Significance
To clarify the evolution of parthenogenesis, given the potential sexual conflict over genetic transmission, identifying the mechanism regulating egg fertilization in females is essential. In the termite Reticulitermes speratus, queens produce their replacements (neotenic queens) parthenogenetically but use sexual reproduction to produce other colony members. We discovered that queens of the termite close micropyles (openings for sperm entry) of their eggs to produce parthenogenetic offspring in the presence of kings. Furthermore, we found that queens control the proportion of micropyleless eggs by regulating of the number of micropyles over time. This study describes a novel route of the evolution of parthenogenesis in favor of females’ interests without interference from males.
Keywords: thelytoky, micropyle, asexual queen succession, egg fertilization, Isoptera
Abstract
Males and females are in conflict over genetic transmission in the evolution of parthenogenesis, because it enhances female reproductive output but deprives the males’ genetic contribution. For males, any trait that coerces females into sexual reproduction should increase their fitness. However, in the termite Reticulitermes speratus, queens produce their replacements (neotenic queens) parthenogenetically while using normal sexual reproduction to produce other colony members. Here, we show that termite queens produce parthenogenetic offspring in the presence of kings by closing the micropyles (sperm gates; i.e., openings for sperm entry) of their eggs. Our field survey showed that termite eggs show large variation in numbers of micropyles, with some having none. Microsatellite analysis showed that embryos of micropyleless eggs develop parthenogenetically, whereas those of eggs with micropyles are fertilized and develop sexually. Surveys of eggs among queens of different age groups showed that queens begin to lay micropyleless eggs when they are older and thus, need to produce their replacements parthenogenetically. In addition, we found clear seasonality in new neotenic queen differentiation and micropyleless egg production. This micropyle-dependent parthenogenesis is the first identification, to our knowledge, of the mechanism through which females control egg fertilization over time in diploid animals, implying a novel route of the evolution of parthenogenesis in favor of female interests without interference from males.
The near ubiquity of sexual reproduction is one of the most enduring puzzles in evolutionary biology, because all else being equal, asexual populations have a twofold fitness advantage over their sexual counterparts and should rapidly outnumber sexual populations (1, 2). A proposed major advantage of sexual reproduction is that it promotes genetic variability across generations, facilitating adaptation to local ecological conditions (3–5). However, mathematical models have revealed that evolution need not favor sexual reproduction, even when it does increase variability that is beneficial, and thus, the prevalence of sexual reproduction remains an enigma (6, 7). Some biologists have approached this question by considering how loss of sex can be achieved in a population of sexual organisms (8–10). The evolution of parthenogenesis may be no exception in that it creates sexual conflict, given that parthenogenetic reproduction enhances female reproductive output but deprives the males’ genetic contribution to future generations. Therefore, any trait in males that coerces parthenogenetic females into sexual reproduction should increase their fitness. A recent theoretical study showed that the evolution of male coercion substantially favors the maintenance of sexual reproduction, although a female barrier against the coercion can evolve (10).
If male counteradaptations interfere with parthenogenesis, females would unlikely be able to switch from sexual to asexual reproduction unless isolated from males. Identifying the mechanism regulating egg fertilization is critical to better understand the evolutionary routes of parthenogenesis given the potential sexual conflict involved. Unlike facultative parthenogenesis, where females use thelytoky only in the absence of males, queens of some ants (11–13) and termites (14–16) selectively use both sexual reproduction and thelytoky when mating with males. In these social insects, queens produce new queens asexually by thelytokous parthenogenesis, but they produce workers through sexual reproduction. This conditional switching of reproductive tactics allow queens to increase the transmission of their genes to the next generation and maintain genetic diversity in the worker force, but it inevitably provokes queen–male sexual conflict by reducing the males’ genetic contribution to future generations (17). This system provides an ideal opportunity to study how and why females switch from sexual to asexual reproduction, even in the presence of males.
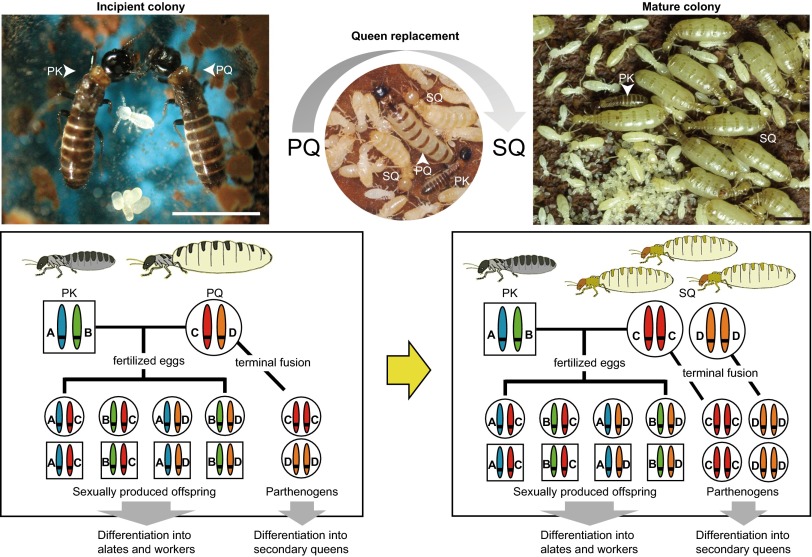
The breeding system of the subterranean termite Reticulitermes speratus is characterized by asexual queen succession (AQS) (Fig. 1 and SI Text). Termite colonies are typically founded by a monogamous pair of primary reproductives (adult winged forms): a king and queen. In Reticulitermes termites, primary queens live more than 11 y (18). As the primary queen senesces and thus, her egg production becomes insufficient to maintain the colony, secondary queens (i.e., neotenic queens) differentiate within the colony and supplement egg production, eventually replacing the primary queen (Fig. 1 and Fig. S1) (18, 19). In AQS species, queens produce their neotenic replacement queens asexually but use normal sexual reproduction to produce other colony members (14–16). Because sex determination in Reticulitermes termites is male heterogamy (20), queens cannot produce secondary kings by parthenogenesis (17). This AQS system enables founding queens to increase their reproductive output while retaining the same transmission rate of their genes to future generations. Therefore, the founder queen can be considered genetically immortal until the colony dies, because neotenic queens are also replaced by subsequent cohorts of parthenogenetically produced neotenic queens.
