Significance
Peripheral nerve injury is a major neurological disorder that can cause multiple motor and sensory disturbances. In this study we found that VEGF-B can be used as a previously unidentified therapeutic for treating peripheral nerve injury. We demonstrated that VEGF-B stimulated nerve regeneration and enhanced the recovery of both tissue sensation and the ability of nerves to enhance healing of innervated tissue. The physiologic relevance of VEGF-B is demonstrated by our findings showing that mice lacking VEGF-B have impaired nerve regeneration and that nerve injury resulted in increased endogenous expression of VEGF-B. We discover that VEGF-B induces strong elongation and branching of neurons and requires specific transmembrane receptors as well as activation of a complex intracellular signaling.
Keywords: VEGF-B, nerve injury, neuronal growth, cornea
Abstract
VEGF-B primarily provides neuroprotection and improves survival in CNS-derived neurons. However, its actions on the peripheral nervous system have been less characterized. We examined whether VEGF-B mediates peripheral nerve repair. We found that VEGF-B induced extensive neurite growth and branching in trigeminal ganglia neurons in a manner that required selective activation of transmembrane receptors and was distinct from VEGF-A–induced neuronal growth. VEGF-B–induced neurite elongation required PI3K and Notch signaling. In vivo, VEGF-B is required for normal nerve regeneration: mice lacking VEGF-B showed impaired nerve repair with concomitant impaired trophic function. VEGF-B treatment increased nerve regeneration, sensation recovery, and trophic functions of injured corneal peripheral nerves in VEGF-B–deficient and wild-type animals, without affecting uninjured nerves. These selective effects of VEGF-B on injured nerves and its lack of angiogenic activity makes VEGF-B a suitable therapeutic target to treat nerve injury.
Nerves can be damaged either through trauma or disease. Nerves from the peripheral nervous system (PNS) have significantly greater capacity to regenerate and reinnervate their original targets after injury, compared with nerves from the CNS. The successful regeneration of PNS neurons requires a number of intrinsic and extrinsic factors, as well as a permissive microenvironment for axonal regrowth (1). Among the numerous growth factors able to induce nerve regeneration, the family of VEGFs has been implicated as a potent mediator of developmental neurogenesis and adult nerve regeneration (2–4). VEGF-A is a well-characterized and potent angiogenic factor but is also a strong inducer of nerve growth. Several studies have demonstrated that both VEGF-A and -B are expressed during peripheral nerve injury (2, 5). In the setting of injury, VEGF-B plays a role in cell survival, nerve protection, and growth (5, 6). The survival effect of VEGF-B on brain cortical neurons, retinal neurons, and motor neurons in the spinal cord is indicative of its pleiotropic role (5). VEGF-B treatment reduced stroke volume in a middle cerebral artery ligation model and increased survival of retinal ganglion cells in an optic nerve crush injury model (7), and VEGF-B knockout mice suffered severe strokes and exacerbated retinal ganglion cell death in both injury models (7–9). VEGF-B has also been used with promising results in Parkinson’s disease (10) and amyotrophic lateral sclerosis models (11).
Given the ability of VEGF-B to regulate both vascular endothelial cells (angiogenesis) as well as axonal growth and survival after injury, it is unclear whether VEGF-B exerts its effects on nerve regeneration through the increase in blood supply or through direct effects on nerve tissue. Indeed, specific studies on its role on peripheral neurons independent of its vascular role are lacking. We have previously reported that VEGF-A can stimulate trigeminal neuronal cell growth and enhance cornea nerve regeneration, resulting in anatomical and functional recovery of peripheral injured nerves independently of its angiogenic effects (12). Here we studied the neuro-regenerative potential of VEGF-B in an avascular model of peripheral nerve injury in mice and the signaling elements involved in the induction of nerve growth. Our results demonstrated tha (i) peripheral nerve regeneration is impaired in mice lacking VEGF-B, (ii) VEGF-B can restore the anatomic and function innervation of target tissues by induction of nerve growth and nerve regeneration, (iii) the effects of VEGF-B are specific for injured nerves and are independent of any vascular effect, and (iv) the effects of VEGF-B on nerve regeneration are distinct from those observed for VEGF-A.
Results
VEGF-B Promotes Trigeminal Ganglion Neuron Growth and Survival in Vitro.
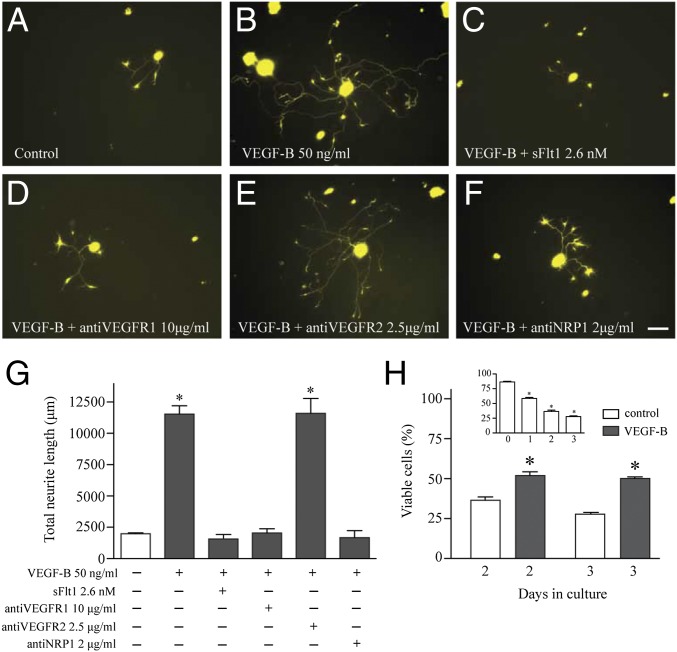
Peripheral nerves such as those that innervate the cornea have their origin far from the site they innervate at the trigeminal ganglia (TG). To evaluate the role of VEGF-B on promoting peripheral nerve growth we treated isolated TG neuronal cells with different concentrations of VEGF-B and found that strong formation and elongation of neurites is induced when cells were incubated with 50 ng/mL VEGF-B; at this concentration the length of neurites is almost fourfold greater than in untreated controls (Fig. 1 A, B, and G). The effect of VEGF-B on inducing neurite growth is dose dependent, as shown in Fig. S1. The VEGF-B signaling leading to neurite growth was inhibited by soluble VEGF receptor 1 (VEGFR1) (sFlt1) (Fig. 1C), antibodies against VEGFR1 (Fig. 1D), or neuropilin 1 (NRP1) (Fig. 1F), but not by antibodies against VEGFR2 (Fig. 1E). VEGFR1 and NRP1 are expressed in TG neurons (Fig. S2), and quantification of the VEGF-B–induced neurite length demonstrated that both VEGFR1 and NRP1 are required for nerve growth and can be inhibited in a dose-dependent manner by specific antibodies (Fig. 1G and Fig. S3). However, VEGF-B action does not require VEGFR2 activation because inhibition of the receptor via antibodies or blocking its tyrosine kinase domains with the specific inhibitor SU 1498 and Ki 8751 does not block the VEGF-B–induced neurite growth (Fig. 1G and Fig. S4). TG neurons cultured without serum died in a time-dependent manner, and only 25% of cells survived after 3 d in culture (Fig. 1H, Inset). However, addition of 50 ng/mL VEGF-B as a single dose during the first day of culture was protective against neuronal death, with 50% of cells surviving at days 2 and 3 (Fig. 1H). Thus, our results show that VEGF-B not only promotes neurite growth but is also neuroprotective in vitro.
