Significance
Endocytic sorting of amyloid precursor protein (APP) governed by the vacuolar protein sorting (Vps10) family of receptors plays a decisive role in controlling the outcome of APP proteolytic processing and the generation of amyloid β (Aβ) peptides, the key pathogenic factor of Alzheimer's disease (AD). This study provides the first evidence to our knowledge that a G protein-coupled receptor, namely, the α2A adrenergic receptor, modulates APP endocytic sorting and promotes Aβ generation through disrupting APP interaction with a Vps10 family protein, sorting-related receptor with A repeat. Significantly, blockade of α2AAR by a clinical antagonist reduces AD-related pathology and ameliorates cognitive deficits in an AD transgenic model, suggesting repurposing clinical α2AR antagonists as a new direction for AD treatment.
Keywords: adrenergic receptor, amyloid, processing, SorLA, sorting
Abstract
Accumulation of amyloid β (Aβ) peptides in the brain is the key pathogenic factor driving Alzheimer’s disease (AD). Endocytic sorting of amyloid precursor protein (APP) mediated by the vacuolar protein sorting (Vps10) family of receptors plays a decisive role in controlling the outcome of APP proteolytic processing and Aβ generation. Here we report for the first time to our knowledge that this process is regulated by a G protein-coupled receptor, the α2A adrenergic receptor (α2AAR). Genetic deficiency of the α2AAR significantly reduces, whereas stimulation of this receptor enhances, Aβ generation and AD-related pathology. Activation of α2AAR signaling disrupts APP interaction with a Vps10 family receptor, sorting-related receptor with A repeat (SorLA), in cells and in the mouse brain. As a consequence, activation of α2AAR reduces Golgi localization of APP and concurrently promotes APP distribution in endosomes and cleavage by β secretase. The α2AAR is a key component of the brain noradrenergic system. Profound noradrenergic dysfunction occurs consistently in patients at the early stages of AD. α2AAR-promoted Aβ generation provides a novel mechanism underlying the connection between noradrenergic dysfunction and AD. Our study also suggests α2AAR as a previously unappreciated therapeutic target for AD. Significantly, pharmacological blockade of the α2AAR by a clinically used antagonist reduces AD-related pathology and ameliorates cognitive deficits in an AD transgenic model, suggesting that repurposing clinical α2AR antagonists would be an effective therapeutic strategy for AD.
Excess amyloid β (Aβ) peptides in the brain are a neuropathological hallmark of Alzheimer’s disease (AD) and are generally accepted as the key pathogenic factor of the disease (1). Aβ is generated by two sequential cleavages of amyloid precursor protein (APP) by β and γ secretase, whereas cleavage by α secretase within the Aβ domain precludes Aβ generation (2, 3). APP and the secretases undergo endocytic sorting into various organelles, such as the trans-Golgi network, the plasma membrane, and endosomes (2–6). The initial step of APP processing by α versus β secretase preferentially occurs in distinct compartments of the cell. Although α secretase-mediated cleavage of APP occurs on the plasma membrane, β secretase primarily interacts with and cleaves APP in endosomes (2–6). Therefore, endocytic sorting of APP into different membranous compartments, causing it to coreside or avoid a particular secretase, plays a decisive role in APP proteolytic processing. Consistent with this notion, abnormalities of the endocytic pathway have been found to precede Aβ deposition in late-onset AD (7).
Retrograde sorting of APP from endosomes to trans-Golgi network mediated by the vacuolar protein sorting-10 (Vps10) family proteins and the retromer complex represents a critical mechanism to prevent amyloidogenic processing of APP (8–10) and has recently emerged as a potential target for therapeutic intervention (11). In particular, the sorting-related receptor with A repeat (SorLA) directs retrograde transport of APP to trans-Golgi network by binding to both APP and the retromer complex (12, 13) and retains APP in the Golgi (14), thus preventing its proteolytic processing. A connection between SorLA and AD was first revealed in patients with late-onset AD, in whom the levels of SorLA at the steady state are markedly reduced (15). Further human genetic studies identified variations of SORL1 (the gene encoding SorLA) resulting in a lower level of expression that are associated with late-onset sporadic AD (12, 16, 17). Moreover, nonsense and missense mutations of SORL1 cause autosomal dominant early-onset AD (18), supporting an etiological role of SorLA in AD. The function of SorLA in inhibiting Aβ production is confirmed by mouse genetic studies showing that loss of SorLA significantly increases Aβ levels in the brain (14) and enhances AD-related early pathology (19). Despite the importance of SorLA-dependent APP sorting in controlling Aβ metabolism and AD pathogenesis, how this process may be targeted by extracellular stimuli, such as neurotransmitters and hormones, to modulate amyloidogenesis remains largely unstudied.
The α2A adrenergic receptor (AR) belongs to the G protein-coupled receptor (GPCR) superfamily and is a crucial component of the brain noradrenergic (NA) system, controlling both NA input to the cerebral cortex and the resulting response in this brain region (20). Profound dysfunction of the NA system consistently occurs at the early stage of AD (21), raising the possibility of involvement of the α2AAR in AD pathogenesis. Here we report for the first time to our knowledge that α2AAR signaling regulates SorLA-dependent APP sorting and promotes amyloidogenic processing of APP by beta-site amyloid precursor protein cleaving enzyme (BACE) 1. The initial cleavage of APP by BACE1 is the rate-limiting factor of Aβ generation (22, 23). Furthermore, blockade of α2AAR by a clinical antagonist reduces AD-related pathology and rescues cognitive deficits in an AD transgenic model, suggesting that repurposing clinical α2AR antagonists would be a novel effective strategy for AD treatment.
