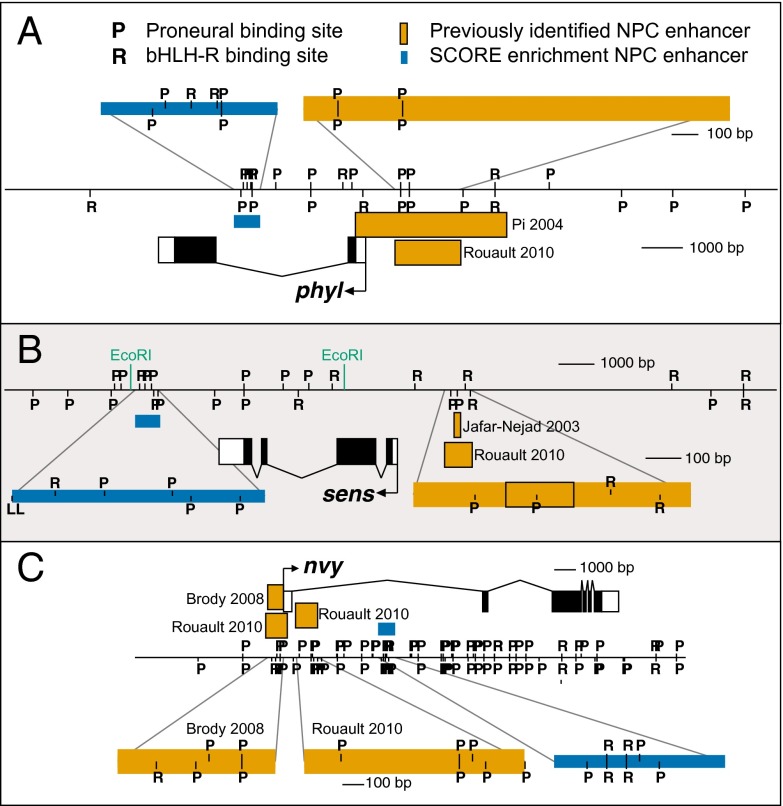
Our finding that neur expression in SOPs depends on the action of two separate CRMs prompted us to investigate whether NP-specific expression of other genes might likewise make use of a dual-enhancer mechanism. Computational methods have already been used successfully to identify NP enhancers genome-wide (1–3); we therefore sought a strategy distinct from those of the previous efforts. The presence of multiple conserved binding sites for proneural bHLH activator proteins (P) and Hes-class bHLH repressor proteins (R) in the neur 4D SOP enhancer (Fig. 1E and Fig. S1) suggested that additional SOP-specific [or, more generally, neural precursor (NP)-specific] enhancer modules might be identifiable on this basis. Accordingly, we used the SCORE computational method (12) to survey the Drosophila genome for statistically significant coclustering of P and R motifs. We detected such clusters in association with several known NP-expressed genes, including phyllopod (phyl), senseless (sens), and nervy (nvy) (Fig. 3 A–C and Table 1). We were interested to find that, for these three genes, the P+R motif cluster is distinct from a previously identified NP enhancer or enhancers (2, 3, 18, 19).
Fig. 3.
P+R motif clusters identified by SCORE analysis (Table 1). (A–C) Diagrams of the phyllopod (phyl) (A), senseless (sens) (B), and nervy (nvy) (C) genes and flanking sequences. Scale is shown at right in each panel. Binding motifs for Ac/Sc proneural proteins (RCAGSTG) and for Hes-class bHLH repressor proteins (CACGYG) are indicated by P and R, respectively. Orange boxes denote previously identified NP enhancer fragments (2, 3, 18, 19). Blue boxes represent fragments containing SCORE-identified P+R motif clusters. Enhancer fragments are also shown enlarged (scale indicated) to clarify motif composition. (B) EcoRI sites mark the boundaries of a 5.9-kb fragment shown previously to drive weak expression in the embryonic PNS (18); L represents Sens binding motifs (AAATCWSW; named after Lyra, the original name of the sens locus) included in the SCORE P+R fragment.