Fig. 1.
Colony development and asexual queen succession in the termite R. speratus. As the primary queen senesces, secondary queens produced asexually by the primary queen differentiate within the colony and supplement egg production, eventually replacing the primary queen (details described in SI Text). Squares indicate males, and circles represent females. PK, primary king; PQ, primary queen; SQ, secondary queen. (Scale bars: 3 mm.)
How can queens of termites (i.e., diploid social insects) control egg fertilization? In haplodiploid social Hymenoptera, unfertilized eggs become males, and fertilized eggs produce females (21), giving queens a potentially powerful mechanism for controlling fertilization (22, 23). However, in diploid insects, sperm release is generally activated through a neural loop whenever an egg passes the genital chamber, with no control over fertilization (24). In this study, we focused on micropyles of eggs to identify a possible mechanism for controlling fertilization in diploid insects. A micropyle is a channel that extends from the external gateway through the chorion and ends in the vitelline membrane, serving as the route of sperm entry into a mature oocyte (25–27). Termite queens are always attended by kings, and thus, the simplest and most effective mechanism to produce parthenogenetic eggs would be to close the micropyles of eggs to prevent sperm entry. Preliminary observations of micropyles of the eggs of R. speratus showed that the number of micropyles varies largely among eggs, even within a colony. If a certain proportion of eggs are micropyleless (i.e., eggs without sperm gates), fertilizing these eggs would be mechanically impossible, regardless of a king’s presence. Unfertilized eggs of R. speratus restore diploidy through automixis with terminal fusion (28), and the embryos develop into parthenogenetic daughters (29). Thus, we hypothesized that queens control the production of parthenogenetic offspring independently of males by regulating the number of micropyles on their eggs.
We tested this hypothesis in a series of micropyle analyses, and DNA genotyping of embryos aimed at confirming the production of micropyleless eggs in field colonies and identifying the genetic profiles of eggs with and without micropyles. We also tested the likelihood of sperm depletion as a potential origin of parthenogenesis. We found that R. speratus queens alter the number of micropyles on their eggs over time and thereby, produce micropyleless eggs to asexually produce their replacement.
Results and Discussion
To test the micropyleless egg hypothesis, we first investigated the number of micropyles of eggs collected from 60 R. speratus field colonies. Micropyles were located on the posterior end of eggs, forming an arc (Fig. 2A). They are funnel-shaped with an opening of 3.23 μm (±0.15 SEM, n = 10) in diameter (Fig. 2B), and the average number of micropyles for all samples (n = 6,000) was 9.48 (±0.04 SEM, range = 0–33) (Fig. 2 C and D). Significant differences were observed in the number of micropyles among colonies (F59, 5,940 = 27.35, P < 0.0001; one-way ANOVA), and 7 of 60 colonies (11.7%) had micropyleless eggs (Table S1). Unmated R. speratus queens lay eggs, and the unfertilized eggs develop parthenogenetically, with a comparably high hatching rate as fertilized eggs (29). All micropyleless eggs showed normal embryonic development as observed in previous studies on parthenogenesis in R. speratus. To test whether the embryos of micropyleless eggs were developing parthenogenetically, we compared microsatellite genotypes of the embryos of eggs with and without micropyles in four colonies (GB130502C, ZE130827B, KW140531A, and AO140511A) that had micropyleless eggs. In all of the colonies, the embryos of micropyleless eggs had only maternal alleles, indicating parthenogenetic development, whereas those of eggs that had micropyles (even only a single micropyle) had both paternal and maternal alleles, indicating sexual development (Table 1 and Table S2). These results clearly indicate that the production of micropyleless eggs functions as a mechanism for parthenogenetic reproduction by the queen in the presence of kings.
Fig. 2.
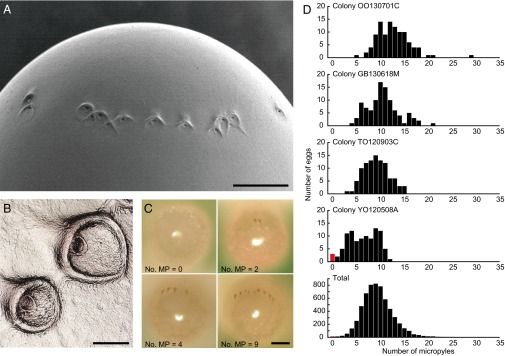
Variation in the number of micropyles of eggs. (A) Scanning electron microscope image of the posterior end of an egg with micropyles (ventral view). (Scale bar: 50 μm.) (B) Confocal scanning laser microscope image of micropyles (close-up view). (Scale bar: 10 μm.) (C) Posterior views (dorsal up) of representative eggs with different numbers of micropyles (zero, two, four, and nine). Micropyles were visualized by eosin Y staining. (Scale bar: 100 μm.) (D) Comparison of the number of micropyles among 4 representative colonies (n = 100 for each colony) and the total of 60 field colonies (n = 6,000). Micropyleless eggs are indicated by red bars. MP, micropyle.