Results
Genetic Deficiency of the α2AAR Lessens AD-Associated Neuropathology in Vivo in an AD Mouse Model.
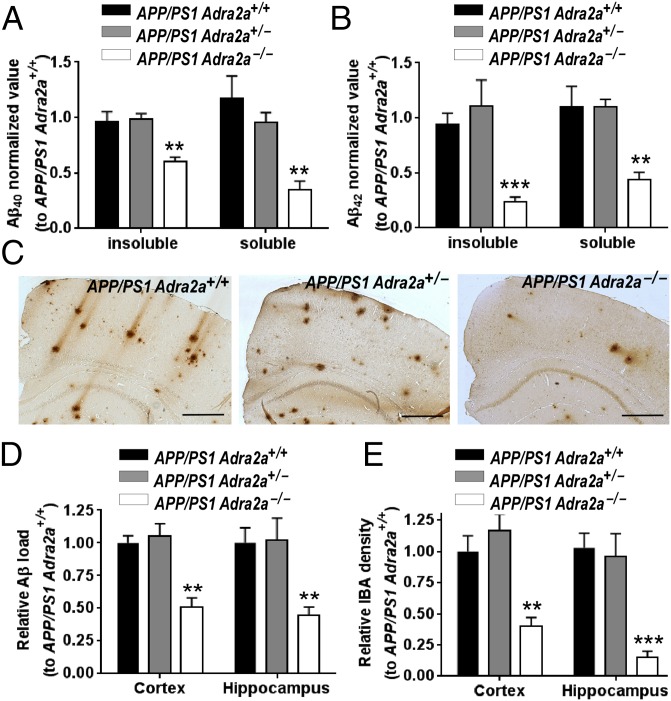
We first addressed whether the α2AAR plays a role in AD pathogenesis using a genetic approach. We crossed the Adra2a‒/‒ line with the APP/PS1 transgenic line to generate APP/PS1,Adra2a+/+, APP/PS1,Adra2a+/‒, and APP/PS1,Adra2a‒/‒ mice and measured the levels of Aβ peptides in the brain. ELISA analysis revealed a reduction in both Aβ40 (Fig. 1A) and Aβ42 (Fig. 1B) content in the cerebrum of 6-mo-old APP/PS1,Adra2a‒/‒ mice compared with age- and sex-matched APP/PS1,Adra2a+/+ and APP/PS1,Adra2a+/‒ mice. In addition, in all APP/PS1,Adra2a‒/‒ mice examined at 6 mo of age, the cerebral Aβ deposits were significantly decreased compared with in sex-matched APP/PS1,Adra2a+/+ and APP/PS1,Adra2a+/‒ mice at the same age (Fig. 1 C and D). These data suggest that the endogenous α2AAR activity enhances brain amyloidosis in APP/PS1 mice. Furthermore, the number of active microglia (Fig. 1E and SI Appendix, Fig. S1) and astrocytes (SI Appendix, Fig. S2) in the brain of APP/PS1,Adra2a‒/‒ mice was greatly decreased, suggesting less neuroinflammation in these mice. Taken together, our data demonstrate a critical role of the α2AAR in promoting AD-associated neuropathology.
Fig. 1.
Genetic deficiency of α2AAR reduces amyloid pathology in APP/PS1 mice. The levels of Aβ40 (A) and Ab42 (B) in carbonate-soluble and carbonate-insoluble fractions of the cerebrum isolated from 6-mo-old male mice with indicated genotypes were measured by ELISA. n = 6 in each genotype measured in triplicates. Data (mean ± SEM) are expressed as the fold change to the Aβ concentration in APP/PS1,Adra2a+/+ mice. **P < 0.01 and ***P < 0.001 compared with APP/PS1,Adra2a+/+ mice. (C) Representative images of Aβ staining of cerebral cortex isolated from 6-mo-old male mice with indicated genotypes. (Scale bar, 500 μm.) (D) Quantification of relative Aβ load. (E) Quantification of ionized calcium-binding adapter molecule 1 density (indicating active microglia). Data (mean ± SEM) are expressed as the fold change to the level in APP/PS1,Adra2a+/+ mice. n = 11 for APP/PS1,Adra2a+/+ and APP/PS1,Adra2a+/‒ mice; n = 8 for APP/PS1,Adra2a‒/‒ mice. **P < 0.01 and ***P < 0.001 compared with APP/PS1,Adra2a+/+ mice.
Activation of the α2AAR Enhances Production of Aβ Peptides in Neurons and Exacerbates Aβ Pathology in the Brain.
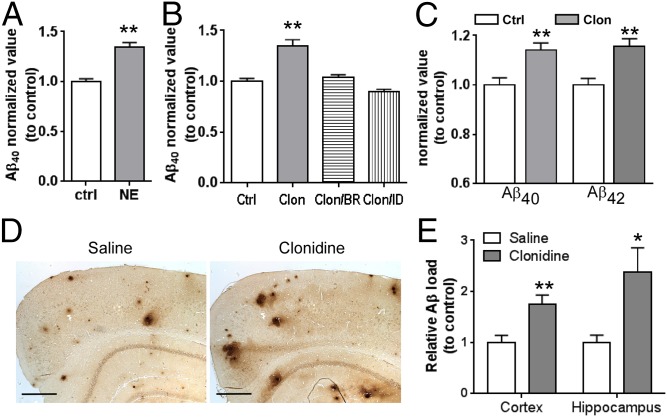
We next determined whether α2AAR activation enhances production of Aβ peptides in neurons. We treated primary cortical neurons with the endogenous ligand, norepinephrine (NE), in the presence of propranolol to block βARs and with prazosin to block α1, α2B, and α2CARs, thus specifically activating the α2AAR. NE treatment significantly increased production of both Aβ40 (Fig. 2A) and Aβ42 peptides (SI Appendix, Fig. S3A). Similarly, NE treatment significantly enhanced Aβ40 generation in neuro-2a (N2a) cells expressing human APP (hAPP) (SI Appendix, Fig. S3B). Treatment with an α2AR-selective agonist, clonidine, which inhibited cAMP production through the α2AAR subtype in cortical neurons (SI Appendix, Fig. S4A), also increased Aβ generation (Fig. 2B and SI Appendix, Fig. S4B). This effect was blocked by an α2AR antagonist, idazoxan, and an α2AAR subtype-selective antagonist, BRL44408 (Fig. 2B), further demonstrating the role of the α2A subtype receptor in promoting Aβ generation.
