Significance
This study demonstrates that the oncoembryonic surface antigen, receptor tyrosine kinase-like orphan receptor 1 (ROR1), is expressed on human ovarian cancer stem cells (CSCs), on which it seems to play a functional role in promoting migration/invasion or spheroid formation in vitro and tumor engraftment in immune-deficient mice. Treatment with a humanized mAb specific for ROR1 (UC-961) could inhibit the capacity of ovarian cancer cells to migrate, form spheroids, or engraft immune-deficient mice. Moreover, such treatment inhibited the growth of tumor xenografts, which in turn had a reduced capacity to engraft immune-deficient mice and were relatively depleted of cells with features of CSC, suggesting that treatment with UC-961 could impair CSC renewal. Collectively, these studies indicate that ovarian CSCs express ROR1, which may be targeted for anti-CSC therapy.
Keywords: ROR1, ovarian cancer stem cell, monoclonal antibody, PDX mice model
Abstract
Although initially responsive to chemotherapy, many patients with ovarian cancer subsequently develop relapsed and potentially fatal metastatic disease, which is thought to develop from cancer stem cells (CSCs) that are relatively resistant to conventional therapy. Here, we show that CSCs express a type I receptor tyrosine kinase-like orphan receptor (ROR1), which is expressed during embryogenesis and by many different cancers, but not normal postpartum tissues. Ovarian cancers with high levels of ROR1 had stem cell-like gene-expression signatures. Furthermore, patients with ovarian cancers with high levels of ROR1 had higher rates of relapse and a shorter median survival than patients with ovarian cancers that expressed low-to-negligible amounts of ROR1. We found that ROR1-positive (ROR1+) cells isolated from primary tumor-derived xenografts (PDXs) also expressed aldehyde dehydrogenase 1 (ALDH1) and had a greater capacity to form spheroids and to engraft immune-deficient mice than did ROR1-negative (ROR1Neg) ovarian cancer cells isolated from the same tumor population. Treatment with UC-961, an anti-ROR1 mAb, or shRNA silencing of ROR1 inhibited expression of the polycomb ring-finger oncogene, Bmi-1, and other genes associated with the epithelial–mesenchymal transition. Moreover, shRNA silencing of ROR1, depletion of ROR1+ cells, or treatment with UC-961 impaired the capacity of ovarian cancer cells to form spheroids or tumor xenografts. More importantly, treatment with anti-ROR1 affected the capacity of the xenograft to reseed a virgin mouse, indicating that targeting ROR1 may affect CSC self-renewal. Collectively, these studies indicate that ovarian CSCs express ROR1, which contributes to their capacity to form tumors, making ROR1 a potential target for the therapy of patients with ovarian cancer.
Although most patients with advanced ovarian cancer initially respond well to paclitaxel- and cisplatin-based therapies (1, 2), ∼85% of patients relapse within a few years after systemic chemotherapy and cytoreductive surgery, including those who had an apparent complete response to therapy (3). Cancer recurrence is thought to reflect the survival of a small percentage of ovarian cancer stem cells (CSCs), which are relatively resistant to chemotherapy, can repopulate the tumor, and can spread to distal sites (4–6).
Studies have identified phenotypic and functional characteristics of CSCs that may distinguish such cells from other neoplastic cells (7, 8). Compared with other ovarian cancer cells, ovarian CSCs have relatively high aldehyde dehydrogenase (ALDH1) enzymatic activity (9–11), which may serve to detoxify intracellular aldehydes or various cytotoxic drugs (12, 13). Ovarian CSCs also can extrude small molecules, such as various chemotherapeutic drugs or fluorescent dyes, allowing for their identification as a less fluorescent “side population” in flow-cytometric analysis of tumor cells stained with the DNA-binding dye Hoechst 33342 (14–16). Furthermore, ovarian CSCs may express various surface antigens, such as CD44, CD117, or CD133 (7, 8, 17–19). Functionally, human ovarian CSCs apparently can form nonadherent cellular spheres, called “spheroids,” which in turn are enriched for ovarian cancer cells that have high ALDH1 activity, are relatively resistant to chemotherapeutic drugs, and have enhanced potential for seeding metastatic sites or engrafting immune-deficient mice (5, 17, 18, 20–22).
Ovarian CSCs may have stem cell-like gene-expression signatures, possibly reflecting their relatively high capacity for self-renewal and capacity to regenerate the entire tumor population (4, 23). Such gene-expression signatures in turn are associated with a subset of ovarian cancers that are less well differentiated, have a higher propensity to form spheroids in vitro or metastasize in vivo, and are associated with poorer prognosis (16, 24). Finally, ovarian cancer spheroids also express genes associated with the epithelial–mesenchymal transition (EMT) that provide the traits required for invasion and metastasis (21, 25).
Another potential marker for CSCs is ROR1. ROR1 is a type I orphan-receptor tyrosine kinase-like surface protein that is found on neoplastic cells of many different types of cancer, but not on normal adult tissues (26–32). Moreover, ROR1 predominately seems to be expressed by less well-differentiated tumors that have high potential for relapse and metastases and that also express markers associated with EMT (31, 33). Conversely, silencing ROR1 in metastasis-prone breast-cancer cell lines could attenuate expression of genes associated with EMT and impair their migration/invasion capacity in vitro and their metastatic potential in vivo (33). Very recently, Zhang et al. reported that high-level expression of ROR1 was an independent prognostic factor for predicting relatively short disease-free survival or overall survival of patients with ovarian cancer (34). In this study, we examined whether ovarian cancer cells that expressed ROR1 also had phenotypic and functional characteristics associated with ovarian CSCs.