Table 1.
SCORE-identified P+R motif clusters associated with NP-expressed genes
| Gene | Location | SCORE Bin | P Value |
| phyllopod (phyl) | Intron | 3P+2R in <350 bp | 0.001 |
| senseless (sens) | Downstream | 3P+1R in <400 bp | 0.003 |
| nervy (nvy) | Intron 1 | 3P+2R in <350 bp | 0.001 |
In the case of the phyl cluster, we verified that the identified P and R motifs do indeed mediate sequence-specific binding by the corresponding transcription factors in vitro (Fig. S2).
P+R Motif Clusters Represent Functional NP Enhancer Modules.
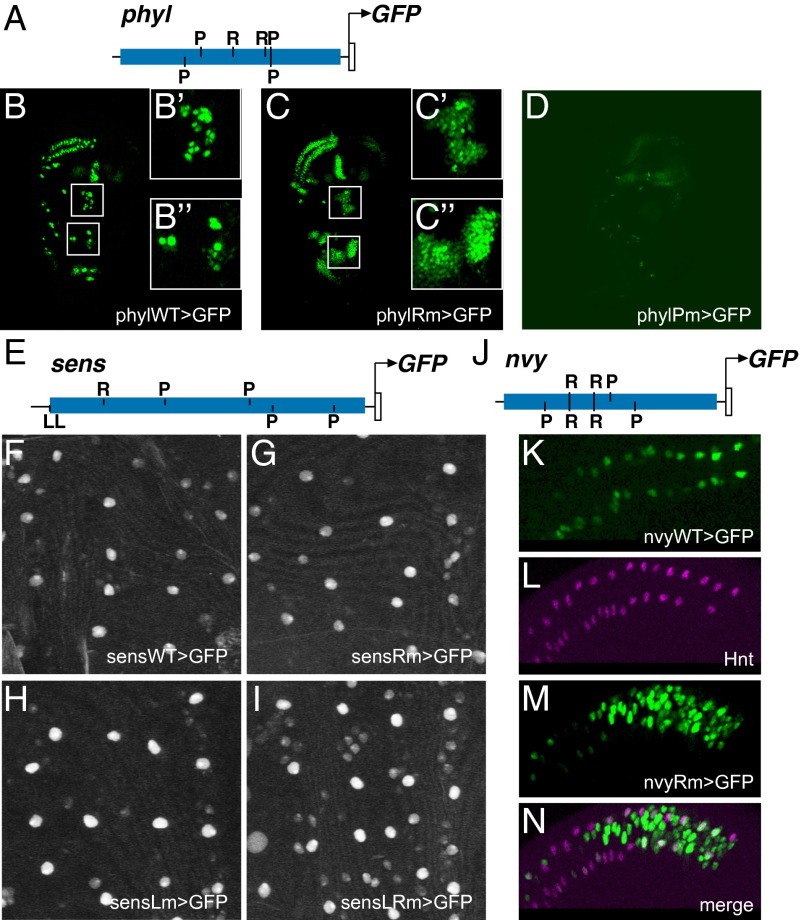
To determine whether the P+R motif clusters identified by the SCORE technique do in fact represent NP-specific CRMs, we tested the activities of genomic DNA fragments bearing these clusters in the context of GFP reporter genes. We found that a 662-bp fragment that includes the intronic P+R cluster from phyl (Figs. 3A and 4A) drives a clear SOP expression pattern in the wing imaginal disc (phylWT-GFP; Fig. 4B). It is noteworthy that this GFP reporter pattern recapitulates with high fidelity the pattern of transcript accumulation from the endogenous phyl gene, in that it includes weak expression in surrounding non-SOP cells in addition to the principal SOP specificity (Fig. 4 B′ and B″) (20). Similarly, a 680-bp fragment from the P+R cluster downstream of sens, including an R site and four P sites (Figs. 3B and 4E), drives microchaete SOP-specific expression in the pupal notum (sensWT-GFP; Fig. 4F and Fig. S3A). Lastly, a 782-bp fragment from the first intron of nvy, containing three P and two R motifs (Figs. 3C and 4J), is active specifically in chemosensory organ SOPs in the margin territory of the third-instar wing disc (nvyWT-GFP; Fig. 4K).