Fig. 2.
Activation of the α2AAR enhances production of Aβ peptides and exacerbates amyloid pathology. (A) Cortical neurons were treated with 10μM NE (plus 1 μM propranolol and 1 μM prazosin) or vehicle for 48 h. The level of Aβ40 in culture medium was measured by ELISA. Data (mean ± SEM) are expressed as the fold change to control. n = 9 for each group. **P < 0.01, NE versus control. (B) The level of Aβ40 secreted from cortical neurons with indicated treatment. The concentration of each drug was 1 μM. BR, BRL44408; Clon, clonidine; ID, idazoxan. Data (mean ± SEM) are expressed as the fold change to vehicle control. n = 6–8 for each group. **P < 0.01 compared with vehicle control. (C) The levels of endogenous Aβ40 and Aβ42 in the cortex of C57BL/6 mice treated with saline or clonidine. Data (mean ± SEM) are expressed as the fold change to the Aβ concentration in saline-treated mice. n = 10–12 in each group. **P < 0.01, clonidine versus control. (D) Representative images of Aβ staining of cerebral cortex isolated from APP/PS1 mice with indicated treatment. (Scale bar, 500 μm.) (E) Quantification of relative Aβ load in APP/PS1 mice with indicated treatments. Data (mean ± SEM) are expressed as the fold change to saline control. n = 8 for each treatment group. *P < 0.05; **P < 0.01, clonidine versus control.
To determine the effect of α2AAR activation on Aβ generation in vivo, we treated C57BL/6 mice with saline or clonidine for 8 wk and examined the levels of endogenous Aβ peptides in the brain. Both Aβ40 and Aβ42 (Fig. 2C) levels in the cerebrum were significantly increased in mice treated with clonidine compared with age- and sex-matched mice treated with saline. We further examined the effect of α2AAR on Aβ deposition in APP/PS1 mice. Clonidine treatment for 8 wk (starting from 4 mo of age) significantly increased Aβ load in both hippocampus and cortex of APP/PS1 mice compared with sex-matched saline-treated controls (Fig. 2 D and E). Taken together, our in vitro and in vivo studies strongly suggest that activation of the α2AAR promotes Aβ production and exacerbates amyloid pathology.
α2AAR Activation Disrupts APP Colocalization and Interaction with SorLA in a G Protein-Dependent Manner.
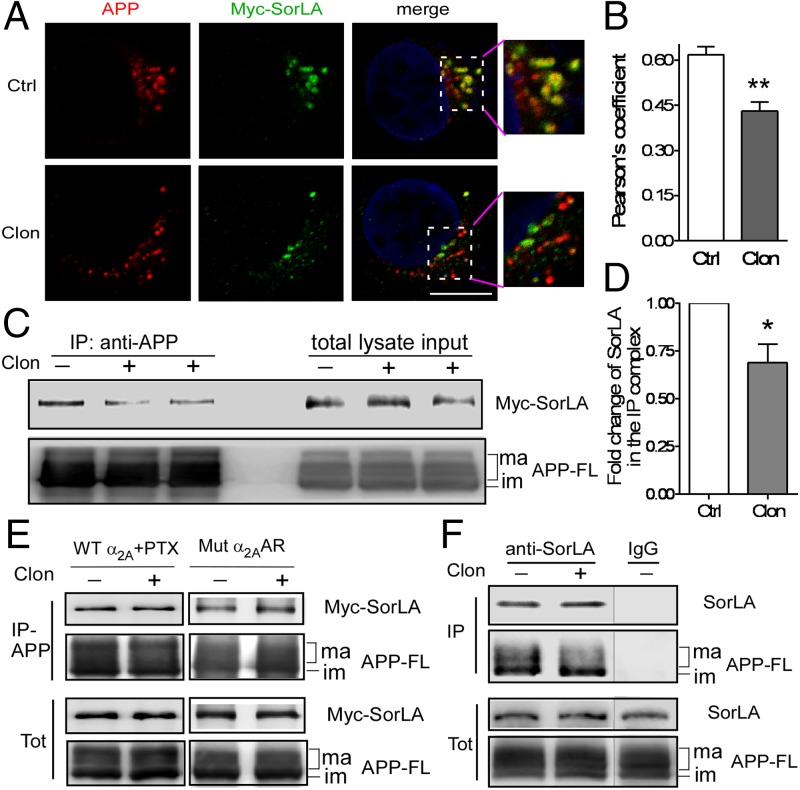
Given the important role of SorLA in controlling APP processing, we sought to address whether α2AAR signaling affects SorLA expression and/or its colocalization and interaction with APP. We found that the protein level of SorLA was not altered by α2AAR activation, nor was the full-length APP level (SI Appendix, Fig. S5). We then examined APP-SorLA colocalization in cells expressing hAPP and Myc-SorLA. In vehicle-treated cells, apparent colocalization between APP and SorLA was observed (Fig. 3A). However, the level of colocalization between these two proteins was significantly reduced after clonidine treatment (Fig. 3 A and B), suggesting that activation of the α2AAR disrupts colocalization of APP with SorLA.