Results
Expression of ROR1 Is Associated with Poor Prognosis and Stem Cell-Like Gene-Expression Signatures in Ovarian Cancer.
We analyzed the PubMed Gene Expression Omnibus (GEO) database on ovarian cancer cells of 285 patients with epithelial ovarian, primary peritoneal, or fallopian tube cancer, which we segregated into three subgroups based upon their relative expression of ROR1 (35). Patients with tumors having the upper-third expression level of ROR1 mRNA (designated as ROR1Hi) had a significantly shorter median progression-free survival (PFS) (1.2 y) or overall survival (OS) (3.8 y) than did patients with lower-third level (called ROR1Low) (PFS = 2.2 y or OS undefined within 5 y) (P = 0.0003 or 0.03, respectively) (Fig. 1A and Table S1). Although most patients included in this cohort had high-grade and advanced-stage serous cancers, there was a small subset of patients who had endometrioid ovarian cancers, serous tumors of low-grade, and/or early-stage tumors with low malignant potential (LMP) (n = 18) (35). We noted that these cases had a significantly lower median level of ROR1 mRNA expression (median = 5.4) than did the other cases of this cohort (median = 6.1, n = 267, P ≤ 0.001). Moreover, a significantly higher percentage of these LMP tumors (72%, n = 13) had expression levels of ROR1 that placed them in the ROR1Low subgroup, and a significantly lower percentage of these cases were in the ROR1Hi subgroup (6%, n = 1) than would be expected by chance (P < 0.0001) (Table S2). Furthermore, segregation of high-grade, late-stage ovarian tumors, described in GSE26712 (36), another PubMed GEO database, into three subgroups by virtue of their relative expression of ROR1 yielded similar findings, identifying patients with ROR1Hi tumors as having a poorer prognosis relative to patients with ovarian cancers in the ROR1Low subgroup (Fig. S1A) (36).
Fig. 1.
Ovarian cancers that express high levels of ROR1 have stem cell-like gene-expression signatures and relatively poor prognosis. (A) Graphs were derived from published data available through the PubMed GEO database (GSE9891). Kaplan–Meier curves depict overall survival (Upper) or progression-free survival (Lower) of patients with ROR1Low (blue line), ROR1Int (black line), or ROR1Hi (red line) ovarian cancers. The P value for the difference between ROR1Low versus ROR1Hi subgroups was determined by the log-rank test. (B) Enrichment plots of side-population gene-expression signatures (23) on ROR1Hi tumors versus ROR1Low cancers in the GSE9891 dataset. Size is the number of genes included in the analysis. NES (normalized enrichment score) accounts for the difference in gene-set size and can be used to compare the analysis results across gene sets. FDR q-val (false discovery rate q value) is the estimated probability that a gene set with a given NES represents a false positive finding. Each gene set is considered significant when the false discovery rate (FDR) is less than 25%. The middle portion of the plot shows where the members of the gene set appear in the list of ranked genes; red and blue colors represent positive and negative correlation with ROR1 expression, respectively.
We ranked the 19,093 genes from the GSE9891 dataset by their relative expression level in ROR1Hi versus ROR1Low ovarian cancer samples for gene-set enrichment analyses (37). Compared with ROR1Low samples, ROR1Hi tumor samples were enriched in expression of gene signatures associated with the “side population” (Fig. 1B, Fig. S1B, and Table S3) (16). ROR1Hi tumors samples also were relatively enriched in expression of gene sets associated with human embryonic stem cells (16, 23, 24) (Fig. 1B, Fig. S1B, and Table S3). Moreover, embryonic stem cell sets of genes targeted by Nanog, Oct4, Sox2, or Nos, Nos tfs, or Myc target 1 genes were enriched or activated in the ROR1Hi ovarian tumor samples relative to ROR1Low samples (Fig. S1B and Table S3). In particular, we noted that four of the nine identified gene sets associated with human embryonic stem cells actually included ROR1 (23). The genes induced by the EMT also were enriched or activated in ROR1Hi tumor samples relative to ROR1Low tumors (Fig. S1C). Finally, the expression level of ALDH1A1, encoding the CSC marker aldehyde dehydrogenase 1, was significantly higher in ROR1Hi ovarian-tumor samples than in ROR1Low ovarian cancer samples (Fig. S1C). However, we did not discern ROR1High ovarian cancers to have relative enrichment in their expression of the 51-gene signature that could distinguish ovarian cancer samples on the basis of morphology (24). Nevertheless, these analyses revealed that high-level expression of ROR1 may be associated with ovarian CSC.
Expression of ROR1 in Primary Ovarian Cancer Cells.