Table 1.
Genotypes of embryos of the eggs with or without micropyles, primary kings, primary queens, and secondary (neotenic) queens in colonies GB130502C and ZE130827B at each of five microsatellite loci
| Individual | Rf24-2 | Rf21-1 | Rs10 | Rs15 | Rf6-1 | P/S* |
| Colony GB130502C | ||||||
| Embryo (no. of MP) | ||||||
| E-1 (0) | 99† 99† | 202† 202† | 152† 152† | 291† 291† | 161 161 | P |
| E-2 (0) | 102 102 | 196† 196† | 152† 152† | 291† 291† | 161 161 | P |
| E-3 (0) | 102 102 | 196† 196† | 152† 152† | 291† 291† | 161 161 | P |
| E-4 (1) | 99† 87‡ | 241‡ 202† | 155‡ 152† | 291† 279‡ | 161 161 | S |
| E-5 (1) | 102 87‡ | 265‡ 196† | 161‡ 152† | 291† 273‡ | 161 161 | S |
| E-6 (1) | 102 99† | 265‡ 196† | 155‡ 152† | 291† 279‡ | 161 152‡ | S |
| E-7 (2) | 102 87‡ | 265‡ 196† | 161‡ 152† | 291† 273‡ | 161 152‡ | S |
| E-8 (2) | 102 99† | 241‡ 196† | 155‡ 152† | 291† 279‡ | 161 152‡ | S |
| E-9 (2) | 102 87‡ | 241‡ 196† | 161‡ 152† | 291† 273‡ | 161 152‡ | S |
| E-10 (3) | 102 99† | 241‡ 202† | 161‡ 152† | 291† 273‡ | 170† 161 | S |
| E-11 (3) | 102 99† | 241‡ 196† | 161‡ 140† | 291† 273‡ | 161 161 | S |
| E-12 (3) | 102 99† | 241‡ 196† | 155‡ 152† | 291† 279‡ | 161 161 | S |
| E-13 (4) | 102 87‡ | 241‡ 196† | 161‡ 152† | 291† 273‡ | 161 152‡ | S |
| E-14 (4) | 102 99† | 265‡ 202† | 161‡ 152† | 291† 273‡ | 161 152‡ | S |
| E-15 (4) | 99† 87‡ | 265‡ 202† | 161‡ 152† | 291† 273‡ | 161 152‡ | S |
| E-16 (5) | 102 99† | 265‡ 196† | 161‡ 140† | 291† 273‡ | 161 152‡ | S |
| E-17 (5) | 99† 87‡ | 241‡ 202† | 161‡ 140† | 291† 273‡ | 161 161 | S |
| E-18 (5) | 102 87‡ | 241‡ 196† | 161‡ 140† | 291† 273‡ | 161 161 | S |
| PK | 102‡ 87‡ | 265‡ 241‡ | 161‡ 155‡ | 279‡ 273‡ | 161‡ 152‡ | |
| PQ§ | 102† 99† | 202† 196† | 152† 140† | 291† 291† | 170† 161† | |
| SQ | ||||||
| SQ-1 | 102† 102† | 196† 196† | 152† 152† | 291† 291† | 161† 161† | |
| SQ-2 | 102† 102† | 196† 196† | 152† 152† | 291† 291† | 161† 161† | |
| SQ-3 | 102† 102† | 196† 196† | 140† 140† | 291† 291† | 161† 161† | |
| SQ-4 | 102† 102† | 202† 202† | 140† 140† | 291† 291† | 170† 170† | |
| SQ-5 | 99† 99† | 202† 202† | 140† 140† | 291† 291† | 161† 161† | |
| SQ-6 | 102† 102† | 196† 196† | 140† 140† | 291† 291† | 161† 161† | |
| SQ-7 | 99† 99† | 196† 196† | 140† 140† | 291† 291† | 170† 170† | |
| SQ-8 | 99† 99† | 202† 202† | 152† 152† | 291† 291† | 161† 161† | |
| SQ-9 | 99† 99† | 196† 196† | 140† 140† | 291† 291† | 161† 161† | |
| SQ-10 | 102† 102† | 196† 196† | 152† 152† | 291† 291† | 161† 161† | |
| Colony ZE130827B | ||||||
| Embryo (no. of MP) | ||||||
| E-1 (0) | 90† 90† | 226† 226† | 140 140 | 270 270 | 167 167 | P |
| E-2 (0) | 90† 90† | 223 223 | 140 140 | 270 270 | 152† 152† | P |
| E-3 (0) | 90† 90† | 223 223 | 140 140 | 255† 255† | 152† 152† | P |
| E-4 (1) | 114‡ 90† | 226† 205‡ | 140 140 | 273‡ 270 | 167 167 | S |
| E-5 (1) | 105† 87‡ | 226† 205‡ | 140 140 | 273‡ 255† | 167 152† | S |
| E-6 (1) | 90† 87‡ | 226† 205‡ | 188‡ 164† | 270 270 | 167 167 | S |