Fig. 1.
VEGF-B–induced neurite growth and survival on TG neurons via VEGFR1 and NRP1. Neurons from TG of thy1-YFP mice were isolated and cultured as indicated in Methods. (A) After 3 d in culture, few neurites grew in neurons cultured only in medium. (B) Addition of VEGF-B immediately after plating the cells induced long and extensive branching of neurites. (C) The neurite growth was competitively inhibited by soluble VEGFR1 at an equimolar dose or (D) by antibodies against VEGFR1 or (F) neuropilin 1, (E) but not by antibodies against VEGFR2. (G) Quantification analysis of neurite length demonstrated that VEGF-B–induced growth requires activation of VEGFR1 and NRP1. (H, Inset) The effect of VEGF-B on neuronal survival, analyzed as described in Methods, showed that there was a time-dependent cell death of TG neurons after 3 d in culture, and only 25% of neurons were found viable at this time point. (H) However, neuronal viability increased to 50% when cells were treated with a single dose of 50 ng/mL of VEGF-B. Data represent the mean ± SEM of three independent experiments, and a P value < 0.05 was considered statistically significant between treatments. (Scale bar, 50 μm.)
VEGF-B Induced Extensive Neurite Elongation and Branching.
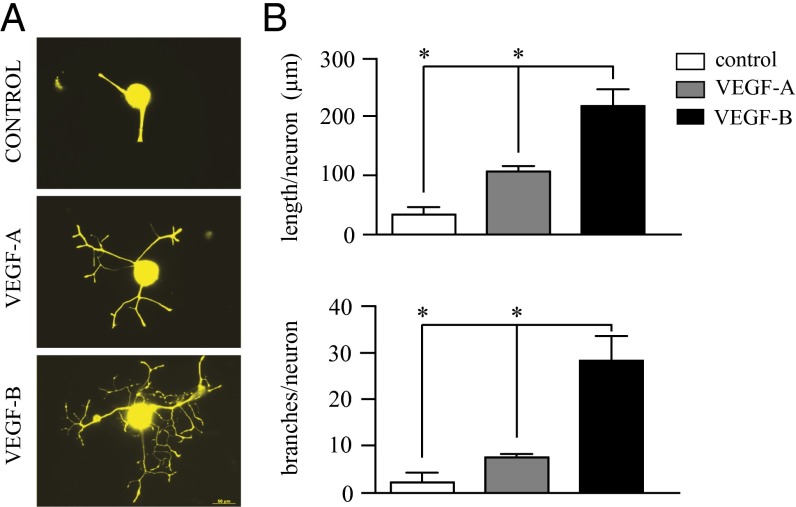
The effect of VEGF-B on neurite growth was significantly different from that observed previously for VEGF-A (12). VEGF-B induced extensive elongation and branching of the neurites, whereas VEGF-A induces mainly neurite elongation, with few branching nodes (Fig. 2A). The VEGF-B–induced neurite elongation was fivefold higher than in untreated controls and more than twofold higher than that induced by VEGF-A treatment (Fig. 2B). Additionally, VEGF-B significantly induced neurite branching in TG neurons: the number of branching nodes was sevenfold higher than in untreated controls and more than fourfold higher than in VEGF-A–treated neurons (Fig. 2B).
Fig. 2.
VEGF-B induces greater neurite elongation and branching than VEGF-A. Isolated trigeminal neurons were treated with either VEGF-A or VEGF-B at 50 ng/mL and neurite growth analyzed after 3 d in culture using Neurolucida software. (A) VEGF-A induced neurite growth with limited branching, whereas VEGF-B induced more extensive elongation and branching. (B) Quantification of the total neurite length demonstrated that VEGF-B induces strong growth with a greater branching area than VEGF-A. Data represent the mean ± SEM of five independent experiments. *P ≤ 0.01. (Scale bar, 50 μm.)
To further investigate the mechanisms by which VEGF-B–stimulated nerve growth differs from that of VEGF-A, we examined the changes in the transcriptome of isolated trigeminal neurons. The gene expression profile in response to the application of VEGF-A or -B showed two- to eightfold differences (90% confidence) on approximately 200 genes (Fig. S5A), and most of these genes were up-regulated compared with untreated TG neurons (Fig. S5B). We found that VEGF-A induced higher expression of VEGF receptors 1, 2, and 3 compared with untreated or VEGF-B–treated neurons, and both VEGF-A and -B induced higher expression of NRP1 compared with controls (Fig. S6A). Although the expression levels of common signaling elements implicated in the VEGF pathway were similar (Fig. S6B), we found significant changes in gene expression in important mediators of the Notch, Wnt, and Semaphorin/Plexin signaling pathways, as well as genes involved in cellular sprouting and neuroprotection (Fig. S7). Target validation using a γ secretase inhibitor demonstrated that activation of the Notch pathway is required for VEGF-induced neurite elongation (Fig. S8), demonstrating the complexity of VEGF-induced neuronal growth signaling.