Fig. 4.
Implementation of a “P+R” cis-regulatory code by SOP enhancers. (A) Diagram of the phyl P+R enhancer fragment (Fig. 3A). Motif symbols as in Fig. 3. (B) The wild-type phyl module (phylWT) drives strong GFP reporter expression in wing imaginal disc SOPs, along with weaker activity in a subset of surrounding PNC cells (B' and B''). This overlaps the activity pattern of a previously identified CRM upstream of the gene (19) (Fig. 3A). (C) Mutation of the two R sites in this module (phylRm) results in extensive expansion of reporter activity into the whole of the PNC (C' and C''). (D) Mutation of the three P sites in this module (phylPm) severely reduces, but does not abolish, reporter activity in SOPs. (E) Diagram of the sens P+R enhancer fragment (Fig. 3B). Motif symbols as in Fig. 3. (F–I) The sens module is subject to combinatorial repression by bHLH repressors and Sens. Lower-magnification, expanded-field versions of F–I, respectively, are shown in Fig. S3 A–D. (F) The wild-type sens module (sensWT) drives SOP-specific expression in the pupal notum. Anterior is toward the top. This overlaps the activity pattern of a previously identified CRM upstream of the gene (18) (Fig. 3B). (G) The spatial pattern of reporter expression is not detectably changed by mutation of the module's R site (sensRm). (H) Mutation of the module's two Sens binding motifs (sensLm) yields weak derepression in non-SOP cells. (I) Clear expansion of reporter gene expression into non-SOPs is observed upon mutation of both R and L sites (sensLRm). (J) Diagram of the nvy P+R enhancer fragment (Fig. 3C). Motif symbols as in Fig. 3. (K) The wild-type nvy module (nvyWT) drives GFP reporter expression in SOPs of chemosensory organs at the wing margin. (L–N) Mutation of the two R sites in this module (nvyRm) results in strong expansion of reporter activity into surrounding PNC cells (M and N). SOPs are marked by anti-Hindsight (Hnt) antibody staining (L and N).
We conclude that computationally defined P+R motif clusters in the genome can indeed correspond to NP-specific enhancer modules. Moreover, the functionality of these motifs is suggested by their conservation among divergent Drosophila species (Fig. S4).
R Sites in P+R Enhancer Modules Function in Transcriptional Repression.
In the proposed logic of a P+R enhancer module, the R motifs would have the role of mediating repression by bHLH repressor proteins in the non-SOP cells of PNCs. We tested this in the case of the phyl, sens, and nvy CRMs by examining the expression patterns of GFP reporter genes in which the R sites (two, one, and two, respectively) have been mutated. As shown in Fig. 4C, the R-mutant phyl reporter (phylRm-GFP) displays strong derepression within PNCs, becoming active in many cells that do not express the wild-type phyl reporter (compare Fig. 4 B′ and B″ with C′ and C″). The corresponding nvy mutant construct (nvyRM-GFP) likewise exhibits greatly expanded expression in the vicinity of the wing margin chemosensory SOPs (Fig. 4 L–N). The results in these two instances are thus consistent with the proposed function of the R motifs in P+R enhancer modules; loss of input from bHLH repressors leads to ectopic module activity in non-SOP cells. However, we obtained a different result with the R-mutant version of the sens reporter gene. In this case, mutation of the single R motif in the 4P+1R cluster did not lead to a significant alteration of the module's activity, despite the strict conservation of this binding site among Drosophila species (sensRm-GFP; Fig. 4G and Figs. S3B and S4B). Such a result suggests that the sens module receives other repressive inputs that contribute strongly to its SOP specificity. One candidate for this role is Sens itself, which can act as a DNA-binding repressor in non-SOP cells (18) (see Fig. S5 for experimental data on direct corepressor recruitment by Sens). Indeed, we found that one end of the sens module as we have defined it includes two adjacent Sens binding motifs (labeled L in Figs. 3B and 4E). Mutation of these sites in the sens reporter gene yields a slight derepression of its activity in non-SOP cells (sensLm-GFP; Fig. 4H and Fig. S3C). However, mutation of both the R and L sites results in a clear expansion of the GFP expression pattern into non-SOPs; this is particularly evident in the two microchaete rows flanking the dorsal midline (sensLRm-GFP; Fig. 4I and Fig. S3D). That the derepression effect is clearly stronger in the double mutant (LRm) than in either single mutant (Rm or Lm) suggests that bHLH repressors synergize with Sens to restrict sens expression to the SOP.