| E-7 (2) | 90† 87‡ | 226† 223 | 188‡ 164† | 270 270 | 167 167 | S |
| E-8 (2) | 90† 87‡ | 226† 205‡ | 188‡ 164† | 270 270 | 167 167 | S |
| E-9 (2) | 114‡ 90† | 226† 205‡ | 140 140 | 273‡ 270 | 167 167 | S |
| E-10 (3) | 114‡ 105† | 223 205‡ | 188‡ 164† | 273‡ 255† | 167 167 | S |
| E-11 (3) | 114‡ 90† | 226† 223 | 140 140 | 270 270 | 167 152† | S |
| E-12 (3) | 90† 87‡ | 223 223 | 140 140 | 273‡ 270 | 167 152† | S |
| E-13 (4) | 114‡ 105† | 223 205‡ | 188‡ 164† | 270 270 | 167 167 | S |
| E-14 (4) | 114‡ 90† | 223 205‡ | 140 140 | 273‡ 255† | 167 152† | S |
| E-15 (4) | 114‡ 90† | 223 205‡ | 140 140 | 270 255† | 167 152† | S |
| E-16 (5) | 105† 87‡ | 223 205‡ | 140 140 | 270 270 | 167 152† | S |
| E-17 (5) | 114‡ 90† | 223 205‡ | 188‡ 140 | 270 270 | 167 152† | S |
| E-18 (5) | 114‡ 90† | 223 205‡ | 140 140 | 273‡ 255† | 167 152† | S |
| PK | 114‡ 87‡ | 223‡ 205‡ | 188‡ 140‡ | 273‡ 270‡ | 167‡ 167‡ | |
| PQ§ | 105† 90† | 226† 223† | 164† 140† | 270† 255† | 167† 152† | |
| SQ | ||||||
| SQ-1 | 105† 105† | 223† 223† | 164† 164† | 270† 270† | 167† 167† | |
| SQ-2 | 105† 105† | 223† 223† | 140† 140† | 270† 270† | 152† 152† | |
| SQ-3 | 90† 90† | 223† 223† | 140† 140† | 270† 270† | 152† 152† | |
| SQ-4 | 105† 105† | 226† 226† | 140† 140† | 255† 255† | 152† 152† | |
| SQ-5 | 105† 105† | 223† 223† | 140† 140† | 270† 270† | 152† 152† | |
| SQ-6 | 90† 90† | 226† 226† | 140† 140† | 270† 270† | 167† 167† | |
| SQ-7 | 105† 105† | 226† 226† | 140† 140† | 270† 270† | 152† 152† | |
| SQ-8 | 105† 105† | 226† 226† | 140† 140† | 270† 270† | 152† 152† | |
| SQ-9 | 90† 90† | 223† 223† | 140† 140† | 270† 270† | 152† 152† | |
| SQ-10 | 105† 105† | 226† 226† | 140† 140† | 270† 270† | 152† 152† | |
E, embryo; MP, micropyle; PK, primary king; PQ, primary queen; SQ, secondary (neotenic) queen.
P/S indicates parthenogenetically developed (P) or sexually developed (S).
Maternal alleles.
Paternal alleles.
The genotype of primary queens was determined from the genotype of offspring, because primary queens had been replaced by parthenogenetically produced secondary queens.
To investigate the occurrence of sperm depletion, we examined the sperm storage of secondary queens by dissecting out the spermathecae (sperm storage organs) and applying microsatellite genotyping. Collections of royal chambers from 54 field colonies revealed that initially monogamous colonies became extremely polygynous as the colonies developed (Table S3). The number of queens per nest was 57.63 (±12.84 SEM, n = 54), and 51 of 54 colonies contained only a single primary king (Table S3). The largest colony (TA090620A) had 676 secondary queens and a single primary king. We detected sperm alleles (i.e., kings’ alleles) from all sampled queens, even in the colony containing 676 secondary queens (Tables S4, S5, and S6), suggesting that kings have the capacity to provide sufficient sperm; thus, parthenogenetic reproduction in colonies containing kings cannot be explained by sperm depletion.