The PI3K-Akt pathway has been implicated in VEGF signaling (13, 14). To identify potential mechanisms for VEGF-B activity on peripheral nerves, we used a specific PI3K inhibitor and demonstrated that the PI3K pathway is required for VEGF-induced neurite elongation (Fig. S9). We examined whether other ligands that activate this pathway could induce neuronal growth. We treated TG neurons with placenta growth factor 2 (PlGF2) (which binds to VEGFR1) and heparin binding-EGF (HB-EGF) (which activates Akt) and analyzed the induction of neurite growth after 4 d incubation. Both ligands induced potent neurite growth (Fig. S10). However, the neurons were less responsive to PlGF2 or HB-EGF than when treated with VEGF-B.
Peripheral Nerve Injury Induces Endogenous VEGF-B Expression.
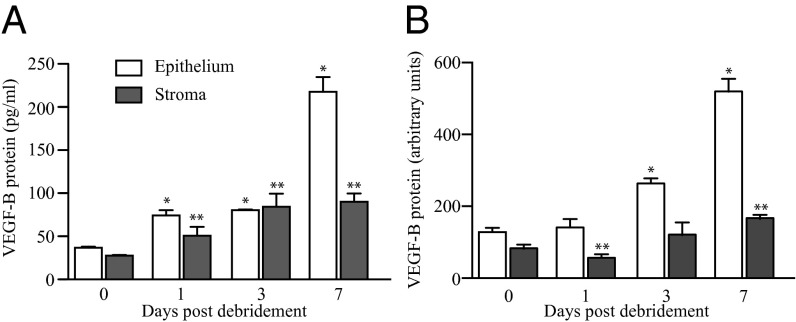
Previous studies have indicated that VEGF-B plays a minimal role under physiologic conditions but may have more profound functions in disease (2, 11). We aimed to determine whether the expression levels of VEGF-B change in sensory nerve innervated tissues after peripheral nerve injury. We used a model of PNS injury in which the subbasal corneal epithelial plexus is removed via epithelial debridement to damage the superficial dense nerve endings. C57BL/6 wild-type mice were subjected to corneal epithelial debridement, and epithelial and stromal cells were collected immediately after the procedure and at 1, 3, and 7 d later to determine protein expression. VEGF-B protein levels analyzed by ELISA showed a concomitant time-dependent increase, with highest amounts of VEGF-B on day 7. After 1 wk the VEGF-B protein expression was sixfold higher in epithelium and threefold higher in the stroma compared with levels observed at the time of debridement (Fig. 3A). These data were corroborated by Western blot that also showed a significant increase of VEGF-B in both epithelium and stroma after 1 wk of cornea injury (Fig. 3B).
Fig. 3.
Endogenous VEGF-B expression increases after peripheral nerve injury. The peripheral nerve endings that innervate the cornea were injured in C57BL/6 mice by central corneal epithelial debridement. Epithelial and stroma samples were collected at the time of debridement or after 1, 3, and 7 d. (A) The ELISA data showed that the VEGF-B protein levels steadily increased in both epithelium and stroma during all days analyzed, with higher level observed in the epithelium at day 7. (B) Western blot analysis from these samples corroborated that the levels of VEGF-B significantly increased after day 7 postinjury in both the epithelium and stroma. Data are expressed as mean ± SEM of two independent experiments (n = 6). *P ≤ 0.05 vs. control epithelium at day 0, **vs. control stroma at day 0.
Impaired Nerve Regeneration in Vegf-b−/− Mice Is Ameliorated by VEGF-B Treatment.
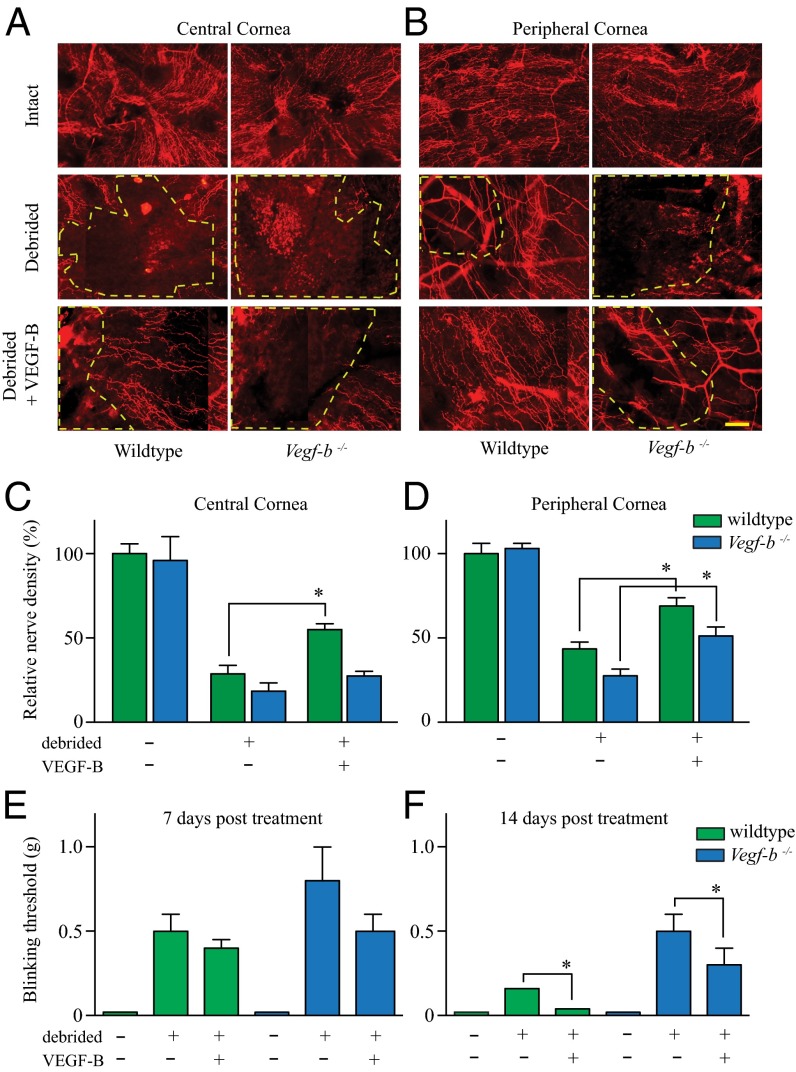
Because peripheral nerve injury induces increasing expression of VEGF-B we aimed to determine if VEGF-B is required for nerve regeneration. Vegf-b−/− mice have no evident neurological deficit and are developmentally healthy (5, 11). First, we analyzed by immunostaining the corneal nerve density and distribution in Vegf-b−/− and wild-type mice and found no differences when both strains were compared (Fig. 4 A and B, Top). We then used the PNS corneal injury model described above to remove via epithelial debridement the trigeminal nerve endings, and after 1 wk we measured the rate of nerve regeneration back into this damaged area. Greater numbers of growing nerve endings into the damaged area were seen in wild-type mice compared with Vegf-b−/−, especially in the peripheral cornea that presented a smaller area without nerve growth (yellow dotted line). We found that Vegf-b−/− mice showed significantly impaired nerve regrowth into the injured zone both at the central and peripheral areas of the cornea (Fig. 4 A and B, Middle). We then examined the ability of exogenous VEGF-B to rescue the impaired corneal nerve regeneration seen in Vegf-b−/− by