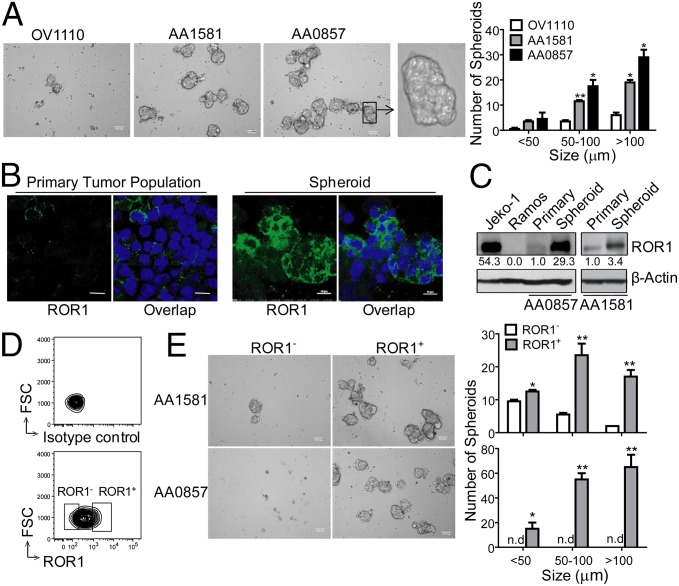
We examined fresh-frozen tumor tissues from each of 14 patients with ovarian cancer for ROR1 protein via immunoblot analysis. As in our previous study using immunohistochemistry (30), we found about half of these ovarian cancers (7 out of 14, 50%) expressed high-level ROR1 by immunoblot analysis (Fig. S2A and Table S4). Similarly, we found that two of three patient-derived xenografts (PDXs) had readily detectable ROR1, as assessed via immunoblot analysis (Fig. S2B), immunohistochemistry, or flow-cytometric analysis (Fig. 2). The PDX sample AA0857 expressed higher levels of ROR1 than did AA1581, which in turn expressed higher levels of ROR1 than did OV1110, which expressed undetectable ROR1 except in a few cells (Fig. 2).
Fig. 2.
ROR1 is expressed on ovarian cancer cells with high ALDH1 activity. (A) OV1110, AA1581, or AA0857 were stained with either HE (Upper) or with the anti-ROR1 mAb 4A5 (Lower). Bound 4A5 is shown in red. (Scale bar: 35 μm.) (B) Flow-cytometric analysis of OV1110, AA1581, or AA0857. The cells were stained with 4A5 or control mAb, and with ALDOFLUOR without (−) or with (+) the ALDH1 inhibitor DEAB, as indicated at the top. The open boxes in the each contour plot indicate the gates for identifying cells with ALDH1 activity, the proportion of which is indicated. The open boxes in the left of the contour plots depict the gates used to identify cells that are certain to lack ALDH1 activity. In the lower row are histograms depicting the fluorescence of cells within these boxes that were negative (left) or positive (right) for ALDH1 activity. The filled histograms depict the fluorescence of cells stained with an isotype-control mAb whereas the open histograms depict the fluorescence of cells stained with 4A5. The number in each plot provides the mean fluorescence intensity ratio (MFIR).
We also examined for expression of ROR1 on cells that had ALDH1 enzymatic activity, which we assessed by treating the cells with ALDEFLUOR. ALDH1 converts ALDEFLUOR into a fluorescent product that can be monitored via flow cytometry. The enhanced fluorescence of cells specifically due to the enzymatic conversion of ALDEFLUOR can be assessed by comparing the fluorescence of stained cells with that of cells stained in parallel with ALDEFLUOR and diethylaminobenzaldehyde (DEAB), an inhibitor of ALDH1-enzymatic activity. We found that the cells that were ALDEFLUOR-positive expressed higher levels of ROR1 than did the ALDEFLUOR-negative cells of the same tumor-cell populations (Fig. 2B and Fig. S2C). Conversely, ROR1+ cells in each of these PDX samples had higher proportions of ALDEFLUOR-staining cells than the tumor cells that did not express ROR1 (Fig. S2D).
Expression of ROR1 in Ovarian Cancer Spheroids.
We cultured single-cell suspensions of primary ovarian cancer cells and monitored their capacity to form spheroids. We did not detect any spheroids in the first week of culture until after cell division had occurred (Fig. S2E). Consistent with previous reports (17, 22), the cells within such spheroids were enriched for cells that had high levels of ALDH1 expression (Fig. S2F). We found that the PDX tumor samples with high proportions of ROR1+ cells (e.g., AA0857) formed significantly greater numbers of spheroids than did the tumor samples with few ROR1+ cells (e.g., OV1110) (Fig. 3A). Moreover, the mean size of the spheroids formed by AA0857 was significantly larger than that of AA1581 or the few spheroids that developed from OV1110. We found that cells that formed spheroids were enriched for cells that expressed high levels of ROR1 relative to that of the original tumor-cell population at the initiation of culture (Fig. 3 B and C).
Fig. 3.