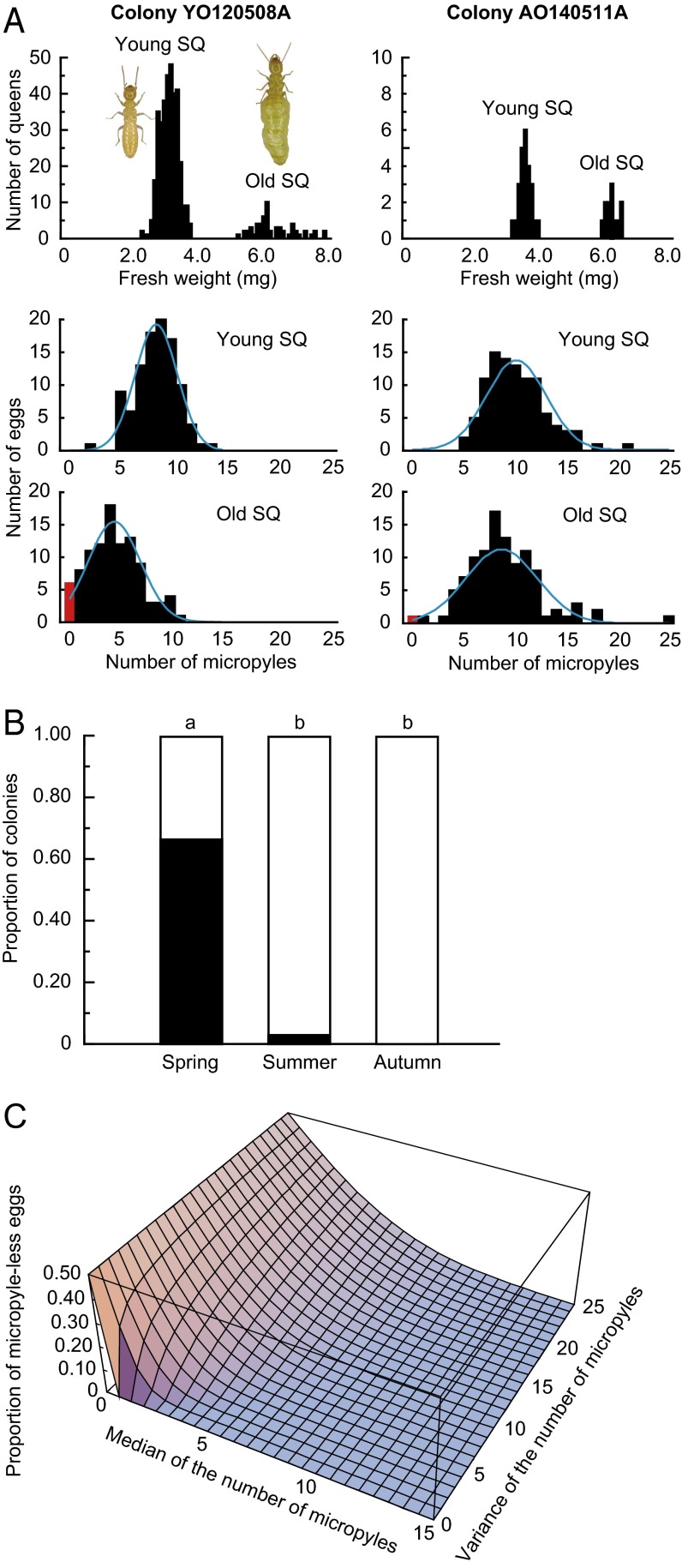
For successful AQS, parthenogenetic daughters must exist in a colony whenever the colony requires supplementary or replacement queens. Conversely, overproduction of parthenogenetic offspring would impose a large cost on colonies, because these individuals are inferior to sexually produced individuals as workers or alates, likely because of their complete loss of heterozygosity (14, 28). Therefore, selection should act against overproduction of parthenogenetic eggs and favor the production of necessary minimum numbers of micropyleless eggs. If queens are able to control the production of micropyleless eggs, we predicted that older queens that require asexual replacements would produce more micropyleless eggs than young queens that will continue to reign for several years. We conducted seasonal sampling of queens from field colonies and identified the season of new secondary queen differentiation. In mid-May, just before swarming, parthenogenetic nymphs with small wing buds (preneotenic brachypterous stage) (30) develop into neotenic queens, and sexual nymphs with long wing buds (alate-destined nymphs) molt into alates simultaneously (Fig. S2). Consequently, two types of secondary queens occur in the colonies under queen replacement in this season (old physogastric queens and first-year small queens), forming a clear bimodal size distribution (Fig. 3A). This seasonality of new secondary queen differentiation seems adaptive to maximize their contribution to egg production, because colonies begin to produce eggs in May and reach maximum output in July followed by cessation in October (31). Among 11 colonies from which we could collect reproductives during the queen replacing season in 2013 and 2014, 2 colonies had new secondary reproductive, but 9 colonies had only old physogastric queens, implying that secondary queen replacement occurs once in 5–6 y. We collected both types of queens from the two colonies (YO120508A and AO140511A) and compared the number of micropyles between eggs laid by old and young secondary queens. A significant difference was observed in the number of micropyles between the eggs laid by old secondary queens and those laid by young secondary queens (colony: F1, 396 = 105.93, P < 0.0001; age group: F1, 396 = 97.39, P < 0.0001; two-way ANOVA), whereby the eggs laid by old secondary queens had significantly fewer micropyles than those laid by young secondary queens [P < 0.0001; Tukey’s honestly significant difference (HSD) test] (Fig. 3A). We also investigated the number of micropyles laid by young primary queens (foundresses) obtained from incipient colonies in the laboratory. A significant difference was observed in the number of micropyles between young primary queens and young secondary queens (colony: F3, 495 = 6.75, P < 0.001; queen type: F1, 495 = 10.24, P < 0.01; nested ANOVA), where the eggs laid by young primary queens had significantly larger numbers of micropyles than those laid by young secondary queens (P < 0.01; Tukey’s HSD) (SI Text and Fig. S3). Neither young primary queens nor young secondary queens produced micropyleless eggs, suggesting that queens begin to lay parthenogenetic eggs to produce their asexual replacements when they are older. Why do young primary queens not produce micropyleless eggs? Considering the high mortality of young queens in early founding stage, producing asexual offspring with the first brood might be adaptive for the queens. Importantly, workers of Reticulitermes termites retain totipotency to differentiate into ergatoid reproductives (worker-derived reproductives) (Fig. S1). Therefore, workers develop into ergatoid queens and continue the colony if there is no parthenogenetic offspring in the colony, although it imposes the costs of inbreeding and reduces the queen’s genetic contribution to the next generations from one-half to one-quarter. Because the number of workers during early founding stage is crucial for colony survivorship, the cost of producing asexual offspring in the first brood would outweigh the cost of ergatoid queen replacements at the accidental death of primary queens. This cost balance could be the reason why we find no micropyleless eggs among the eggs laid by young primary queens. In addition, micropyleless egg production was observed almost exclusively in the spring (Fig. 3B), suggesting that queens are also able to regulate the number of micropyles seasonally.