injecting a single bolus of recombinant VEGF-B in the adjacent subconjunctival space immediately after the time of epithelial debridement. By 1 wk after injury the exogenous VEGF-B induced greater nerve regeneration in both wild-type and Vegf-b−/− mice compared with untreated mice (Fig. 4 A and B, Bottom). The quantification of the newly regenerated nerves growing into the injured zone demonstrated that the application of VEGF-B to wild-type mice enhanced nerve regeneration in both the central and peripheral corneas, whereas the neuroregenerative effects of VEGF-B on Vegf-b−/− mice seemed confined to the peripheral cornea at the 7-d time point (Fig. 4 C and D).
Fig. 4.
VEGF-B is required for anatomical and functional nerve regeneration. C57BL/6 and Vegf-b−/− mice were left untreated or subjected to corneal epithelial debridement. Mice were injected subconjuntivally with 0.5 ng of recombinant mouse (rm) VEGF-B or an equal volume of 0.1% BSA as control. After 1 wk corneas were harvested, flat mounted, and stained with β3-tubulin antibody. Composite images were obtained using an automated microscope stage and Axiovision software (Mosaic module, Zeiss). Nerve density was obtained by analyzing composite images of the central and peripheral cornea using Neurolucida software. (A and B, Top) Normal central and peripheral nerve density and distribution was observed in both wild-type and Vegf-b−/− untreated mice. (B, Middle) In the debrided corneas, the nerves regenerated in the peripheral area in wild-type but not in Vegf-b−/− mice, as indicated by the yellow dashed lines that zoned the area without superficial nerves, and (A, Middle) scarce nerve regeneration was seen in the central cornea in both types of mice. (A and B, Bottom) The administration of VEGF-B induced rapid nerve regeneration in the central and peripheral cornea in wild-type mice but only in the peripheral cornea in Vegf-b−/− mice. (C and D) Nerve tracing analysis shows that Vegf-b−/− mice have a delay in nerve growth compared with wild type, and (C) VEGF-B induced a significant increase in central nerve regeneration only in wild-type mice but (D) significantly increased peripheral nerve regeneration in both types of mice. The functional sensation of the regenerated nerves in the ocular surface was evaluated by the von Frey Hair technique (Methods) in both wild-type and Vegf-b−/− mice. (E) After 1 wk postinjury, corneal sensation was similarly decreased in wild-type and Vegf-b−/− mice. (F) However, after 2 wk postinjury there was a significant recovery of sensation in mice treated with VEGF-B. In wild-type mice the regenerated nerves reached the same sensation level as normal untreated controls, whereas Vegf-b−/− mice demonstrated a delay in recovering complete sensation. Data are expressed as mean ± SEM of three independent experiments (n = 8). *P ≤ 0.05. (Scale bar, 20 μm.)
VEGF-B Improves Functional Sensation of Injured Nerves.
The cornea is a highly sensitive tissue, and we next investigated whether the anatomic recovery of corneal innervation in response to VEGF-B has functional consequences. For this, the corneas of mice subjected to epithelial debridement were gently touched with a set of thin filaments known as von Frey hairs, which assess the sensitivity to mechanical stimulation. After epithelial debridement and the loss of superficial sensory nerves, the mechanosensation of the ocular surface was dramatically impaired. Our analysis showed that VEGF-B treatment began to improve the functional recovery sensation of the corneas in both wild-type and Vegf-b−/− mice after only 7 d of treatment (Fig. 4E). After 2 wk the VEGF-B treatment significantly improved the sensation levels in both wild-type mice and Vegf-b−/− mice. Sensation in VEGF-B–treated wild-type animals was completely restored at this time point, whereas VEGF-B–treated Vegf-b−/− mice had significantly improved sensation compared with untreated Vegf-b−/− mice (Fig. 4F).
VEGF-B Stimulates Neuronal Growth Only in Injured Nerves.