P Sites in the phyl P+R Intron Enhancer Mediate a Critical Positive Input.
In the “P+R” code model, P binding sites for the proneural bHLH activators are expected to play a critical role in driving enhancer module activity in NP cells. We tested this proposition by mutating all three P sites in the phyl 3P+2R intronic NP enhancer (phylPm-GFP), and observed a very strong reduction of SOP/PNC reporter gene expression in the wing imaginal disc (Fig. 4D). The activity of the module was clearly not eliminated entirely, suggesting that another factor or factors contribute weak positive inputs as well. Such additional factors evidently require proneural gene function for their expression or activity, because expression of the wild-type enhancer-reporter gene (phylWT-GFP), like that of the endogenous phyl gene (20), is lost when it is placed in a sc10-1 mutant background, which eliminates ac and sc activity (Fig. S6).
Discussion
We have shown here that NP-specific expression of the neur gene, a key member of the Notch cell–cell signaling pathway, is directed by at least two separate enhancers with overlapping function. We find that each module is sufficient to support rescue of neur expression and function in the SOPs that give rise to the mechanosensory bristles of the adult thorax, whereas deletion of both modules leads to widespread failure of the neur-dependent lateral inhibition process by which the SOP normally prevents neighboring cells in the PNC from adopting the SOP fate. As with previous instances of apparently functionally “redundant” enhancers, it may be that phenotypic robustness is the principal fitness advantage conferred by neur’s use of dual enhancers (13, 21, 22). Another very plausible advantage of a dual-enhancer strategy is achieving precision in the final gene expression output (23, 24); in this case, for example, a sharp distinction in neur transcript levels in NP versus non-NP cells of the PNC.
That the dual enhancer paradigm may be a common one for controlling NP-specific expression is suggested by our identification of multiple additional instances of NP-expressed genes that are served by at least two separate modules with this specificity. Statistically significant clustering of TF binding sites has proven to be a valuable criterion for the identification of functional CRMs (12, 25). We applied a two-motif version of the SCORE computational technique (12) to recognize several NP-specific enhancers in the fly genome. In the case of three genes (phyl, sens, and nvy), the newly identified NP module is distinct from another one previously reported, establishing the existence of a dual-enhancer configuration.
It is noteworthy that, in contrast to several described examples of “shadow” enhancers (13, 14), the dual-enhancer configurations reported here (in neur, phyl, sens, and nvy) all consist of a pair of NP-specific modules that lie close to the corresponding gene. Moreover, there may be functional significance to the observation that in all four cases one of the enhancers in the pair lies upstream of a promoter, whereas the other lies downstream. This arrangement may, for example, facilitate cooperative (synergistic) interaction of the two modules with the promoter (23), by allowing them to “loop” independently and simultaneously.