Fig. 3.
Regulation of the production of micropyleless eggs by the queens. (A) Comparisons of (Top) the size of newly differentiated secondary queens and old physogastric secondary queens in colonies (Left) YO120508A and (Right) AO140511A and frequency distributions of the numbers of micropyles of the eggs laid by (Middle) young and (Bottom) old queens. Micropyleless eggs are indicated by red bars. Blue lines show the fitted normal distributions. (B) Comparison of the proportion of the colonies with (black bars) and without (white bars) micropyleless eggs among the colonies collected in the spring (n = 9), summer (n = 35), and autumn (n = 16). Different letters on the bars indicate significant differences (P < 0.01; Fisher’s exact probability test with sequential Bonferroni correction). (C) Expectation of the proportion of micropyleless eggs based on the median and the variance of the distribution of the number of micropyles. SQ, secondary queen.
Our results confirm that queens of AQS termites control egg fertilization and thereby, regulate the proportion of sexual and asexual offspring. The proportion of parthenogenetic eggs P is determined by the cumulative distribution function:
where μ (μ ≥ 0) and σ2 are the median and variance, respectively, of the distribution of the number of micropyles (Fig. 3C). This function indicates that parthenogenetic egg production increases as the median of the distribution decreases and that larger variance yields more micropyleless eggs. Each micropyle is formed by a single cytoplasmic projection from a micropylar channel-forming cell (MCFC), and thus, the variation in the number of the MCFCs is proximately responsible for the differing number of micropyles in insects (26, 27). Consequently, the expression of the gene that regulates the differentiation of MCFCs determines the switch between sexual and asexual reproduction of termites. It is known that the gene hemipterous (hep) is required in the follicle cells for morphogenesis of micropyles during oogenesis in Drosophila (32). Comparison of hep expression between sexual and asexual strains in Drosophila species, such as D. mercatorum, might illuminate the evolutionary linkage of parthenogenesis and micropyle formation in insects.
Considering the evolutionary process of parthenogenesis and AQS in termites, this switching mechanism based on the number of micropyles seems most parsimonious, because reduction in the number of MCFCs as queens’ age automatically results in the production of parthenogenetic offspring. AQS seems to have evolved in at least three Reticulitermes species independently; we would expect the other two AQS species, R. virginicus (15) and R. lucifugus (16), to have the same switching mechanism between sexual and asexual reproduction. The production of secondary queens through parthenogenesis effectively extends the reproductive life of the primary queen, greatly expanding her reproductive capacity. Coercion by kings cannot impede queens’ parthenogenesis through micropyleless egg production, although parthenogenesis reduces reproductive output by kings. This lack of counteradaptation by kings may have facilitated the evolution of AQS systems in termites.
In conclusion, this study showed that queens of the termite R. speratus produce asexual offspring even in the presence of kings by laying micropyleless eggs. The production of micropyleless eggs gives females a powerful mechanism to control egg fertilization under sexual conflict over parthenogenesis. This study provides a mechanism for the evolution of parthenogenesis in favor of female interests independently of male interests.
Methods
Egg Collection from Field Colonies.
Sixty mature colonies of the subterranean termite R. speratus were collected from pine forests in Kyoto, Shiga, and Wakayama Prefectures, Kinki District and Aomori Prefecture, Tohoku District in Japan from May of 2012 to May of 2014. All sampling was conducted from May to October (Table S1), because a previous study revealed a seasonal pattern of egg production in R. speratus—colonies begin to produce eggs in May and reach the maximum level in July followed by cessation in October (31). We very carefully dismantled nest wood and extracted eggs using an aspirator. The reproductives (kings and queens) were also collected if they were present. The eggs and reproductives collected from each colony were placed in a moist unwoven cloth in a 90-mm Petri dish together with nursing workers and transported to the laboratory.
Micropyle Analysis.
We investigated the micropyle morphology and distribution on eggs of R. speratus using a scanning electron microscope (VE-8800; Keyence) and a laser 3D measurement microscope (VK-X200; Keyence). Size measurements of 10 eggs randomly selected from the eggs extracted from field colony OO140529B (Kyoto) were obtained using the scanning electron microscope.
We developed a micropyle staining method to observe micropyles without damaging DNA. Live eggs collected from each colony were placed in a sterile 1.5-mL tube containing 500 μL 1% (wt/vol) eosin Y solution (Wako Pure Chemical Industries) and kept for 3 h at room temperature before observation. Eggs preserved in eosin Y solution can be used for micropyle observation and microsatellite analysis for 2 wk if kept at 4 °C. Each focal egg was placed on a moist unwoven cloth in a 35-mm Petri dish in a posterior-up position using a stereomicroscope (SZX7; Olympus), and the number of micropyles was counted under a digital microscope (VHX-900; Keyence). One hundred eggs were randomly chosen from each colony (6,000 eggs in total) to determine the number of micropyles, and these data were analyzed using one-way ANOVA. To determine the seasonal pattern of micropyleless egg production, we also compared the proportion of colonies with and without micropyleless eggs among the colonies collected in May (spring), from June to August (summer), and from September to October (autumn) using Fisher’s exact probability tests with sequential Bonferroni correction.