ROR1+ cells give rise to more spheroids than ROR1Neg cells. (A) Photomicrographs of spheroids that developed from cultured single cells isolated from OV1110, AA1581, or AA0857. (Scale bar: 100 µm, except for Far Right, which displays the spheroid in the box under higher magnification.) The bar graph to the Right depicts the average numbers of small (<50 µm), medium (50–100 µm), or large (>100 µm) spheroids formed by cells of OV1110 (open), AA1581 (gray), or AA0857 (black) in triplicate wells ± SEM. Asterisks (*) indicate the statistical significance of differences in the number of spheroids of cells from OV1110 versus AA1581 or AA0857 (*P < 0.05, **P < 0.01, using Student’s t test). (B) Confocal microscopy of spheroids stained with 4A5 (green). Nuclear staining is in blue. (Scale bar: 10 μm.) (C) Immunoblot analyses of cell lysates prepared from cell lines Jeko-1 (ROR1+) or Ramos (ROR1Neg), the primary tumor (Primary), or spheroids, as indicated on the top of each lane; the tumor from which the lysates were derived is indicated at the bottom. The blots were probed for ROR1 or β-Actin, as indicated on the right. The numbers between the panels provide the ratio of band intensities for blots probed with anti-ROR1 versus β-Actin, using Image J software. (D) Strategy for sorting ROR1+ versus ROR1Neg cells. The open boxes indicate the gates used to select ROR1Neg (left) or ROR1+ (right) cells. (E) Photomicrographs of ROR1+ or ROR1Neg cells isolated from AA1581 (Upper) or AA0857 (Lower), as indicated on the left margin. (Scale bar: 100 µm.) The bar graph (Right) depicts the average numbers of small (<50 µm), medium (50–100 µm), or large (>100 µm) spheroids formed in three separate culture wells containing ROR1+ cells (filled bars) or ROR1Neg cells (open bars), as indicated at the bottom of histograms (n.d., not detectable). Error bars indicate SEM. Asterisks (*) indicate the statistical significance of differences between the number of spheroids that formed by ROR1+ cells versus ROR1Neg cells of AA0857 (Upper) or AA1581 (Lower) (*P < 0.05, **P < 0.01, using Student’s t test). FSC, forward light scatter.
ROR1+ and ROR1Neg ovarian cancer cells were isolated from the AA0857 or AA1581 PDXs by staining single-cell suspensions with a noninhibitory anti-ROR1 mAb, 4A5, for FACS sorting using the gates depicted in the bottom contour plot of Fig. 3D. The sorted ROR1+ cancer cells formed significant greater numbers of spheroids and larger spheroids than ROR1Neg tumor cells (Fig. 3E and Fig. S2 E and G). Furthermore, sorted ROR1+ cancer cells were more invasive into Matrigel than the ROR1Neg cancer cells isolated from the same tumor population (Fig. S3 A and B).
Ovarian Cancer Cells That Express ROR1 Have Enhanced Capacity for Engraftment.
PDX tumor samples with high proportions of ROR1+ cells (e.g., AA0857) displayed faster growth in immune-deficient mice than did the tumor samples with intermediate expression of ROR1 or few ROR1+ cells (e.g., OV1110) (Fig. 4A). We isolated ROR1+ and ROR1Neg cells from AA0857 or AA1581 by FACS and performed a tumorigenicity assay in immune-deficient mice using limiting numbers of tumor cells. As shown in Table 1, as few as 500 purified ROR1+ cells from either AA0857 or AA1581 could initiate a tumor in most engrafted mice (Table 1). In contrast, the same number of ROR1Neg tumor cells from either AA0857 or AA1581 did not form tumors except in a few animals, which developed smaller PDX tumors than did the ROR1+ cells (Table 1 and Fig. 4 B and C). We also evaluated the capacity of ROR1+ versus ROR1Neg cells from the AA0857 PDX population to form tumors after orthotopic transfer to the ovary. Although 500 ROR1+ cells could give rise to tumors in the ovary, the ROR1Neg cells were not able to give rise to such tumors (Fig. 4C). Moreover, the percentage of cells in these tumors that expressed ROR1 was similar to that noted in the parental tumors (Fig. 4D), suggesting that ROR1+ cells were able to give rise to ROR1Neg cells.
Fig. 4.
ROR1+ ovarian cancer cells more effectively engraft immune-deficient mice. (A) Tumor growth over time resulting from injection of 1 × 106 OV1110 (filled boxes), AA1581 (filled circles), or AA0857 (filled inverted triangles) per mouse. Asterisks indicate the statistical significance of differences between the mean sizes of the tumors that developed in mice engrafted with AA0857 versus OV1110 (black), AA1581 versus OV1110 (black), or AA0857 versus AA1581 (red) (*P < 0.05, **P < 0.01, ***P < 0.001, using Student’s t test, n = 5 for each group). (B) Gating strategy used for sorting ROR1+ versus ROR1Neg cells, as in Fig. 3D. (C) Photographs of representative tumors extirpated from mice engrafted with ROR1+ or ROR1Neg ovarian cancer cells of AA0857. (Upper) Representative tumors that formed on the flanks of engrafted mice. (Lower) Ovarian tumors orthotopically implanted with sorted ROR1+ or ROR1Neg cells. (D) The fluorescence of single-cell suspensions of unsorted tumor (Initial Tumor), freshly sorted ROR1+ cells before engraftment (Sorted Cell), or cells isolated from tumors that developed in mice engrafted with ROR1+ cells (Tumor of ROR1+ Cells), as indicated at the top, for cells derived from AA1581 (Upper) or AA0857 (Lower), as indicated on the left margin. The cells were stained with either 4A5 (open histograms) or control mAb (filled histograms). The bar indicates the gate used to calculate the proportion of ROR1+ cells, which is indicated in the top right of each histogram.