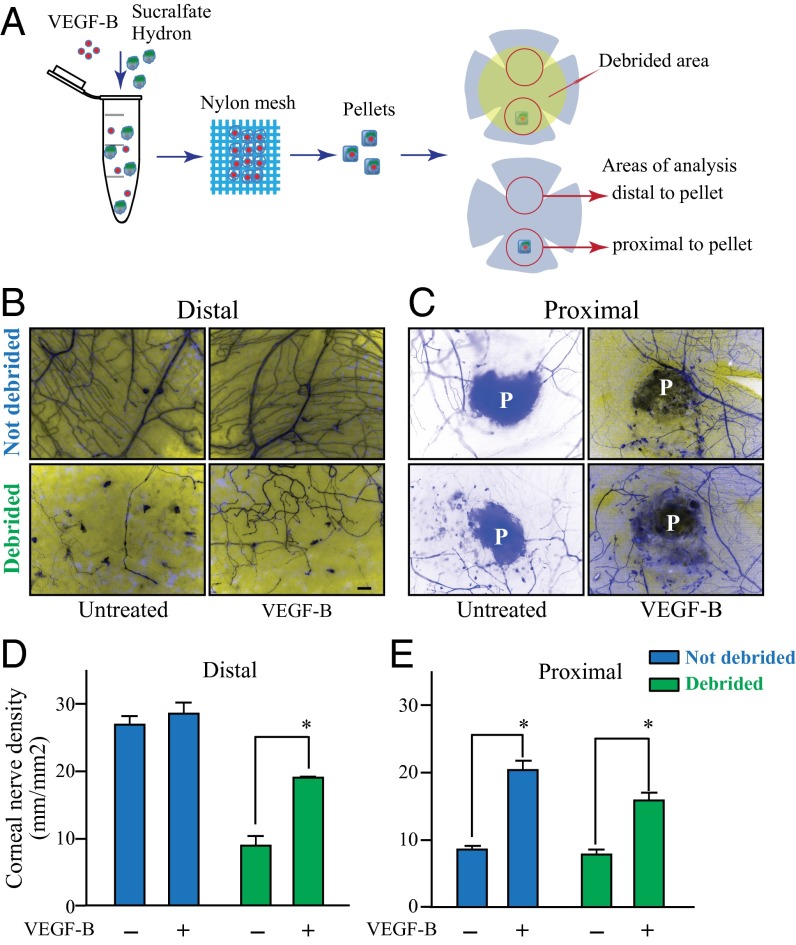
Given previous observations that VEGF-B has differential effects on intact vs. injured tissues (2, 11), we evaluated whether the VEGF-B–induced peripheral sensory neuron regeneration was specific to injured neurons. We used thy1-YFP neurofluorescent mice to evaluate the in vivo effects of sustained VEGF-B release at the site of nerve injury on corneal nerve regeneration. For this we induced two types of injury in the cornea and measured the nerve growth into the damaged areas by using nerve tracing software. To ensure the slow release of VEGF-B into the cornea, a hydron pellet containing either vehicle or VEGF-B was implanted into the corneal stroma, a procedure that results in a localized injury. After recovery, a subset of mice was subjected to epithelium debridement to injure the superficial nerves, resulting in a wider nerve injury (Fig. 5A). Analysis by fluorescence microcopy of cornea whole mounts taken from these animals after 3 d demonstrated that there was no alteration of corneal nerve density or patterning in the absence of injury in mice treated with vehicle or VEGF-B (Fig. 5B, Upper, and Fig. 5D, blue bars). However, there was significantly increased nerve density in response to VEGF-B at the site of pellet implantation, at which there was limited injury (Fig. 5C, Upper, and Fig. 5E, blue bars). When mice receiving VEGF-B–releasing pellets were also subjected to superficial corneal debridement, we observed a more profound effect, with significantly increased innervation throughout sites of injury compared with control animals receiving vehicle (Fig. 5B and C, Lower, and Fig. 5 D and E, green bars).
Fig. 5.
Slow release of VEGF-B into the cornea selectively enhances regeneration of injured nerves. A hydron pellet containing VEGF-B or vehicle was inserted into a corneal stroma micropocket in thy1-YFP mice. The pellet ensures the slow release of VEGF-B into the cornea. (A) One day after the procedure a subset of mice received epithelial debridement, and 3 d later corneas were collected and flat mounted for immunofluorescence analysis. Nerve growth was traced using Neurolucida software. (B, Upper and D, blue bars) In uninjured corneas, VEGF-B treatment did not induce any alteration in corneal nerve density or patterning. (C, Upper and E, blue bars) We observed a significant increase in nerve regeneration at the site of pellet implantation, which corresponded to a site of focal injury, in animals receiving VEGF-B treatment compared with vehicle controls. (B and C, Lower and D and E, green bars) When mice receiving VEGF-B–releasing pellets were also subjected to superficial corneal debridement, a more extensive injury, we observed a more profound effect, with significantly increased innervation throughout sites of injury compared with control animals. Data are expressed as mean ± SEM of two independent experiments (n = 8). *P ≤ 0.05. (Scale bar, 100 μm.)
Impaired Corneal Epithelial Wound Healing in Vegf-b−/− Mice Is Improved by VEGF-B.