Fig. 3.
α2AAR signaling disrupts APP-SorLA colocalization and interaction. (A) HEK293 cells expressing α2AAR, Myc-SorLA, and hAPP were treated with vehicle or 1 μM clonidine for 15 min. Representative images showing localization of hAPP (by Y188 antibody) and Myc-SorLA. (Scale bar, 10 μm.) (B) Quantification of colocalization between APP and SorLA. Data are mean ± SEM. n = 17–18 cells in each group. **P < 0.01 compared with vehicle-treated cells. (C) After vehicle or clonidine treatment, cell lysates were subjected to IP assays using Y188 antibody or preimmune IgG. Neither APP nor Myc-SorLA could be isolated by preimmune IgG. The input represents 1/20 of the total lysate used for each IP. APP-FL, full-length APP; im, immature form; ma, mature forms. (D) Quantification of the amount of SorLA in the IP complex with hAPP. Data (mean ± SEM) are expressed as fold change to vehicle control (defined as 1.0). n = 5 in each group. *P < 0.05, clonidine versus control. (E, Left) Cells expressing α2AAR, Myc-SorLA, and hAPP were pretreated with pertussis toxin (PTX, a Gi/o protein blocker, 200 ng/mL) overnight and then treated with vehicle or clonidine. (Right) Cells expressing a G protein-coupling deficient α2AAR, Myc-SorLA, and hAPP were treated with vehicle or clonidine. In these cells, clonidine treatment failed to alter the APP-SorLA interaction. Representative blots from three independent experiments are shown. (F) Interaction of endogenous APP and SorLA in mouse brain. Mice were treated with saline or clonidine for 30 min and lysates of cerebral cortex were subjected to IP assays using a SorLA antibody or preimmune IgG. Representative blots from three independent experiments were shown.
We examined APP-SorLA interaction in these cells by coimmunoprecipitation (IP) assays. In cells treated with clonidine, the amount of Myc-SorLA coimmunoisolated with hAPP was significantly reduced compared with that in vehicle-treated cells (Fig. 3 C and D). However, when cells were pretreated with pertussis toxin (a Gi/o protein blocker) or expressing a mutant α2AAR that does not couple to Gi/o proteins (24), clonidine failed to alter APP interaction with SorLA (Fig. 3E). These data suggest that activation of the α2AAR disrupts APP-SorLA interaction in a G protein-dependent manner. Furthermore, clonidine treatment markedly decreased the amount of endogenous SorLA coimmunoisolated with APP in the brain (SI Appendix, Fig. S6A), suggesting that α2AAR activation disrupts APP-SorLA interaction in vivo.
When examining the level of APP in the SorLA IP complex from both cell and brain lysates, we found that clonidine treatment caused a marked reduction in the amount of mature APP coisolated with SorLA, whereas it had a marginal effect on the amount of immature APP in the SorLA IP complex compared with vehicle treatment (Fig. 3F and SI Appendix, Fig. S6B). These data suggest that α2AAR activation primarily disrupts SorLA interaction with the mature forms of APP. In the IP assays, two irrelevant membrane proteins expressed in the brain, adenosine A1 receptor and Semaphorin 6D, could not be detected in the SorLA IP complex (SI Appendix, Fig. S7), demonstrating the specificity of APP-SorLA interaction.
α2AAR Activation Reduces APP Localization to the Golgi and Increases Its Colocalization with BACE1 in Endosomes.
Because SorLA mediates retrograde sorting of APP to the Golgi and retains APP there, we predicted that the decrease in APP-SorLA interaction induced by α2AAR signaling would lead to reduced localization of APP in the Golgi. Indeed, in N2a cells treated with clonidine, colocalization of the endogenous APP with a Golgi marker, Golgi 58K protein (Golgi58K), was dramatically decreased compared with that in vehicle-treated control cells (Fig. 4 A and B). Concurrent with the reduction of APP localization in the Golgi, we observed a significant increase in colocalization of APP with both an early endosomal marker, early endosome antigen 1 (EEA1) (Fig. 4 C and D), and a recycling endosomal marker, Rab GTPase 11 (Rab11) (SI Appendix, Fig. S8 A and B), in clonidine-treated cells compared with in control cells. Furthermore, in primary cortical neurons, clonidine treatment led to a significant decrease in colocalization of endogenous APP with Golgi58K (SI Appendix, Fig. S8 A and B) and a simultaneous increase in colocalization of APP with EEA1 (SI Appendix, Fig. S9 C and D). These data suggest that activation of the α2AAR drives APP exit from the Golgi to localize in endosomes, a phenotype that is consistent with diminished SorLA regulation of APP sorting.
Fig. 4.
Activation of the α2AAR reduces APP localization in Golgi and enhances its localization in endosomes. N2a cells expressing the α2AAR were treated with vehicle or 1 μM clonidine for 15 min. Representative images showing localization of the endogenous APP (by Y188 antibody) together with Golgi58K (A) and EEA1 (C). (Scale bar, 10 μm.) Colocalization was quantified by Pearson’s correlation coefficient (B and D). n = 13–18 cells for each group. **P < 0.01; ***P < 0.001, clonidine versus vehicle.