Mutational analysis of the phyl and nvy enhancers has provided strong evidence for the in vivo functionality of a “P+R” cis-regulatory code in driving NP-specific gene expression in Drosophila. The logic underlying this simple code is that activation by the proneural factors will generate expression both in the NP (where proneural protein levels are typically highest) and in the surrounding non-NP cells of the PNC, whereas repression by bHLH repressors (which are expressed selectively in the non-NP cells under the control of Notch signaling) (10, 11, 26) will overcome the latter activity and confine the module's output to the NP. As predicted, we did indeed observe a strong expansion of the expression of the phyl and nvy enhancers when their R sites were mutated. Conversely, mutation of the P sites in the phyl enhancer led as expected to a severe reduction in its activity. Thus, our findings validate the in vivo utility of the P+R code for generating NP-specific expression, and define, to our knowledge for the first time, direct targets (phyl and nvy) of bHLH repressor action in the non-NP cells of PNCs.
However, our results also reveal that CRMs using this code can embody additional functional complexity. Thus, for example, loss of direct proneural protein input in the P site-mutant phyl enhancer greatly decreased, but did not eliminate, its activity in wing disc SOPs. This is in contrast to the effect of removing both direct and indirect proneural input in the sc10-1 mutant background, wherein phyl-GFP reporter expression is lost entirely from ac/sc-dependent (but not atonal-dependent) SOP positions. Perhaps the most likely interpretation of these observations is that the phyl module relies on direct positive inputs by both the proneural proteins and at least one other factor, NP expression of which is activated by the proneurals — a coherent feed-forward loop (27). There is also the possibility that this enhancer includes additional unrecognized binding sites for the proneural proteins. Again in contrast to a prediction of the simplest version of the P+R code model, we observed no effect of mutating only the R motif in the sens downstream NP enhancer. We note that a similar result has been reported previously for three other NP-specific enhancers, including the upstream sens module (3). However, in the case of the sens downstream CRM, we were able to identify a second repressive input — Sens itself — that works synergistically with the bHLH repressor input to generate the module's NP specificity. We conclude, then, that some NP enhancers, while exploiting the inherent logic of the P+R code, may integrate additional positive and/or negative inputs in creating their NP output.
Materials and Methods
Fly Strains.
neurIF65 (28), also known as neur11, is an EMS-generated amorphic allele. Df(3R)ED5330 was generated as part of the DrosDel project (29), and removes sequence between the coordinates 3R:4495308–5055517, including the neur locus. The sc10-1 allele is an X-ray-induced deficiency affecting the coding sequences of both ac and sc (30, 31).
Motif Definitions.
Binding sites for Achaete/Scute-class proneural proteins (P sites) were identified by searching with the motif definition RCAGSTG (32). In the SCORE analysis, binding sites for Hes-class bHLH repressor proteins (R sites) were identified by searching with the motif definition CACGYG (32). R sites identified in neur fit the more restricted definition GGCACGYGHY (33), with allowance for a one-base mismatch at any of the four positions flanking the hexamer core. Binding sites for the Senseless (Sens) protein (L sites) were identified by searching with the motif definition AAATCWSW (34).
SCORE Analysis.
Statistically significant clusters of P (proneural protein) + R (bHLH repressor) binding motifs were detected by the SCORE technique (12). Random clustering probabilities (p) were estimated by running 1000 Monte Carlo simulations. See Table 1 for bins and probability values. Bin purities: phyl and nvy, 55%; sens, 25%.
Reporter Constructs.