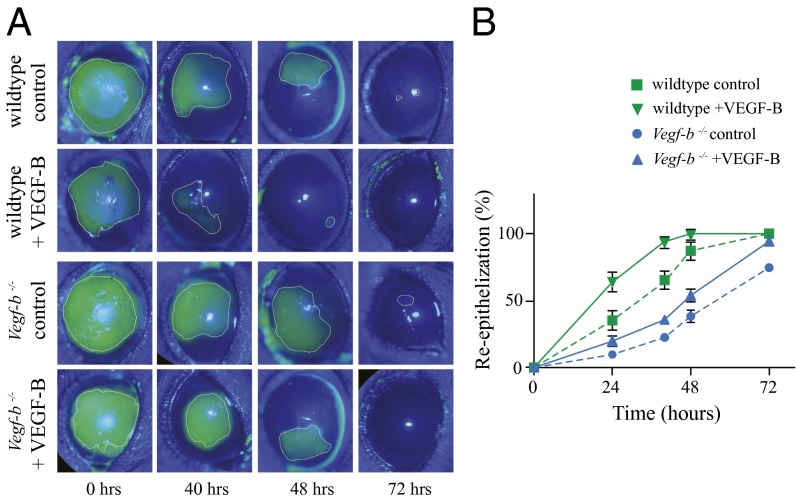
The well-known trophic and functional relationship between nerves and epithelium in the cornea led us to study whether VEGF-B has any effect on epithelial wound healing. We analyzed whether VEGF-B influences the wound healing in wild-type and Vegf-b−/− mice subjected to corneal epithelial debridement. We used fluorescein staining to demarcate the epithelial wounded area and found that in untreated control mice there was a delay in the reepithelization of the injured cornea in Vegf-b−/− compared with wild-type mice. At 48 h a significant area of the injured epithelium was healed in wild-type mice, but a larger injured area was still present in Vegf-b−/−. Subconjunctival injection of VEGF-B clearly accelerated the epithelium regrowth in both wild-type and Vegf-b−/− mice. There was almost complete healing in VEGF-B–treated wild-type mice and only half the injured area in Vegf-b−/− mice compared with untreated controls at 48 h (Fig. 6A). Quantification analysis of the wounded areas showed that in wild-type mice, VEGF-B treatment induced 90% epithelial healing at 40 h, whereas untreated mice displayed only 60% closure. Similarly, VEGF-B improved epithelial wound healing in Vegf-b−/−, with more than 90% closure at 72 h compared with 70% closure in untreated mice (Fig. 6B).
Fig. 6.
VEGF-B enhances corneal epithelial wound healing. Wild-type C57BL/6 and Vegf-b−/− mice were subjected to 2-mm central corneal epithelial debridement and immediately injected subconjunctivally with 0.5 ng of rm VEGF-B or an equal volume of 0.1% BSA as a control. Fluorescein staining was used to follow the reepithelialization of the injured corneas using a slit lamp biomicroscope. (A) In animals receiving control injections, we observed a persistent delay in epithelial wound closure in Vegf-b−/− compared with wild-type mice. (A) The reepithelialization was accelerated in both groups of VEGF-B–treated mice, but the epithelium healed rapidly in wild-type mice. (B) Quantification of the images confirms that epithelial wound closure is accelerated upon VEGF-B treatment. (B) In wild-type mice the epithelium was almost healed at 40 h in VEGF-B–treated animals but only 60% healed in untreated mice. In Vegf-b−/− mice only 75% of the injured epithelium healed after 72 h, compared with almost 100% when treated with VEGF-B. Data are expressed as mean ± SEM of two independent experiments (n = 6). *P ≤ 0.01.
Given the pleiotropic roles of VEGF-B, we analyzed whether the faster reepithelization seen upon VEGF-B administration is a direct effect on the epithelial cells or due to indirect effects of VEGF-B on corneal nerve regeneration and the subsequent trophic action of these nerves on the epithelium. We cultured human corneal limbal epithelial cells to confluency and made an epithelial injury using a scratch wound assay. Cells treated with VEGF-B showed similar reepithelization compared with untreated control cells after 48 h incubation, whereas cells treated with epidermal growth factor, a known inducer of epithelial cell proliferation, completely closed the scratch area before 24 h (Fig. S11). Thus, VEGF-B has no direct effect on corneal epithelial wound healing, and the effect induced by VEGF-B in vivo is likely due to the trophic influence of regenerated corneal nerves.
Discussion
The VEGF family of growth factors has been increasingly implicated in developmental neurogenesis and pathological nerve regeneration (2–4). VEGF-A, the most studied ligand, possesses a strong angiogenic effect and has been therapeutically inhibited in cancer and ophthalmic diseases (15–18). VEGF-A is also a potent neuroregenerative factor, but its induction of concomitant neovascularization is a potential major impediment to its use as a neuroregenerative agent. In contrast, VEGF-B, which weakly induces angiogenesis and has a seemingly minor role in normal neurogenesis, is considered as a survival factor and an inducer of nerve growth in several models of CNS nerve injury (5, 19, 20). Here we studied the effect of VEGF-B on neuronal growth and peripheral nerve regeneration independently of its angiogenic effect, by using isolated TG neurons and the cornea as a model that not only contains the highest density of sensory innervation of the human body but also is avascular.
VEGF-B induced extensive neurite growth in isolated TG neurons, a finding that, to our knowledge, has not been described before. The effects of VEGF-B on total neurite elongation were comparable to that seen for VEGF-A. However, a closer examination of VEGF-B effects demonstrated a dramatically enhanced induction on neurite branching compared with VEGF-A. This differential effect of VEGF-B on branching could be partially explained by our finding that VEGF-B signaling requires the activation of both VEGFR1 and NRP1. We have previously shown that VEGF-A action on trigeminal neurons requires concomitant activation of VEGFR2 in addition to VEGFR1 and NRP1 (12). Our gene expression array showed that the changes induced by VEGF-B are distinct from those induced by VEGF-A. We found that higher expression of VEGFR1/R2/R3 occurs in TG neurons treated with VEGF-A compared with those treated with VEGF-B, but without differences in the expression level of common mediators of VEGF signaling. Interestingly, significant overexpression differences were found in some elements of the Notch, Wnt, and Semaphorin/Plexin signaling pathways. It has been demonstrated that activation of Notch signaling results in increased microtubule stability and changes in axonal morphology and branching (21). Similarly, Wnt2 promotes cortical dendrite growth and dendritic spine formation and Wnt11 early neurogenesis (22, 23). Because VEGF-induced signaling is complex and intersects with multiple pathways, we tested whether inhibition of PI3K or Notch signaling resulted in functional changes of VEGF-induced neurite growth. Indeed, both pathways are required for neurite elongation but not for neurite formation or growth initiation. Additionally, we found other candidate genes that may be involved in VEGF signaling, such as Endothelial cell-specific molecule 1 (Esm1), which is a VEGF pathway mediator (24), and Apelin (Apln) a neuropeptide that acts as a neuroprotectant (25, 26). Furthermore, we found that distinct ligands that bind the VEGF receptor (such as PlGF) or interact with similar intracellular mediators (i.e., HB-EGF) also induced neurite growth in TG neurons but with less potency than VEGF-B. In our study VEGF-B not only promoted neurite extension and branching but also increased the survival of TG neurons. Thus, VEGF-B has dual effects: it protects neurons from death and induces strong neuronal growth through a complex signaling cascade that requires further study.