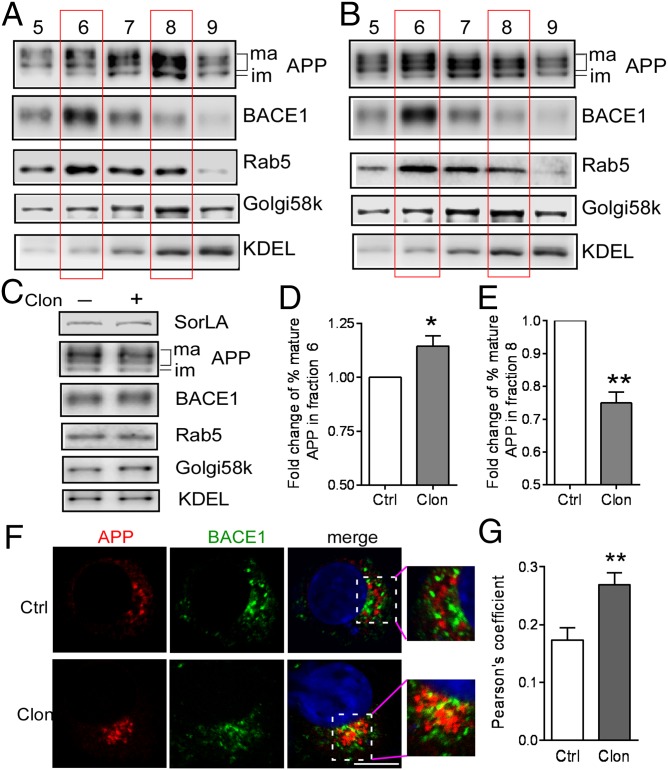
To confirm that distribution of the full-length mature form of APP in different organelles is altered after α2AAR activation, we performed subcellular fractionation of cells treated with or without clonidine. BACE1 was mainly distributed in endosomal fractions in both vehicle- and clonidine-treated (Fig. 5 A and B and SI Appendix, Fig. S10) cells. However, distribution of the mature APP appeared to be shifted from Golgi-enriched fractions to endosomal fractions after clonidine treatment (comparing Fig. 5B with Fig. 5A), but such treatment did not change the overall expression of full-length APP and BACE1 (Fig. 5C). The input levels of APP, BACE1, and different organelle markers are nearly identical between samples treated with vehicle and samples treated with clonidine (Fig. 5C). Quantitation of the percentage of mature APP distributed in different fractions revealed a significant increase of mature APP in the endosomal and BACE1-enriched fraction (fraction 6) in clonidine-treated cells compared with in control cells (Fig. 5D). In contrast, the percentage distribution of mature APP in the Golgi-enriched fraction (fraction 8) is significantly decreased by clonidine treatment (Fig. 5E).
Fig. 5.
α2AAR activation alters subcellular localization of APP and increases its colocalization with BACE1. (A–E) N2a cells expressing α2AAR were treated with vehicle (A) or clonidine (B), and cell lysates were subject to density gradient fractionation. Representative Western blots showing endogenous APP, BACE1, Rab GTPase 5 (Rab5), Golgi58K, and a KDEL (Lys-Asp-Glu-Leu) motif containing protein in fractions 5–9. The full blots with all fractions are provided in SI Appendix, Fig. S10. The intensity of mature APP in each fraction was quantified, and the percentage of the mature APP distributed in each fraction was calculated, with the total of all fractions equal to 100%. (C) Input of the total lysate subjected to fractionation in A (−Clon) and B (+Clon). (D) Fold change of the percentage of the mature APP in fraction 6 (where BACE1 and Rab5 peak) in clonidine-treated cells compared with control cells (arbitrarily defined as 1.0). (E) Fold change of the percentage of mature APP in fraction 8 (where Golgi58K peaks) in clonidine-treated cells over that in control cells (defined as 1.0). Data are mean ± SEM. n = 6 for each group. *P < 0.05; **P < 0.01 compared with control. (F) N2a cells expressing α2AAR and BACE1-CFP were treated with vehicle or clonidine. The low level of BACE1-CFP expression was detected by an anti-GFP antibody. Representative images showing localization of the endogenous APP and BACE1-CFP. (G) Colocalization between APP and BACE1 was quantified. Data are mean ± SEM. n = 14–18 cells in each group. **P < 0.01 clonidine versus vehicle.
We further examined whether α2AAR activation promotes colocalization and interaction of APP with BACE1. In cells treated with clonidine, colocalization of APP and BACE1 was significantly enhanced compared with that in control cells (Fig. 5 F and G). Moreover, clonidine treatment markedly increased the amount of BACE1 coimmunoisolated with APP compared with vehicle treatment both in cultured cells and in mouse brain (SI Appendix, Fig. S11). Collectively, our data suggest that by diminishing the APP-SorLA interaction, α2AAR signaling promotes APP sorting to endosomal compartments, where it is colocalized and interacts with BACE1.
Activation of the α2AAR Promotes Amyloidogenic Processing of APP in Vitro and in Vivo.
On the basis of the data shown here, we predicted that APP cleavage by BACE1 would be increased in cells after α2AAR stimulation. To test this prediction, we used cells expressing human APP carrying the Swedish mutation (hAPPsw), which produced an excess amount of the C-terminal fragment derived from BACE1 cleavage (C99) that can be readily detected by Western blot analysis. Indeed, clonidine treatment dramatically enhanced the level of C99 (Fig. 6 A and B), while having no obvious effect on the level of C83, the C-terminal fragment derived from α secretase cleavage (SI Appendix, Fig. S12A). Consistently, the amount of secreted APP ectodomain derived from BACE1 cleavage (sAPPβ; Fig. 6 C and D), but not that derived from α secretase (sAPPα; SI Appendix, Fig. S12B), was significantly enhanced by clonidine treatment. Furthermore, chronic treatment with clonidine led to a marked increase in accumulation of C99 (Fig. 6 E and F) and sAPPβ (Fig. 6 G and H), but not C83 and sAPPα (SI Appendix, Fig. S12 C and D), in the cerebral cortex of APP/PS1 mice, suggesting that α2AAR activation promotes BACE1 cleavage of APP in vivo.