Wild-type and mutant enhancer-reporter constructs for neur, phyl, and nvy were cloned into pH-Stinger (35) or pH-RedStinger (36). At least three independent transformant lines were analyzed before a representative line was selected for all further analysis. Constructs were injected using standard transformation techniques (37), with w1118 as the recipient strain. sens enhancer-reporter constructs were cloned through a pCR8/GW/TOPO intermediate into pGreenFriend, a variant of pBPGUw (38) in which the GAL4 coding sequence was removed via a FseI/HindIII double digest and replaced with an FseI/HindIII-linkered eGFP coding sequence amplified from pH-Stinger (35). These constructs were integrated into the attP2 docking site (39) using a germ-line ΦC31 integrase source (40). Mutations were generated by overlap extension PCR (41). Factor binding motifs were mutated as follows: P motifs, RCANNTG > RAANNGG; R motifs, CACGYG > CCCTYT; L motifs, AAATCWSW > AGGTCWSW. Fragment boundary primers are shown below:
| neur4D: | CCAAGACCCAAATTTAGTTGGTATTCAAGC |
| AATAGGCCCCAATCCAGTACACGTATGTGC | |
| neur1B: | TCCCAGTTTTGAAACCATTAGCTTACACAG |
| AAAGACAATTGTGAGGCCAGAGGGTAATGC | |
| neurNRS1: | AGCGCGACGTCGCTGACGCAGCTCAGCTGG |
| TGATTTAACATGGGATGTCGCGTAAGGAGG | |
| neurNRS2: | GTAACTCTAGAAGCTTAGCAAGAATGGACG |
| GGCTAAGAATCGGGCTAAGGATATCGATGG | |
| sensDSCD1: | GTGATTTTGAGATTTTTTCTGAGTGC |
| CCACGTACCATCCTCTGAATCGGT | |
| phylSCORE: | TACGCCTATGCACTTAGTGATCGC |
| TTTGCACCTGGCCTGCAATTTGCA | |
| nvySCORE: | ATGCACTACATCCTGCTGGTGG |
| CCCTCGATTCACCACCCTTCATTT |
Rescue Constructs.
neurRC-WT-P[acman] constructs were generated by BACR09F04-mediated gap repair of attB-P[acman]-AmpR via recombineering, as described (42). The region cloned extends to the EagI sites on either side of the neur locus (from sequence CGGCCGCCTCCAGGATAAGATGCT to sequence GATATACCCGCTGTGAATCGGCCG, a 21-kb region). These constructs were subsequently injected into the docking sites attP40 and attP16 (43) by Genetic Services, using the ΦC31 integrase system (40). Mutant variants of this starting construct were generated by recombineering, using galK-mediated selection (44), and injected into the attP40 docking site by Genetic Services.
Immunohistochemistry.
Antibodies used in this study include guinea pig anti-Sens (1:2,000; a gift of Hugo Bellen, Baylor College of Medicine, Waco, TX), mouse anti-Hindsight (1:100; 1G9, Developmental Studies Hybridoma Bank), mouse anti-Pros (1:10; MR1A, DSHB), and rat anti-Elav (1:100; 7E8A10, DSHB). Third-instar larvae were collected and dissected in 1X PBS, fixed [1× PBS, 0.1% Triton X-100 (PBT); 4% (wt/vol) paraformaldehyde] for 30 min at room temperature, and washed five times in PBT for 10 min each. Carcasses were incubated in primary antibody for 1 h at room temperature or 4 °C overnight, followed by washes and similar secondary antibody incubation (diluted 1:1,000 in PBT; goat anti-guinea pig or goat anti-mouse Alexa555, goat anti-mouse Alexa488, or goat anti-rat Alexa647). After final washes, carcasses were placed in 70% glycerol-30% PBT, and imaginal discs were mounted on slides and visualized by confocal microscopy.
Supplementary Material
Acknowledgments
Scott Barolo conducted the initial experiments that demonstrated the presence of an SOP-specific enhancer in the 4D region of neur. Dylan Relyea assisted in experiments examining the phenotypic effects of overexpressing wild-type and mutant variants of Sens. Tammie Stone performed the EMSAs. We thank Hugo Bellen for providing the anti-Sens antibody and Konrad Basler for the gift of pUASTattB. The mouse anti-Hindsight monoclonal antibody was developed by Howard Lipshitz; the mouse anti-Prospero antibody was developed by Chris Doe; the rat anti-Elav antibody was developed by Gerald Rubin; these three were obtained from the Developmental Studies Hybridoma Bank (created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology). J.E.A. is supported by NIH Predoctoral Training Grant 5T32GM007240. This research was supported by NIH Grant R01GM046993 (to J.W.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415308111/-/DCSupplemental.