Table 1.
Tumor incidence in animals implanted with ROR1+ Or ROR1Neg cells isolated from ovarian PDX samples
| Number of cells | ||||||
| Tissue ID | Group | 5,000 | 1,500 | 500 | Frequency | P value |
| AA0857 | ROR1+ | 6/6 (100%) | 2/3 (67%) | 6/7 (86%) | 1/515 | 0.0004 |
| AA0857 | ROR1Neg | 4/5 (80%) | 0/3 (0%) | 1/7 (14%) | 1/4,158 | |
| AA1581 | ROR1+ | n.d. | 4/5 (80%) | 3/5 (60%) | 1/747 | 0.003 |
| AA1581 | ROR1Neg | n.d. | 1/5 (20%) | 0/5 (0%) | 1/9,230 | |
Frequency of tumorigenic cell and probability estimates were computed using Extreme Limiting Dilution Analysis (ELDA) software. n.d., not done.
Silencing Expression of ROR1 Reduces Ovarian Cancer Cell Expression Of Bmi-1 and Markers of EMT.
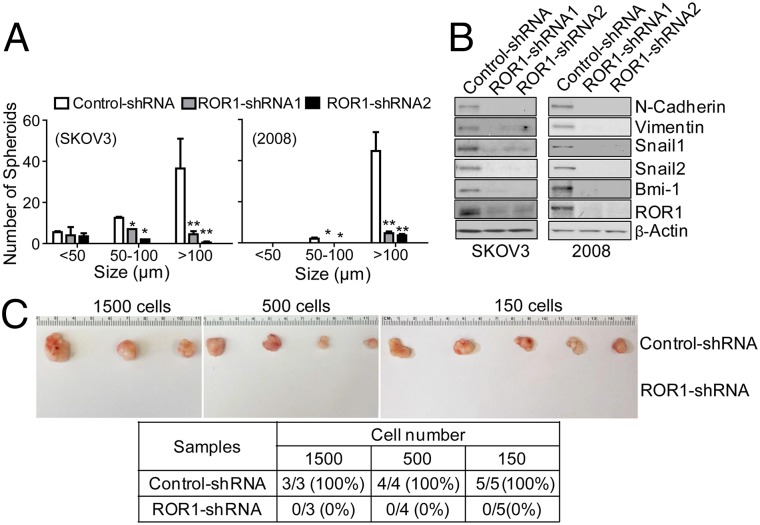
We silenced expression of ROR1 in ovarian adenocarcinoma cell lines (e.g., SKOV3 or 2008) using short-hairpin RNAs (shRNAs), which targeted either of two different sequences of ROR1 (31). Ovarian cancer cells transduced with ROR1-shRNAs have reduced expression of ROR1, formed significantly fewer spheroids and migrated significantly less well into Matrigel compared with the same cell lines transduced with a control shRNA (Fig. 5A and Fig. S3 C and D). Furthermore, silencing ROR1 reduced expression of Bmi-1, the polycomb ring-finger oncogene that regulates CSC self-renewal (38, 39). Moreover, silencing ROR1 protein expression inhibited the expression level of EMT markers (e.g., N-cadherin and vimentin) and SNAIL1/2, master regulators of EMT (Fig. 5B). Finally, cells silenced for ROR1 lost their capacity to form tumors in immune-deficient mice (Fig. 5C).
Fig. 5.
Silencing ROR1 inhibits spheroid formation, tumor engraftment, and the expression of Bmi-1 or markers associated with EMT. (A) The average numbers of spheroids assessed in each of three separate wells (±SEM) for SKOV3 (Left) or 2008 (Right) transfected with control-shRNA (open bars), ROR1-shRNA1 (gray bars), or ROR1-shRNA2 (black bars) are depicted in the histograms, as in Fig. 3E. (B) Immunoblot analysis of lysates made from SKOV3 or 2008 transfected with control-shRNA, ROR1-shRNA1, or ROR1-shRNA2, as indicated at the top of each lane. The blots were probed for the proteins listed on the right. (C) Representative tumors extirpated from immune-deficient mice engrafted with defined numbers of 2008 cells transfected with either control-shRNA (top row) or ROR1-shRNA (bottom row). (Lower) A table providing the numbers of animals that had tumor at 5 wk post tumor-cell injection. The numbers of injected tumor cells are indicated at the top of the columns, which provide the numbers of mice that developed tumor over the number of mice injected with 2008 cells transfected with control-shRNA or ROR1-shRNA (bottom row). The percentage of injected animals that developed tumor is provided in parentheses.
A Humanized Antibody Specific for ROR1 Could Inhibit the Capacity of Ovarian Cancer Cells to Form Spheroids in Vitro or Engraft Immune-Deficient Mice.