Fig. 6.
α2AAR activation promotes amyloidogenic processing of APP. (A–D) N2a cells expressing α2AAR and hAPPsw were treated with clonidine or vehicle for 36 h. Representative blots of APP-FL (by Y188 antibody), C99 (by 6E10 antibody), and C83 (by Y188 antibody) separated by a tricine gel are shown in A. Representative blots of APP-FL, sAPPβ (by sAPPβ antibody), and sAPPα (by 6E10 antibody) separated by a glycine gel are shown in C. Quantitation of C99 (B) and sAPPβ (D) as a ratio over APP-FL was measured. Data represent mean ± SEM. n = 5–6 for each group. *P < 0.05; **P < 0.01, clonidine versus control. (E–H) APP/PS1 mice treated with clonidine or saline for 8 wk. Representative blots of APP-FL, C99, and C83 in cortical lysates separated by a tricine gel are shown in E. Representative blots of APP-FL, sAPPα, and sAPPβ separated by a glycine gel are shown in G. Quantitation of C99 (F) and sAPPβ (H) as a ratio over APP-FL was measured. n = 5–6 for each treatment. *P < 0.05, **P < 0.01 clonidine versus control.
α2AAR-promoted Aβ production may also result from enhancement of γ secretase cleavage after agonist treatment. To investigate this possibility, we examined Aβ production in cells expressing α2AAR and the C99 fragment of hAPP. Unlike in cells expressing the full-length hAPP (SI Appendix, Fig. S3B), neither NE nor clonidine had an effect on Aβ production in cells expressing C99 (SI Appendix, Fig. S12E). These data suggest that activation of the α2AAR does not affect γ secretase cleavage of APP. Taken together, our data suggest that activation of the α2AAR enhances the initial processing of APP by BACE1, leading to an increase in Aβ generation.
Pharmacologic Blockade of the α2AAR at the Early Disease Stage Reduces Amyloid Pathology and Rescues Cognitive Deficits in APP/PS1 Mice.
To directly explore the therapeutic potential of the α2AAR, we examined whether pharmacological blockade of this receptor delays the onset of AD-related pathology in AD transgenic mice. We treated APP/PS1 mice and sex-matched nontransgenic (nTg) littermates with a clinically used α2AR antagonist, idazoxan, starting at 10 wk of age, a time before Aβ deposition can be detected in the brain of APP/PS1 mice. Idazoxan is highly selective and potent for blocking α2ARs (25) and has been used to treat mood disorders (26). After 10 wk of saline (control) or idazoxan treatment, mice were untreated for a week to allow drug washout and were then evaluated in behavioral tests for 2 wk before being killed and analyzed for pathological changes. We found that the levels of human Aβ peptides, especially Aβ42, were dramatically reduced in the cerebrum of APP/PS1 mice with idazoxan treatment compared with those of mice with saline treatment (Fig. 7 A and B). Strikingly, idazoxan treatment also significantly reduced endogenous Aβ40 and Aβ42 levels in the cortex of nTg mice compared with saline treatment (SI Appendix, Fig. S13). In addition, in the idazoxan-treated APP/PS1 mice, Aβ deposition in both hippocampus and cortex was significantly decreased compared with saline-treated controls (Fig. 7 C and D). These data suggest that α2AR antagonist treatment is effective in reducing Aβ generation and AD-related pathology.
Fig. 7.
Idazoxan treatment reduces Aβ production and deposition and ameliorates cognitive deficits in APP/PS1 mice. The levels of Aβ40 (A) and Aβ42 (B) in carbonate-soluble and carbonate-insoluble fractions of the cerebrum isolated from APP/PS1 mice with indicated treatment were measured by ELISA. n = 6 in each group. Data (mean ± SEM) are expressed as the fold change to saline treatment. *P < 0.05; **P < 0.01; ***P < 0.001, idazoxan versus saline. (C) Representative images of Aβ staining in cerebral cortex. (Scale bar, 500 μm.) (D) Quantification of the Aβ load in different brain regions. Data (mean ± SEM) are the fold change to saline treatment. n = 6 in each group. *P < 0.05, idazoxan versus saline. (E) Performance of nontransgenic (nTg) and APP/PS1 mice treated with saline or clonidine in novel object recognition tests. The dashed line indicates the 50% level of recognition index. Data represent mean ± SEM. n = 11 for nTg + saline (Sal); n = 9 for nTg + idazoxan (Ida); n = 11 for APP/PS1 + saline; n = 8 for APP/PS1 + idazoxan. *P < 0.05, saline-treated versus clonidine-treated APP/PS1. †P < 0.05, saline-treated APP/PS1 versus nTg. (F) Measurement of escape latency on each day in Morris water maze tests. Data represent mean ± SEM n = 11 for nTg + saline and APP/PS1 + saline; n = 9 for nTg + idazoxan; n = 8 for APP/PS1 + idazoxan. *P < 0.05; ***P < 0.001, saline-treated versus idazoxan-treated APP/PS1 mice. †P < 0.05; †††P < 0.001, saline-treated APP/PS1 versus nTg.