References
- 1.Aerts S, et al. Robust target gene discovery through transcriptome perturbations and genome-wide enhancer predictions in Drosophila uncovers a regulatory basis for sensory specification. PLoS Biol. 2010;8(7):e1000435. doi: 10.1371/journal.pbio.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody T, et al. Sequence conservation and combinatorial complexity of Drosophila neural precursor cell enhancers. BMC Genomics. 2008;9:371. doi: 10.1186/1471-2164-9-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouault H, Mazouni K, Couturier L, Hakim V, Schweisguth F. Genome-wide identification of cis-regulatory motifs and modules underlying gene coregulation using statistics and phylogeny. Proc Natl Acad Sci USA. 2010;107(33):14615–14620. doi: 10.1073/pnas.1002876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey AM, Posakony JW. Suppressor of Hairless directly activates transcription of Enhancer of split Complex genes in response to Notch receptor activity. Genes Dev. 1995;9(21):2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 5.Jarriault S, et al. Signalling downstream of activated mammalian Notch. Nature. 1995;377(6547):355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 6.Lecourtois M, Schweisguth F. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split Complex genes triggered by Notch signaling. Genes Dev. 1995;9(21):2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 7.Culí J, Modolell J. Proneural gene self-stimulation in neural precursors: An essential mechanism for sense organ development that is regulated by Notch signaling. Genes Dev. 1998;12(13):2036–2047. doi: 10.1101/gad.12.13.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Doren M, Bailey AM, Esnayra J, Ede K, Posakony JW. Negative regulation of proneural gene activity: Hairy is a direct transcriptional repressor of achaete. Genes Dev. 1994;8(22):2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- 9.Singson A, Leviten MW, Bang AG, Hua XH, Posakony JW. Direct downstream targets of proneural activators in the imaginal disc include genes involved in lateral inhibitory signaling. Genes Dev. 1994;8(17):2058–2071. doi: 10.1101/gad.8.17.2058. [DOI] [PubMed] [Google Scholar]
- 10.Jennings B, Preiss A, Delidakis C, Bray S. The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development. 1994;120(12):3537–3548. doi: 10.1242/dev.120.12.3537. [DOI] [PubMed] [Google Scholar]
- 11.Jennings B, de Celis JF, Delidakis C, Preiss A, Bray S. Role of Notch and achaete-scute complex in the expression of Enhancer of split bHLH proteins. Development. 1995;121:3745–3752. [Google Scholar]
- 12.Rebeiz M, Reeves NL, Posakony JW. SCORE: A computational approach to the identification of cis-regulatory modules and target genes in whole-genome sequence data. Site clustering over random expectation. Proc Natl Acad Sci USA. 2002;99(15):9888–9893. doi: 10.1073/pnas.152320899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barolo S. Shadow enhancers: Frequently asked questions about distributed cis-regulatory information and enhancer redundancy. BioEssays. 2012;34(2):135–141. doi: 10.1002/bies.201100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong JW, Hendrix DA, Levine MS. Shadow enhancers as a source of evolutionary novelty. Science. 2008;321(5894):1314. doi: 10.1126/science.1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiúza UM, Arias AM. Cell and molecular biology of Notch. J Endocrinol. 2007;194(3):459–474. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 16.Hartenstein V, Posakony JW. A dual function of the Notch gene in Drosophila sensillum development. Dev Biol. 1990;142(1):13–30. doi: 10.1016/0012-1606(90)90147-b. [DOI] [PubMed] [Google Scholar]
- 17.Leviten MW, Posakony JW. Gain-of-function alleles of Bearded interfere with alternative cell fate decisions in Drosophila adult sensory organ development. Dev Biol. 1996;176(2):264–283. doi: 10.1006/dbio.1996.0133. [DOI] [PubMed] [Google Scholar]