Microsatellite DNA Genotyping.
We used four field colonies collected in Wakayama (colony GB130502C), Shiga (ZE130827B), Kyoto (KW140531A), and Aomori (AO140511A) for microsatellite analysis of embryos. After micropyle analysis, we sorted the eggs based on the number of micropyles; three eggs were randomly chosen from each egg group with zero, one, two, three, four, or five micropyles for each colony (Table 1 and Table S2). Eggs with embryos of developmental stage II or later (29) were used for microsatellite analysis to exclude any nondeveloping eggs. We also genotyped the single primary king and 10 randomly chosen secondary queens from each colony to assign parentage of the eggs (Table S3 shows the royal composition of each colony). All individuals used in this analysis were placed in vials containing 99.5% (vol/vol) ethanol and stored until DNA extraction. Whole eggs or heads of individual termites were ground in Chelex-100 resin solution (Bio-Rad), and DNA was extracted and purified in accordance with standard Chelex-based protocols (14). Individuals were genotyped at five highly polymorphic microsatellite loci: Rf6-1, Rf21-1, Rf24-2 (33), Rs10, and Rs15 (34). PCR conditions are detailed in a previous study (33, 34); fluorescently labeled PCR products were analyzed in a 3500 Genetic Analyzer (Applied Biosystems) with the internal GeneScan-600 LIZ size standard (Applied Biosystems). Allele sizes were determined using GeneMapper 5 (Applied Biosystems). Embryos were determined as sexual (containing both paternal and maternal alleles) or asexual (only containing maternal alleles) offspring based on the genotypes of five microsatellite loci.
Micropyle Comparison Between Old and New Queens.
Differentiation of secondary queens (neotenic queens) occurs simultaneously in mid-May just before swarming (Fig. S2). Consequently, two types of secondary queens occur in the colonies under queen replacement in this season, first-year small queens (2.5–4.1 mg fresh weight) and old physogastric queens (5.5–8.3 mg fresh weight), forming a clear bimodal size distribution (Fig. 3A). To compare the number of micropyles between the queen age groups, we used two field colonies (YO120508A and AO140511A), which had both types of queens, collected in Kyoto and Aomori, respectively. The new and old secondary queens were separated from each colony and placed on a moist unwoven cloth in a 90-mm Petri dish together with 100 workers. The Petri dishes were kept at 25 °C for 1 wk to obtain the eggs of the queens of each age group. One hundred eggs laid by the queens were randomly chosen from each age group, and we counted the number of micropyles using a digital microscope (VHX-900; Keyence) after staining. The numbers of micropyles of eggs were analyzed using two-way ANOVA followed by Tukey’s HSD test.
We also examined the numbers of micropyles of the eggs laid by young primary queens. The nests of three R. speratus colonies (UR140513F, OO140531A, and OO140603A) were collected in Kyoto, Japan just before swarming in 2014. After alates emerged from the nest, they were separated by sex and maintained in Petri dishes containing moist filter paper until they shed their wings. Then, a male and a female were randomly chosen from each colony and placed in a 90-mm Petri dish that contained mixed sawdust bait blocks. Eight replications were made for each colony. The Petri dishes were kept at 25 °C under constant darkness. After 35 d, the nests in bait blocks were dissected to collect eggs from the incipient colonies. Because the number of eggs in each incipient colony was limited (9–19 eggs), 100 eggs were randomly chosen from eight incipient colonies with founding pairs that were originated from the same colony. To compare the numbers of micropyles between the eggs laid by young primary queens and those laid by young secondary queens, we used two-way nested ANOVA followed by Tukey’s HSD test.
Supplementary Material
Acknowledgments
We thank Y. Namba, K. Kobayashi, Y. Yamamoto, W. Suehiro, Y. Mitaka, N. Mizumoto, C. Iwata, and T. Nozaki for research assistance; J. Yoshimura, X. Zhou, and A. S. Mikheyev for helpful advice; the editor and two anonymous referees for valuable comments on the manuscript; and E. L. Vargo for helpful comments in revising the manuscript. This work was supported by Japanese Society for the Promotion of Science Kiban Kenkyu S Grant 25221206 (to K.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412481111/-/DCSupplemental.