We assessed basic and cognitive behaviors of APP/PS1 mice and their age- and sex-matched nTg littermates after saline or idazoxan treatment. We did not observe any significant differences in baseline activity (SI Appendix, Fig. S14) or anxiety levels (SI Appendix, Fig. S15) between different genotypes or between different treatments. In the novel object recognition paradigm, APP/PS1 mice treated with saline exhibited a significant deficit in recognition memory compared with saline-treated nTg littermates (Fig. 7E). In contrast, the performance of APP/PS1 mice treated with idazoxan was comparable to that of their nTg littermates in this paradigm (Fig. 7E), suggesting that idazoxan treatment enhanced cognitive function in these mice. We further evaluated APP/PS1 and nTg mice in the Morris water maze task. Saline-treated APP/PS1 mice showed greatly prolonged escape latency (Fig. 7F) and travel distance (SI Appendix, Fig. S16A) compared with their nTg littermates receiving the same treatment. Idazoxan treatment fully rescued this spatial learning deficit in APP/PS1 mice (Fig. 7F and SI Appendix, Fig. S16A). In addition, idazoxan rescued the spatial memory deficit in APP/PS1 mice, as revealed by increased time spent in the target quadrant by APP/PS1 mice treated with idazoxan compared with APP/PS1 mice treated with saline (SI Appendix, Fig. S16 B and C). These data strongly suggest that α2AR antagonist treatment is effective in ameliorating AD-related cognitive deficits. Because we incorporated a 1-wk washout period before behavioral assessment, the effect of idazoxan on behavior is unlikely to be attributed to an acute symptomatic response to this drug but, rather, is a result of underlying changes in pathology caused by the chronic treatment. Taken together, our preclinical studies using a clinical α2AR antagonist strongly support pharmacological blockade of α2AR as a novel therapeutic strategy for AD treatment.
Discussion
SorLA-dependent endocytic sorting of APP has emerged as a central regulatory mechanism of APP proteolytic processing and Aβ production (8). Our current study reveals for the first time to our knowledge that this process can be regulated by a GPCR; namely, the α2AAR, which disrupts colocalization and interaction between SorLA and the mature APP through G protein-dependent signaling and thus diminishes SorLA-dependent regulation of mature APP sorting. As illustrated in SI Appendix, Fig. S17, SorLA can govern APP sorting in multiple ways along the APP trafficking route. SorLA can retain APP in the Golgi, thus impeding its sorting to plasma membrane and endocytic organelles (14). In endosomes, SorLA can mediate APP retrograde sorting back to the Golgi through interacting with the retromer complex (12, 13). Consistent with diminished SorLA regulation of APP sorting, activation of the α2AAR promotes APP to exit the Golgi and concurrently increases its localization in endosomes and convergence with BACE1. As a result, more APP is processed by BACE1, and more Aβ is generated after α2AAR activation. Our current study provides the first example to our knowledge that a GPCR promotes APP amyloidogenic processing through modulating its endocytic sorting by Vps10 family receptors. A few GPCRs, including β2AR (27) and GPR3 (28), have been reported to promote Aβ generation by enhancing γ secretase activity. However, α2AAR activation has no effect on γ secretase cleavage of the C99 fragment of APP. Modulation of APP-SorLA interaction by α2AAR represents a novel mechanism by which extracellular stimuli regulate APP processing and Aβ production.
We coisolated both mature and immature forms of APP in a complex with SorLA from cell and mouse brain lysates, suggesting that association of these two proteins can occur in the early secretary pathway. Interestingly, α2AAR activation primarily disrupted SorLA interaction with the mature forms of APP but only had a marginal effect on its interaction with immature APP. This result is consistent with our observation that α2AAR signaling does not alter APP maturation but, rather, regulates mature APP distribution between Golgi and endosomes. These data provide additional support to the specific role for α2AAR in modulation of SorLA-dependent mature APP trafficking. α2AAR-induced modulation of APP-SorLA interaction requires activation of the Gi/o subfamily of heterotrimeric G proteins, which may regulate multiple downstream signaling effectors to modify APP and/or SorLA. How the α2AAR-induced G protein signaling cascade regulates the APP-SorLA interaction warrants further investigation.
Familial AD with mutations in genes encoding APP or PS1 (a subunit of γ secretase) accounts for less than 10% of AD. In contrast, late-onset sporadic AD likely involves multiple genetic and environmental risk factors that lead to disruption of Aβ homeostasis. The NA system plays a pivotal role in supporting interactions with and responses to environmental stimuli, and modulates cognitive activities in the cortex, including attention, perception, and memory retrieval. Profound NA degeneration and adaptation occur consistently in patients at the early stage of AD, suggesting a potential connection between alteration of the NA system and AD. The potential involvement of NA dysfunction and AD is much more complicated than a simple loss of NE input to cerebral cortex. In fact, normal, or even elevated, NE levels have been found in patients with advanced AD, likely as a result of compensatory changes in the remaining locus coeruleus neurons, such as an increase in sprouting and NE synthesis and a decrease in NE transporter expression (29). Our current study suggests that activation of the α2AAR by NE promotes Aβ generation and exacerbates AD-related pathology. Blockade of α2AAR activity would reduce the detrimental effects of NE on AD-related pathology through this receptor subtype and would further boost NE, given that the α2AAR is also an autoreceptor, to improve cognition. Increased α2AAR density and/or activity have been associated with type 2 diabetes mellitus and depression (30, 31), both of which are risk factors for AD. α2AAR-promoted Aβ generation likely acts as a key contributor driving AD-related pathophysiology under these disease conditions. Consistent with a role of α2AAR in promoting AD, clonidine treatment has been reported to worsen attention and memory deficit in patients with AD (32).
To date, there are no effective treatments to prevent, cure, or even slow the progression of AD. Our studies suggest that the α2AAR is a previously unappreciated therapeutic target for AD treatment. Of particular significance, several α2AR antagonists, such as idazoxan (used in this study), mirtazapine, and yohimbine, have been widely used clinically for the treatment of mood disorders and sexual dysfunction, as well as for body fat loss. Redirection of these existing therapeutics to treat AD would greatly expedite clinical trials, as their safety, brain bioavailability, and pharmacokinetic/pharmacodynamic parameters have been well addressed previously, thus saving the time and cost associated with new drug development.