Our in vitro findings led us to determine whether VEGF-B could play a role in nerve regeneration in vivo. Previous reports indicated that VEGF-B is not fundamental for normal homeostasis but is overexpressed during disease states (6, 8, 27, 28). We have found that the level of VEGF-B protein expression increases consistently and significantly after injury in the cornea. This increased endogenous expression may play an important physiologic role in nerve repair. Our studies using Vegf-b−/− mice indicate that during normal conditions no outstanding phenotype is seen in these mice. Some cardiac abnormalities and impaired protection against brain ischemic injury have been described (8, 29–31). However, when Vegf-b−/− mice were challenged by superficial nerve injury, they showed a delayed nerve and epithelium recovery compared with wild-type mice, indicating that the presence of VEGF-B is necessary for a normal healing response. This requirement of VEGF-B for normal nerve recovery has also been observed in retrograde degeneration of sensory neurons (6) and adult brain neurogenesis (20).
The actions of VEGF-B on the vasculature and CNS seem to depend on the presence of disease or injury. Thus, we used a corneal nerve-wounding model to examine the differential effects of VEGF-B on PNS innervation in the absence or presence of nerve injury. This wounding model also excludes any angiogenic influence, owing to the avascular nature of the cornea. We found that the slow release of VEGF-B within the target tissue (intrastromal pellet) or from an adjacent site (subconjunctival) induced nerve regeneration in the injured zone without altering the innervation of healthy tissue. Thus, VEGF-B induced nerve repair of injured nerves in vivo without affecting preexisting uninjured nerves. Exogenous VEGF-B application not only accelerated the recovery of the injured nerves in wild-type mice but also alleviated the impairment of reinnervation seen in Vegf-b−/− mice. Importantly, VEGF-B did not produce any unwanted neovascularization, whereas it promoted potent nerve regeneration.
We investigated whether the improvement in anatomic reinnervation of the PNS in response to VEGF-B was accompanied by an improvement in PNS function at the target tissue. We observed that the faster regeneration observed in the injured nerves upon VEGF-B treatment was accompanied by improved cornea sensation in these animals. Sensory neurons of the PNS may have functions beyond sensation, including trophic influences on target tissues. VEGF-B also induced accelerated reepithelialization of the debrided epithelium in the cornea in wild-type and reversed the delay in reepithelialization in Vegf-b−/− mice. This effect of VEGF-B on epithelial wound healing seems to be indirect, resulting from the neurotrophic effect rather than the direct action on epithelial cells, because our epithelial scratch assays demonstrated that VEGF-B treatment induced no improvement in wound closure in cultured corneal epithelial cells. Our data suggest that VEGF-B–mediated nerve regeneration not only restores sensation but also restores the critical trophic function of PNS sensory nerves.
Taken together, our data demonstrate that VEGF-B is required for proper nerve regeneration in the cornea. The increased endogenous VEGF-B expression in response to injury or the application of exogenous VEGF-B promotes nerve regeneration without affecting undamaged nerves or inducing unwanted neovascularization. VEGF-B–mediated nerve regeneration restored the sensation and healing responses of PNS innervated tissues. The relative inactivity of VEGF-B on intact neurons, its lack of significant angiogenic activity, and its potent action in restoring PNS function to damaged tissues make VEGF-B an important therapeutic target for treating injured peripheral nerves.
Methods
Animals.
All experiments were performed according to the guidelines of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision. C57BL/6 and neurofluorescent thy1-YFP mice were purchased from Jackson Laboratories, and the VEGF-B knockout (Vegf-b−/−) mice were a generous gift from David A. Greenberg (Buck Institute for Research on Aging, Novato, CA).
TG Neuronal Growth Assay.
TG neuronal cells were obtained and cultured as described previously (12). To evaluate the effect of VEGF-B on neuronal growth, TG neuronal cells were treated with 10–100 ng/mL recombinant mouse VEGF-B186 (R&D 767-VE-010). The VEGF-B treatments were replenished every other day. Neurite formation and growth were followed up to 3 d. Inhibitions studies to determine VEGF-B–receptor interactions were carried out as follow: VEGF-B availability was competitively inhibited by treating the cells at the same time with 50 ng/mL VEGF-B and an equal molar concentration (2.6 nM) of recombinant human-soluble VEGFR1 (sVEGFR1/sFlt1, CellSciences CRF103). VEGF receptors were inhibited by using neutralizing antibodies against VEGFR1 (R&D AF471), VEGFR2 (R&D MAB4431), and NRP1 (R&D MAB566). TG cells were incubated in a dose-dependent manner with anti-VEGFR1, anti-VEGFR2, or anti-NRP1 for 1 h before the addition of 50 ng/mL VEGF-B. The tyrosine kinase domains of VEGFR2 were inhibited with specific inhibitors by incubating TG neuronal cells with 10 μM SU 1498 (Sigma T4192) or 5 nM Ki 8751 (Sigma K1265) for 1 h before adding 50 ng/mL VEGF-B.
Corneal Micropocket Assay.
A sucralfate/hydron pellet impregnated with VEGF-B (100 ng per pellet) or vehicle was implanted into a corneal micropocket in anesthetized thy1-YFP mice subjected to a partial lamellar incision parallel to the limbus (32). Mice were allowed to recover for 1 d, after which a subset received corneal debridement that removed a circular 2-mm region of central epithelium and the underlying subbasal nerves. The corneas were harvested 3 d later and processed as previously described (12).
Subconjunctival Injection of VEGF.