We developed a humanized anti-ROR1 antibody, named cirmtuzumab (UC-961), which has high binding affinity and can recognize the same epitope as D10, a lower affinity mouse mAb specific for ROR1 that had functional activity against ROR1+ tumor cells in vitro and in vivo (33, 40). Treatment with UC-961 blocked the capacity of AA0857 tumor cells to form spheroids and reduced their expression of ROR1 and vimentin (Fig. 6). UC-961–treated tumor cells also lost their capacity to migrate into Matrigel (Fig. S4). We examined whether treatment with UC-961 could inhibit the capacity of AA0857 cells to form tumors in immune-deficient mice. We treated mice engrafted with AA0857 cells with biweekly i.v. infusions of UC-961 (at 10 mg/kg) or control human immunoglobulin G (hIgG) and found that UC-961 significantly suppressed the development and growth of the PDX tumors (Fig. 7 A–C and Fig. S5 A–C).
Fig. 6.
ROR1 antibody UC-961 can inhibit spheroid formation of ROR1+ cancer cells. (A, Left) Representative images of spheroids formed by AA0857 ovarian cancer cells cultured for 3 wk in media containing control hIgG or UC-961, each at 50 µg/mL. (Scale bar: 100 µm.) Average numbers of spheroids formed in each of three separate wells with either treatment (±SEM) are indicated in the bar graph (Right), as in Fig. 3D. (B) Graph showing the average numbers of detected spheroids over time in three separate wells with either treatment (±SEM). (C) Confocal microscopy of ovarian cancer cell spheroids stained with mAb specific for ROR1 (green) or vimentin (red), after treatment with UC-961 for 48 h. Nuclear staining is in blue. (Scale bar: 10 μm.)
Fig. 7.
ROR1 MAb inhibits tumor engraftment. (A) AA0857 cells (2.5 × 104) were engrafted into Rag2−/−γc−/− mice, which were subsequently treated with control hIgG or UC-961 at times indicated by the arrows. The line graph provides the mean tumor volume over time of control hIgG-treated (red) or UC-961–treated (black) mice ± SEM (n = 3 for each group). Asterisks indicate a significant difference between the mean volume measured in control-treated versus UC-961–treated mice (*P < 0.05, **P < 0.01, ***P < 0.001, using Student’s t test). (B) Representative images of tumors extirpated from mice treated with control hIgG (Upper) or UC-961 (Lower). (C) Average weights of extirpated tumors from mice treated with control hIgG or UC-961 (n = 3). Error bars indicate SEM. (D) Flow-cytometric analysis of cells isolated from tumors extirpated from mice treated with either control hIgG or UC-961 upon staining with ALDOFLOUR ± the ALDH1-inhibitor DEAB, as indicated at the top of each contour plot. The box in the contour plot indicates the gate used to detect cells with ALDH1 activity. The bar graph (Right) provides the average proportion of ALDOFLUOR-staining cells in each tumor population (n = 3) with error bars indicating the SEM. (E) Single-cell suspensions of tumors from AA0857-engrafted immune-deficient mice that were treated with either a control antibody or UC-961 were sorted into ALDH1+ versus ALDH1Neg subgroups by FACS. Defined numbers of ALDH1+ or ALDH1Neg human tumor cells were engrafted into immune-deficient mice, and the development of tumor xenografts was assessed up to 5 mo postengraftment. Frequency of tumorigenic cell and probability estimates were computed using ELDA software.
Single-cell suspensions were made from tumors that developed in control-treated mice or the few mice among those that were treated with UC-961. Compared with the tumors that developed in control-treated mice, the tumors that developed in UC-961–treated mice had lower levels of ROR1 by immunoblot analysis (Fig. S5D), reduced proportions of ALDH1+ cells by flow cytometry (Fig. 7D), and reduced expression of markers associated with EMT (e.g., vimentin) (Fig. S5D). We isolated ALDH1-positive (ALDH1+) versus ALDH1-negative (ALDH1Neg) cells from tumors that developed in mice treated with either UC-961 or control hIgG and examined their capacity to form tumors upon adoptive transfer into immune-deficient mice. As noted in prior studies (40), ALDH1+ cells from tumors of control-treated mice engrafted more effectively than ALDH1Neg cells of the same tumor population (Fig. 7E). However, the ALDH1+ cells from the tumors of UC-961–treated mice did not have an apparent advantage over ALDH1Neg cells in engrafting such immune-deficient mice (Fig. 7E). Moreover, the tumor cells isolated from UC-961–treated mice had lower expression of Bmi-1 and markers associated with EMT (e.g., vimentin) (Fig. S5 E and F) than the tumors that developed in control-treated mice. Collectively, these data suggest that treatment with UC-961 may inhibit the growth and self-renewal of CSC.
Discussion
Analysis of gene-expression data revealed that patients with ovarian cancers that expressed high levels of ROR1 had a shorter median progression-free survival and overall survival than patients with tumors that had low-level expression of ROR1. Moreover, cases of ovarian cancer that had low malignant potential generally had low-to-negligible expression of ROR1. Consistent with these observations, we noted that ovarian cancers that expressed ROR1 by immunohistochemistry more commonly had a high-grade, less-differentiated histology (30), which typically is associated with aggressive disease (41). This also is consistent with a recently published study indicating that high-level expression of ROR1, assessed by immunohistochemistry of primary tumor tissue, was an independent prognostic factor for predicting relatively short disease-free survival or overall survival of patients with ovarian cancer (34).