- 18.Jafar-Nejad H, et al. Senseless acts as a binary switch during sensory organ precursor selection. Genes Dev. 2003;17(23):2966–2978. doi: 10.1101/gad.1122403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi H, Huang SK, Tang CY, Sun YH, Chien CT. phyllopod is a target gene of proneural proteins in Drosophila external sensory organ development. Proc Natl Acad Sci USA. 2004;101(22):8378–8383. doi: 10.1073/pnas.0306010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves N, Posakony JW. Genetic programs activated by proneural proteins in the developing Drosophila PNS. Dev Cell. 2005;8(3):413–425. doi: 10.1016/j.devcel.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Frankel N, et al. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature. 2010;466(7305):490–493. doi: 10.1038/nature09158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry MW, Boettiger AN, Bothma JP, Levine M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol. 2010;20(17):1562–1567. doi: 10.1016/j.cub.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry MW, Boettiger AN, Levine M. Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc Natl Acad Sci USA. 2011;108(33):13570–13575. doi: 10.1073/pnas.1109873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry MW, Bothma JP, Luu RD, Levine M. Precision of Hunchback expression in the Drosophila embryo. Curr Biol. 2012;22(23):2247–2252. doi: 10.1016/j.cub.2012.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berman BP, et al. Exploiting transcription factor binding site clustering to identify cis-regulatory modules involved in pattern formation in the Drosophila genome. Proc Natl Acad Sci USA. 2002;99(2):757–762. doi: 10.1073/pnas.231608898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro B, Barolo S, Bailey AM, Posakony JW. Lateral inhibition in proneural clusters: Cis-regulatory logic and default repression by Suppressor of Hairless. Development. 2005;132(15):3333–3344. doi: 10.1242/dev.01920. [DOI] [PubMed] [Google Scholar]
- 27.Alon U. Network motifs: Theory and experimental approaches. Nat Rev Genet. 2007;8(6):450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann R, Jiménez F, Dietrich U, Campos-Ortega JA. On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Rouxs Arch Dev Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- 29.Ryder E, et al. The DrosDel collection: A set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics. 2004;167(2):797–813. doi: 10.1534/genetics.104.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez I, Hernández R, Modolell J, Ruiz-Gómez M. Competence to develop sensory organs is temporally and spatially regulated in Drosophila epidermal primordia. EMBO J. 1990;9(11):3583–3592. doi: 10.1002/j.1460-2075.1990.tb07569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villares R, Cabrera CV. The achaete-scute gene complex of D. melanogaster: Conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- 32.Nellesen DT, Lai EC, Posakony JW. Discrete enhancer elements mediate selective responsiveness of Enhancer of split Complex genes to common transcriptional activators. Dev Biol. 1999;213(1):33–53. doi: 10.1006/dbio.1999.9324. [DOI] [PubMed] [Google Scholar]
- 33.Rebeiz M, Stone T, Posakony JW. An ancient transcriptional regulatory linkage. Dev Biol. 2005;281(2):299–308. doi: 10.1016/j.ydbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Zweidler-Mckay PA, Grimes HL, Flubacher MM, Tsichlis PN. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol Cell Biol. 1996;16(8):4024–4034. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barolo S, Carver LA, Posakony JW. GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29(4):726–732, 728, 730, 732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- 36.Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: Insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36(3):436–440, 442. doi: 10.2144/04363ST03. [DOI] [PubMed] [Google Scholar]
- 37.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA. 2008;105(28):9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166(4):1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104(9):3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 42.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314(5806):1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 43.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet. 2008;40(4):476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33(4):e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitsouli C, Delidakis C. The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development. 2005;132(18):4041–4050. doi: 10.1242/dev.01979. [DOI] [PubMed] [Google Scholar]
- 46.Commisso C, Boulianne GL. The NHR1 domain of Neuralized binds Delta and mediates Delta trafficking and Notch signaling. Mol Biol Cell. 2007;18(1):1–13. doi: 10.1091/mbc.E06-08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.