Wild-type C57BL/6 and Vegf-b−/− mice were subject to corneal epithelial debridement as described above or left intact. A 5-μL volume containing 0.5 ng VEGF-B186 in PBS was injected subconjunctivally, at the lateral temporal bulbar conjunctiva, using a 10-μL microsyringe (Hamilton Co.). An equal volume of 0.1% BSA was used in control mice. The recovery of the corneal epithelial injury was imaged at 24, 40, and 48 h by applying 1% fluorescein solution to the corneal surface and wound closure evaluated as previously described (12). Corneas were harvested after 1 wk for immunodetection of nerves. Possible growth of blood vessel into the cornea was monitored by slit lamp examination.
Corneal Whole Mount and Immunofluorescence.
Harvested corneas from thy1-YFP mice were fixed in 4% (vol/vol) paraformaldehyde (PFA) for 1 h and then stained with DAPI to label nuclei before mounting. The YFP fluorescent nerves were imaged with a fluorescence microscope at the site of pellet implantation and at a site 180° opposite to the pellet. Images were analyzed using Neurolucida. C57BL/6 wild-type and Vegf-b−/− mouse corneas were flat mounted and fixed as previously described (12). Samples were stained with rabbit anti-β3 tubulin antibody (Abcam 18207) overnight at 4 °C, followed by Alexa Fluor 568-conjugated goat anti-rabbit IgG (Life Technologies A11011) at room temperature for 1 h and counterstained with DAPI.
Statistics.
Data are presented as mean ± SEM. The significance of differences was evaluated using ANOVA followed by post hoc test for multiple comparisons, with P < 0.05 considered statistically significant unless otherwise stated.
Supplementary Material
Acknowledgments
This work was supported by National Eye Institute/National Institutes of Health Grants R01EY018594 and K08EY015829, and a Research to Prevent Blindness Career Development Award.
Footnotes
Conflict of interest statement: V.H.G., Z.P., N.K., and M.I.R. have disclosed intellectual property related to the use of vascular endothelial growth factor in peripheral nerve repair.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407227111/-/DCSupplemental.
References
- 1.Bosse F. Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res. 2012;349(1):5–14. doi: 10.1007/s00441-012-1389-5. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Ruiz de Almodovar C. VEGF ligands and receptors: Implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci. 2013;70(10):1763–1778. doi: 10.1007/s00018-013-1283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci. 2013;70(10):1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139(8):1371–1380. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Kumar A, Zhang F, Lee C, Tang Z. Complicated life, complicated VEGF-B. Trends Mol Med. 2012;18(2):119–127. doi: 10.1016/j.molmed.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Dhondt J, et al. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. 2011;25(5):1461–1473. doi: 10.1096/fj.10-170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118(3):913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, et al. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J Cereb Blood Flow Metab. 2004;24(10):1146–1152. doi: 10.1097/01.WCB.0000134477.38980.38. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70(10):1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falk T, et al. Vascular endothelial growth factor-B is neuroprotective in an in vivo rat model of Parkinson’s disease. Neurosci Lett. 2011;496(1):43–47. doi: 10.1016/j.neulet.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 11.Poesen K, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci. 2008;28(42):10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Z, Fukuoka S, Karagianni N, Guaiquil VH, Rosenblatt MI. Vascular endothelial growth factor promotes anatomical and functional recovery of injured peripheral nerves in the avascular cornea. FASEB J. 2013;27(7):2756–2767. doi: 10.1096/fj.12-225185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63(4):642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilic E, et al. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF’s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20(8):1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- 15.Campbell RJ, Bell CM, Campbell EdeL, Gill SS. Systemic effects of intravitreal vascular endothelial growth factor inhibitors. Curr Opin Ophthalmol. 2013;24(3):197–204. doi: 10.1097/ICU.0b013e32835f8bbe. [DOI] [PubMed] [Google Scholar]
- 16.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120(1):106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):5–13. doi: 10.1517/14728222.2011.641951. [DOI] [PubMed] [Google Scholar]
- 18.Crawford Y, Ferrara N. VEGF inhibition: Insights from preclinical and clinical studies. Cell Tissue Res. 2009;335(1):261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- 19.Nash AD, Baca M, Wright C, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006;19(1):61–69. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, et al. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: Evidence from knockout mice and growth factor administration. Dev Biol. 2006;289(2):329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Bonini SA, Ferrari-Toninelli G, Montinaro M, Memo M. Notch signalling in adult neurons: A potential target for microtubule stabilization. Ther Adv Neurol Disord. 2013;6(6):375–385. doi: 10.1177/1756285613490051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiester BG, Galati DF, Salinas PC, Jones KR. Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol Cell Neurosci. 2013;56:115–127. doi: 10.1016/j.mcn.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elizalde C, et al. Distinct roles for Wnt-4 and Wnt-11 during retinoic acid-induced neuronal differentiation. Stem Cells. 2011;29(1):141–153. doi: 10.1002/stem.562. [DOI] [PubMed] [Google Scholar]
- 24.Rocha SF, et al. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ Res. 2014;115(6):581-90. doi: 10.1161/CIRCRESAHA.115.304718. [DOI] [PubMed] [Google Scholar]
- 25.Cheng B, Chen J, Bai B, Xin Q. Neuroprotection of apelin and its signaling pathway. Peptides. 2012;37(1):171–173. doi: 10.1016/j.peptides.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Cook DR, et al. NMDA receptor modulation by the neuropeptide apelin: Implications for excitotoxic injury. J Neurochem. 2011;118(6):1113–1123. doi: 10.1111/j.1471-4159.2011.07383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boer K, et al. Cellular distribution of vascular endothelial growth factor A (VEGFA) and B (VEGFB) and VEGF receptors 1 and 2 in focal cortical dysplasia type IIB. Acta Neuropathol. 2008;115(6):683–696. doi: 10.1007/s00401-008-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie L, Mao X, Jin K, Greenberg DA. Vascular endothelial growth factor-B expression in postischemic rat brain. Vasc Cell. 2013;5(1):8. doi: 10.1186/2045-824X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aase K, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104(3):358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 30.Bellomo D, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86(2):E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 31.Mould AW, et al. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003;48(9):2660–2669. doi: 10.1002/art.11232. [DOI] [PubMed] [Google Scholar]
- 32.Rogers MS, Birsner AE, D’Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2(10):2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.