We found ovarian cancers that expressed high levels of ROR1 had gene-expression signatures associated with CSCs. Compared with ROR1Low cases, ROR1Hi ovarian cancers had higher expression of gene signatures associated with the side population, which may contain CSCs (42). Moreover, ROR1Hi ovarian cancers were enriched for expression of genes associated with embryonic stem cells and EMT (17, 25), which facilitates the capacity of tumor cells to migrate and seed metastatic sites (21, 43). We also observed that two primary ovarian cancers that expressed ROR1 were better able to grow as xenografts in immune-deficient mice than one that lacked expression of ROR1. Furthermore, within any one tumor population, the cells that expressed ALDH1, a marker of ovarian CSCs, expressed higher levels of ROR1, as did ovarian cancer cells that formed tumor spheroids, a functional characteristic associated with CSCs and EMT (43). Finally, we found that ROR1+ cells were better able to form spheroids, invade ECM, or engraft immune-deficient mice than ROR1Neg cells from the same tumor population. Collectively, these studies indicate that ovarian CSCs may express relatively high levels of ROR1.
Prior studies found that expression of ROR1 could enhance tumor-cell proliferation, migration/invasion, and tumorigenicity (30, 33, 40). Conversely, silencing ROR1, or treatment with an anti-ROR1 mAb, could enhance apoptosis, inhibit cell proliferation and migration/invasion, and reduce the capacity of tumor cells to develop metastatic foci (30, 31, 33). Similarly, silencing ROR1 could inhibit the capacity of primary ovarian cancer cells to form spheroids, invade ECM, or to develop tumor xenografts, which are functional characteristics associated with CSCs. As such, the present study demonstrates that ovarian CSCs also may have a dependency upon ROR1.
In the present study, we examined whether UC-961 could target ovarian CSCs. UC-961 is a humanized IgG1 mAb currently in clinical trials that binds with high affinity to the same epitope of ROR1 as the low-affinity mAb D10, which, in prior studies, was found capable of down-modulating ROR1, impairing ROR1+ leukemia-cell engraftment, and inhibiting breast-cancer cell metastasis (33, 40). We found that treatment of primary ovarian PDX tumor cells with an anti-ROR1 mAb, UC-961, could inhibit spheroid formation and migration in vitro and engraftment in immune-deficient mice. Furthermore, the small tumors that did develop in mice treated with UC-961 had reduced expression of Bmi-1, ALDH1, ROR1, and genes associated with EMT. Moreover, treatment with anti-ROR1 impaired the capacity of tumor xenografts to re-engraft a virgin mouse. These studies suggest that treatment with this anti-ROR1 mAb could impair the self-renewal capacity of CSC cells in vivo. As such, these studies demonstrate that UC-961 might inhibit the maintenance and/or self-renewal of ovarian CSCs, which otherwise may be resistant to chemotherapy and responsible for relapse after conventional anticancer treatment (4–6).
Although several putative ovarian CSC markers have been identified (e.g., CD133, ALDH1, CD44, and CD117) (8, 18, 19, 44, 45), such markers also can be found on normal adult tissues, including normal stem cells (10, 46, 47). Therefore, targeting such antigens with mAbs or small molecules also may affect the normal tissues that express these antigens (48, 49). However, with few exceptions (50), the postpartum expression of ROR1 seems restricted to cancer cells. Furthermore, due to its apparent functional role in promoting tumor-cell growth, metastasis, and tumor initiation, ROR1 seems to be a promising target for specific therapy directed against ovarian CSCs.
Materials and Methods
Primary ovarian-tumor specimens were collected from patients, who provided written informed consent on a protocol approved by the Institutional Review Board of the University of California, San Diego (HRPP 090401), in accordance with the Declaration of Helsinki. The animal study protocol was approved by the University of California, San Diego and Medical Experimental Animal Care Committee. The PDX models were established using mechanically minced fresh ovarian cancer specimens. Early passages (1–5) of primary-tumor tissues from these PDX models were mechanically minced and enzymatically and mechanically dissociated using GentleMACS Dissociator (Miltenyi Biotec) in accordance to the manufacturer’s protocol. Dead cells and erythrocytes were removed through density gradient centrifugation using Percoll Plus (CC-17-5442-01; GE Healthcare Life Sciences) following the manufacturer’s protocol.
Supplementary Material
Acknowledgments
We thank Dennis Young for flow-cytometry analysis and sorting, the University of California, San Diego (UCSD) Histology Core laboratory for technical help with processing tissue specimens, Dr. Nissi Varki for histology and immunohistochemistry assessments, UCSD Moores Cancer Center Biorepository and Tissue Technology Shared Resource for PDX samples, and Dr. Lei Bao for statistical analysis assistance. This study was supported by NIH Grant P01-CA081534, the California Institute for Regenerative Medicine, and the Blood Cancer Research Fund, UCSD Foundation, Cancer Center Support Grant P30CA23100.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419599111/-/DCSupplemental.
