Abstract
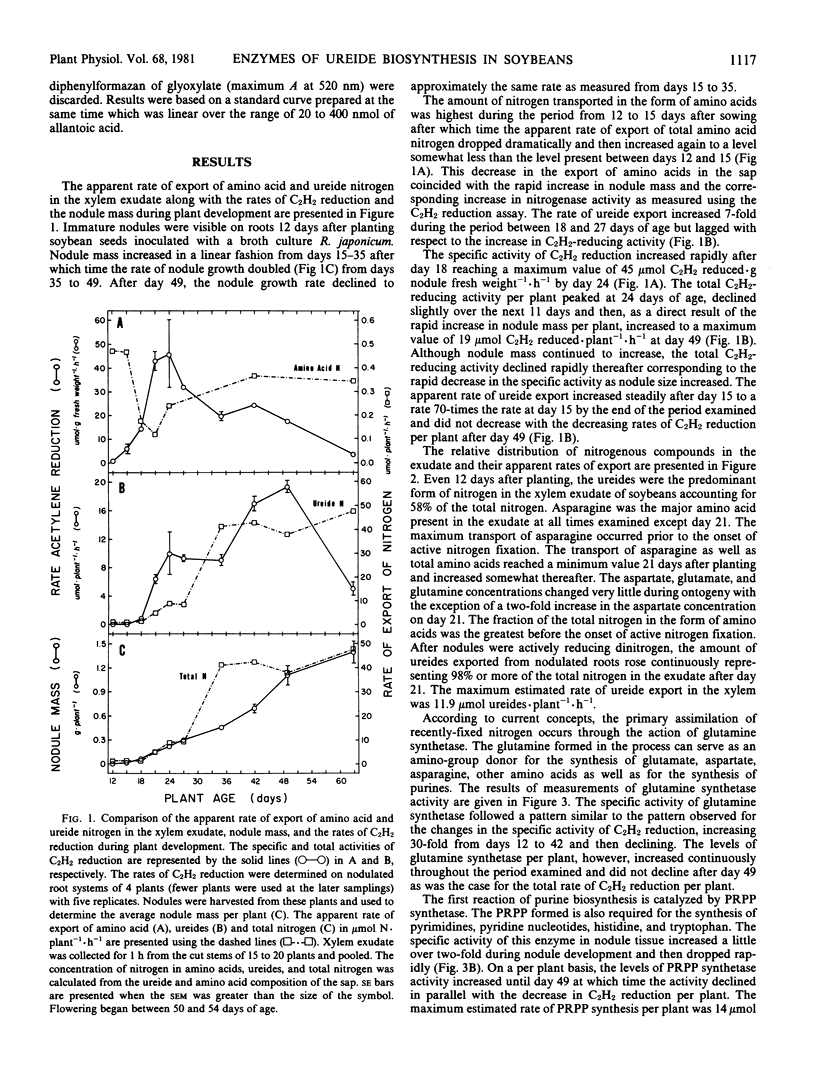
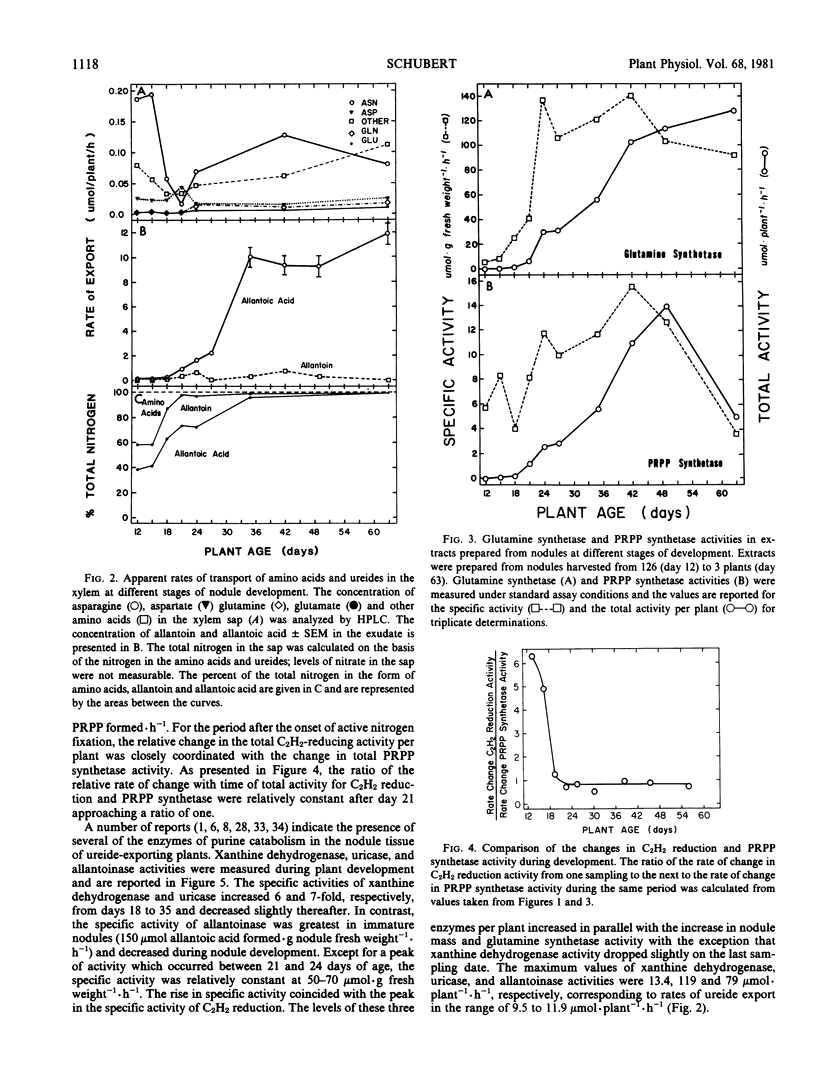
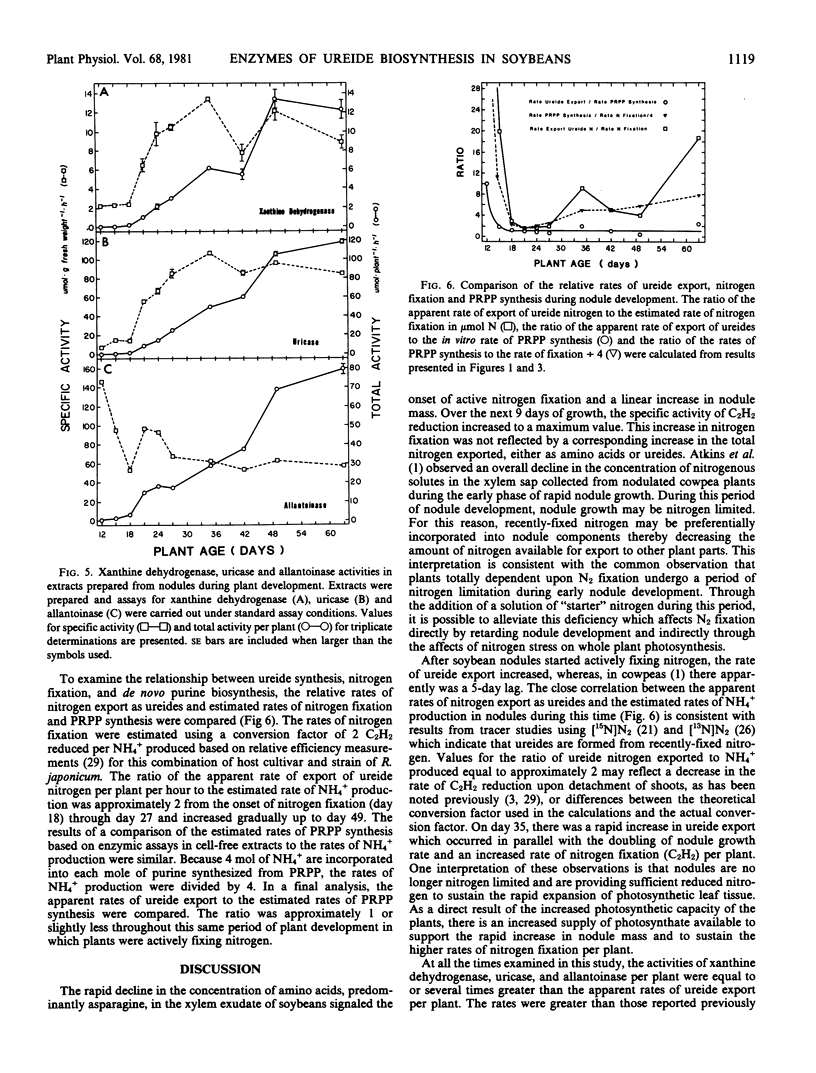
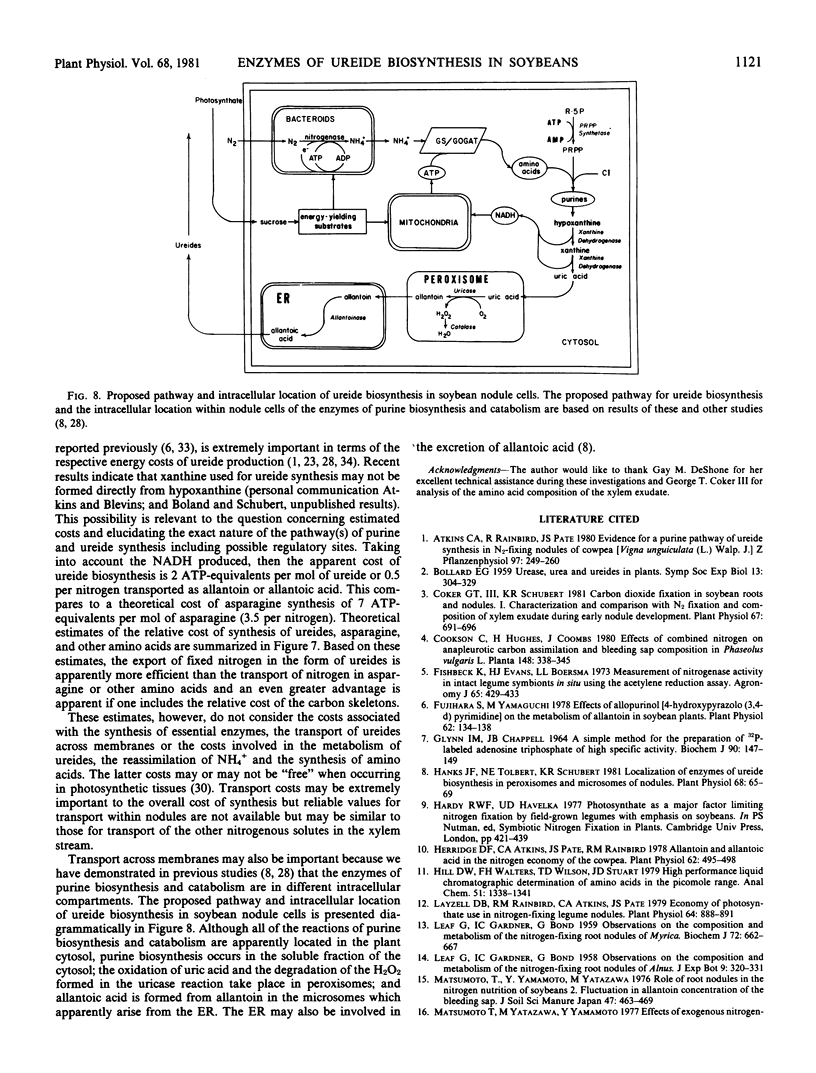
During the period examined from 12 to 63 days after planting, the ureides, allantoin and allantoic acid, were the predominant nitrogenous solutes in the xylem exudate of soybeans (Glycine max [L.]) growing solely on symbiotically fixed nitrogen, accounting for approximately 60% and greater than 95% of the total nitrogen in the xylem exudate before and after the onset of active nitrogen fixation, respectively. For plants between 18 and 49 days of age, the apparent rate of ureide export estimated from concentrations of ureides in xylem exudate collected over a period of one hour was closely related to the rate of nitrogen fixation estimated from measurements of C2H2 reduction by nodulated root systems. After this time, the apparent rate of ureide export per plant continued to increase, reaching a maximum value at day 63 of 12 micromoles per plant per hour, even though the rate of C2H2 reduction per plant declined approximately four-fold. The most probable pathway for the biosynthesis of ureides involves the catabolism of purines. The levels of phosphoribosylpyrophosphate (PRPP) synthetase, which catalyzes the formation of the PRPP required for purine synthesis, increased in parallel with the rates of nitrogen fixation (C2H2) from day 18 reaching a maximum value of 13.9 micromoles per plant per hour at day 49, and then both activities declined rapidly. During the period of active nitrogen fixation the ratio of PRPP synthesis estimated from measurements of PRPP synthetase activity in cell-free extracts to the apparent rate of ureide export was between 1 and 2. The activities of the enzymes of purine catabolism, xanthine dehydrogenase, uricase, and allantoinase, increased in parallel with the increases in nodule mass and the export of ureides with maximum activities of 13, 119, and 79 micromoles per plant per hour, corresponding with apparent rates of ureide export in the range of 9.5 to 11.9 micromoles per plant per hour. These results demonstrate that there is a close association between nitrogen fixation, PRPP synthetase activity, and ureide export in soybeans and support the proposal that recently-fixed nitrogen is utilized in the de novo synthesis of purines which are subsequently catabolized to produce the ureides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S., Yamaguchi M. Effects of Allopurinol [4-Hydroxypyrazolo(3,4-d)Pyrimidine] on the Metabolism of Allantoin in Soybean Plants. Plant Physiol. 1978 Jul;62(1):134–138. doi: 10.1104/pp.62.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- LEAF G., GARDNER I. C., BOND G. Observations on the composition and metabolism of the nitrogen-fixing root nodules of Myrica. Biochem J. 1959 Aug;72:662–667. doi: 10.1042/bj0720662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil D. L., Atkins C. A., Pate J. S. Uptake and Utilization of Xylem-borne Amino Compounds by Shoot Organs of a Legume. Plant Physiol. 1979 Jun;63(6):1076–1081. doi: 10.1104/pp.63.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Coker G. T. Ammonia Assimilation in Alnus glutinosa and Glycine max: SHORT-TERM STUDIES USING [N]AMMONIUM. Plant Physiol. 1981 Apr;67(4):662–665. doi: 10.1104/pp.67.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Engelke J. A., Russell S. A., Evans H. J. Hydrogen reactions of nodulated leguminous plants: I. Effect of rhizobial strain and plant age. Plant Physiol. 1977 Nov;60(5):651–654. doi: 10.1104/pp.60.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem. 1969 Jun 10;244(11):2854–2863. [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]