Materials and Methods
Mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Animal Resources Program facility at the University of Alabama at Birmingham. All strains of mice have been backcrossed for more than 12 generations to C57BL/6 background. Experimental details on drug treatment, behavioral assessment, and immunohistochemistry for amyloid plaques and activated astrocytes/microglia are provided in SI Appendix, Materials and Methods. The levels of Aβ1-42 and Aβ1-40 were determined using specific ELISA kits, as described in SI Appendix, Materials and Methods. Detailed information on cell culture, immunocytochemistry, immunoprecipitation, subcellular fractionation, and Western blot are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Guangyu Wu (Georgia Regents University) for providing the GFP-Rab11 construct and Stephan Real and Jin Huang for technical support. We also thank Dr. Erik Roberson, Dr. Elizabeth Sztul, and Dr. Christopher Cottingham (Morehead State University) for helpful discussion and critical reading of the manuscript and Dr. Christopher Cottingham for editing the manuscript. This study is supported by NIH Grants AG042716 (to Q.W.) and MH081917 (to Q.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409513111/-/DCSupplemental.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2(5):a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang S, et al. Trafficking regulation of proteins in Alzheimer’s disease. Mol Neurodegener. 2014;9:6. doi: 10.1186/1750-1326-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: Intracellular pathways to pathogenesis. Neuron. 2006;52(1):15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283(44):29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26(3):373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Willnow TE, Andersen OM. Sorting receptor SORLA—a trafficking path to avoid Alzheimer disease. J Cell Sci. 2013;126(Pt 13):2751–2760. doi: 10.1242/jcs.125393. [DOI] [PubMed] [Google Scholar]
- 9.Reitz C. The role of intracellular trafficking and the VPS10d receptors in Alzheimer’s disease. Future Neurol. 2012;7(4):423–431. doi: 10.2217/fnl.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane RF, et al. Vps10 family proteins and the retromer complex in aging-related neurodegeneration and diabetes. J Neurosci. 2012;32(41):14080–14086. doi: 10.1523/JNEUROSCI.3359-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mecozzi VJ, et al. Pharmacological chaperones stabilize retromer to limit APP processing. Nat Chem Biol. 2014;10(6):443–449. doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fjorback AW, et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci. 2012;32(4):1467–1480. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen OM, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102(38):13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherzer CR, et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61(8):1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- 16.Grear KE, et al. Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain. Mol Neurodegener. 2009;4:46. doi: 10.1186/1750-1326-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caglayan S, et al. Identification of Alzheimer disease risk genotype that predicts efficiency of SORL1 expression in the brain. Arch Neurol. 2012;69(3):373–379. doi: 10.1001/archneurol.2011.788. [DOI] [PubMed] [Google Scholar]
- 18.Pottier C, et al. PHRC GMAJ Collaborators High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry. 2012;17(9):875–879. doi: 10.1038/mp.2012.15. [DOI] [PubMed] [Google Scholar]
- 19.Dodson SE, et al. Loss of LR11/SORLA enhances early pathology in a mouse model of amyloidosis: Evidence for a proximal role in Alzheimer’s disease. J Neurosci. 2008;28(48):12877–12886. doi: 10.1523/JNEUROSCI.4582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 2006;326(2):541–551. doi: 10.1007/s00441-006-0285-2. [DOI] [PubMed] [Google Scholar]
- 21.Haglund M, Sjobeck M, Englund E. Locus ceruleus degeneration is ubiquitous in Alzheimer's disease: Possible implications for diagnosis and treatment. Neuropathology. 2006;26(6):528–532. doi: 10.1111/j.1440-1789.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 22.Vassar R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 23.Yan R, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402(6761):533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 24.Eason MG, Liggett SB. Chimeric mutagenesis of putative G-protein coupling domains of the alpha2A-adrenergic receptor. Localization of two redundant and fully competent gi coupling domains. J Biol Chem. 1996;271(22):12826–12832. doi: 10.1074/jbc.271.22.12826. [DOI] [PubMed] [Google Scholar]
- 25.Doxey JC, Roach AG, Smith CF. Studies on RX 781094: A selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman F, Potter WZ, Brown EA, Maislin G. A double-blind study comparing idazoxan and bupropion in bipolar depressed patients. J Affect Disord. 1999;56(2-3):237–243. doi: 10.1016/s0165-0327(99)00041-5. [DOI] [PubMed] [Google Scholar]
- 27.Ni Y, et al. Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med. 2006;12(12):1390–1396. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- 28.Thathiah A, et al. The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science. 2009;323(5916):946–951. doi: 10.1126/science.1160649. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald PJ. Is elevated norepinephrine an etiological factor in some cases of Alzheimer’s disease? Curr Alzheimer Res. 2010;7(6):506–516. doi: 10.2174/156720510792231775. [DOI] [PubMed] [Google Scholar]
- 30.Cottingham C, Wang Q. α2 adrenergic receptor dysregulation in depressive disorders: Implications for the neurobiology of depression and antidepressant therapy. Neurosci Biobehav Rev. 2012;36(10):2214–2225. doi: 10.1016/j.neubiorev.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cottingham C, Chen H, Chen Y, Peng Y, Wang Q. Genetic variations of α(2)-adrenergic receptors illuminate the diversity of receptor functions. Curr Top Membr 2011;67:161–190. [DOI] [PubMed]
- 32.Riekkinen M, Laakso MP, Jäkälä P. Clonidine impairs sustained attention and memory in Alzheimer’s disease. Neuroscience. 1999;92(3):975–982. doi: 10.1016/s0306-4522(99)00070-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.