References
- 1.McGuire WP, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 2.Bookman MA, et al. Carboplatin and paclitaxel in ovarian carcinoma: A phase I study of the Gynecologic Oncology Group. J Clin Oncol. 1996;14(6):1895–1902. doi: 10.1200/JCO.1996.14.6.1895. [DOI] [PubMed] [Google Scholar]
- 3.Pliarchopoulou K, Pectasides D. Epithelial ovarian cancer: Focus on targeted therapy. Crit Rev Oncol Hematol. 2011;79(1):17–23. doi: 10.1016/j.critrevonc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Clarke MF, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 5.Alvero AB, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8(1):158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curley MD, Garrett LA, Schorge JO, Foster R, Rueda BR. Evidence for cancer stem cells contributing to the pathogenesis of ovarian cancer. Front Biosci (Landmark Ed) 2011;16:368–392. doi: 10.2741/3693. [DOI] [PubMed] [Google Scholar]
- 7.Baba T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28(2):209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 8.Curley MD, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27(12):2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 9.Landen CN, Jr, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng S, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS ONE. 2010;5(4):e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penumatsa K, Edassery SL, Barua A, Bradaric MJ, Luborsky JL. Differential expression of aldehyde dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian tumors. J Ovarian Res. 2010;3:28. doi: 10.1186/1757-2215-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida A, Rzhetsky A, Hsu LC, Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem. 1998;251(3):549–557. doi: 10.1046/j.1432-1327.1998.2510549.x. [DOI] [PubMed] [Google Scholar]
- 13.Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: Problems for therapy? J Pathol. 2011;223(2):147–161. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- 14.Szotek PP, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moserle L, et al. The side population of ovarian cancer cells is a primary target of IFN-alpha antitumor effects. Cancer Res. 2008;68(14):5658–5668. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- 16.Vathipadiekal V, et al. Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS ONE. 2012;7(1):e29079. doi: 10.1371/journal.pone.0029079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YC, et al. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am J Pathol. 2012;180(3):1159–1169. doi: 10.1016/j.ajpath.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrandina G, et al. CD133 antigen expression in ovarian cancer. BMC Cancer. 2009;9:221. doi: 10.1186/1471-2407-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abubaker K, et al. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden. Mol Cancer. 2013;12:24. doi: 10.1186/1476-4598-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidowitz RA, et al. Mesenchymal gene program-expressing ovarian cancer spheroids exhibit enhanced mesothelial clearance. J Clin Invest. 2014;124(6):2611–2625. doi: 10.1172/JCI69815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao J, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9(1):e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwede M, et al. Stem cell-like gene expression in ovarian cancer predicts type II subtype and prognosis. PLoS ONE. 2013;8(3):e57799. doi: 10.1371/journal.pone.0057799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin Cancer Biol. 2012;22(5-6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda T, et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci USA. 2008;105(8):3047–3052. doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabbani H, et al. Expression of ROR1 in patients with renal cancer: A potential diagnostic marker. Iran Biomed J. 2010;14(3):77–82. [PMC free article] [PubMed] [Google Scholar]
- 28.Barna G, et al. ROR1 expression is not a unique marker of CLL. Hematol Oncol. 2011;29(1):17–21. doi: 10.1002/hon.948. [DOI] [PubMed] [Google Scholar]
- 29.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 2011;71(8):3132–3141. doi: 10.1158/0008-5472.CAN-10-2662. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, et al. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181(6):1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS ONE. 2012;7(3):e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi T, et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21(3):348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Cui B, et al. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013;73(12):3649–3660. doi: 10.1158/0008-5472.CAN-12-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, et al. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep. 2014;4:5811. doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tothill RW, et al. Australian Ovarian Cancer Study Group Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 36.Bonome T, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68(13):5478–5486. doi: 10.1158/0008-5472.CAN-07-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113(2):175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreso A, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20(1):29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 40.Widhopf GF, 2nd, et al. ROR1 can interact with TCL1 and enhance leukemogenesis in Eμ-TCL1 transgenic mice. Proc Natl Acad Sci USA. 2014;111(2):793–798. doi: 10.1073/pnas.1308374111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter WE, 3rd, et al. Gynecologic Oncology Group Study Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(24):3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo S, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10(2):325–335. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinogradov S, Wei X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine (Lond) 2012;7(4):597–615. doi: 10.2217/nnm.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricci F, et al. ALDH enzymatic activity and CD133 positivity and response to chemotherapy in ovarian cancer patients. Am J Cancer Res. 2013;3(2):221–229. [PMC free article] [PubMed] [Google Scholar]
- 45.Silva IA, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71(11):3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer N, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133) Cells Tissues Organs. 2008;188(1-2):127–138. doi: 10.1159/000112847. [DOI] [PubMed] [Google Scholar]
- 47.Arber DA, Tamayo R, Weiss LM. Paraffin section detection of the c-kit gene product (CD117) in human tissues: Value in the diagnosis of mast cell disorders. Hum Pathol. 1998;29(5):498–504. doi: 10.1016/s0046-8177(98)90066-1. [DOI] [PubMed] [Google Scholar]
- 48.Slomiany MG, et al. Inhibition of functional hyaluronan-CD44 interactions in CD133-positive primary human ovarian carcinoma cells by small hyaluronan oligosaccharides. Clin Cancer Res. 2009;15(24):7593–7601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casagrande F, et al. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer. 2011;117(24):5519–5528. doi: 10.1002/cncr.26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broome HE, Rassenti LZ, Wang HY, Meyer LM, Kipps TJ. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk Res. 2011;35(10):1390–1394. doi: 10.1016/j.leukres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.