References
- 1.Maynard Smith J. The Evolution of Sex. Cambridge Univ Press; Cambridge, United Kingdom: 1978. [Google Scholar]
- 2.Williams GC. Sex and Evolution. Princeton Univ Press; Princeton: 1975. [Google Scholar]
- 3.Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. University of California Press; San Francisco: 1982. [Google Scholar]
- 4.Barton NH, Charlesworth B. Why sex and recombination? Science. 1998;281(5385):1986–1990. [PubMed] [Google Scholar]
- 5.Hamilton WD, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc Natl Acad Sci USA. 1990;87(9):3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto SP, Lenormand T. Resolving the paradox of sex and recombination. Nat Rev Genet. 2002;3(4):252–261. doi: 10.1038/nrg761. [DOI] [PubMed] [Google Scholar]
- 7.Otto SP, Nuismer SL. Species interactions and the evolution of sex. Science. 2004;304(5673):1018–1020. doi: 10.1126/science.1094072. [DOI] [PubMed] [Google Scholar]
- 8.Simon JC, Delmotte F, Rispe C, Crease T. Phylogenetic relationships between parthenogens and their sexual relatives: The possible routes to parthenogenesis in animals. Biol J Linn Soc Lond. 2003;79(1):151–163. [Google Scholar]
- 9.Schön I, Martens K, van Dijk P. Lost Sex: The Evolutionary Biology of Parthenogenesis. Springer; New York: 2009. [Google Scholar]
- 10.Kawatsu K. Sexual conflict over the maintenance of sex: Effects of sexually antagonistic coevolution for reproductive isolation of parthenogenesis. PLoS ONE. 2013;8(2):e58141. doi: 10.1371/journal.pone.0058141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearcy M, Aron S, Doums C, Keller L. Conditional use of sex and parthenogenesis for worker and queen production in ants. Science. 2004;306(5702):1780–1783. doi: 10.1126/science.1105453. [DOI] [PubMed] [Google Scholar]
- 12.Fournier D, et al. Clonal reproduction by males and females in the little fire ant. Nature. 2005;435(7046):1230–1234. doi: 10.1038/nature03705. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawara K, Nakayama M, Satoh A, Trindl A, Heinze J. Clonal reproduction and genetic caste differences in a queen-polymorphic ant, Vollenhovia emeryi. Biol Lett. 2006;2(3):359–363. doi: 10.1098/rsbl.2006.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuura K, et al. Queen succession through asexual reproduction in termites. Science. 2009;323(5922):1687. doi: 10.1126/science.1169702. [DOI] [PubMed] [Google Scholar]
- 15.Vargo EL, Labadie PE, Matsuura K. Asexual queen succession in the subterranean termite Reticulitermes virginicus. Proc R Soc Lond B Biol Sci. 2012;279(1729):813–819. doi: 10.1098/rspb.2011.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luchetti A, Velonà A, Mueller M, Mantovani B. Breeding systems and reproductive strategies in Italian Reticulitermes colonies (Isoptera: Rhinotermitidae) Insectes Soc. 2013;60(2):203–211. [Google Scholar]
- 17.Kobayashi K, et al. Sex ratio biases in termites provide evidence for kin selection. Nat Commun. 2013;4:2048. doi: 10.1038/ncomms3048. [DOI] [PubMed] [Google Scholar]
- 18.Vargo EL, Husseneder C. Biology of subterranean termites: Insights from molecular studies of Reticulitermes and Coptotermes. Annu Rev Entomol. 2009;54:379–403. doi: 10.1146/annurev.ento.54.110807.090443. [DOI] [PubMed] [Google Scholar]
- 19.Thorne BL, Traniello JFA, Adams ES, Bulmer M. Reproductive dynamics and colony structure of subterranean termites of the genus Reticulitermes (Isoptera Rhinotermitidae): A review of the evidence from behavioral, ecological, and genetic studies. Ethol Ecol Evol. 1999;11(2):149–169. [Google Scholar]
- 20.Matsuura K. A test of the haplodiploid analogy hypothesis in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae) Ann Entomol Soc Am. 2002;95(5):646–649. [Google Scholar]
- 21.Crozier RH, Pamilo P. Evolution of Social Insect Colonies: Sex Allocation and Kin Selection. Oxford Univ Press; Oxford: 1996. [Google Scholar]
- 22.Ratnieks FL, Keller L. Queen control of egg fertilization in the honey bee. Behav Ecol Sociobiol. 1998;44(1):57–61. [Google Scholar]
- 23.Aron S, Timmermans I, Pearcy M. Ant queens adjust egg fertilization to benefit from both sexual and asexual reproduction. Biol Lett. 2011;7(4):571–573. doi: 10.1098/rsbl.2010.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark J, Lange AB. Evidence of a neural loop involved in controlling spermathecal contractions in Locusta migratoria. J Insect Physiol. 2001;47(6):607–616. doi: 10.1016/s0022-1910(00)00149-9. [DOI] [PubMed] [Google Scholar]
- 25.Akai H. Observations of the micropylar region of the chorion in the silkworm, Bombyx mori L. Acta Sericol. 1958;26:19–21. [Google Scholar]
- 26.Kubrakiewicz J, Jędrzejowska I, Szymańska B, Biliński SM. Micropyle in neuropterid insects. Structure and late stages of morphogenesis. Arthropod Struct Dev. 2005;34(2):179–188. [Google Scholar]
- 27.Yamauchi H, Yoshitake N. Formation and ultrastructure of the micropylar apparatus in Bombyx mori ovarian follicles. J Morphol. 1984;179(1):47–58. doi: 10.1002/jmor.1051790106. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura K, Fujimoto M, Goka K. Sexual and asexual colony foundation and the mechanism of facultative parthenogenesis in the termite Reticulitermes speratus (Isoptera, Rhinotermitidae) Insectes Soc. 2004;51(4):325–332. [Google Scholar]
- 29.Matsuura K, Kobayashi N. Size, hatching rate, and hatching period of sexually and asexually produced eggs in the facultatively parthenogenetic termite Reticulitermes speratus (Isoptera: Rhinotermitidae) Appl Entomol Zool. 2007;42(2):241–246. [Google Scholar]
- 30.Lainé LV, Lainé LV, Wright DJ. The life cycle of Reticulitermes spp. (Isoptera: Rhinotermitidae): What do we know? Bull Entomol Res. 2003;93(4):267–378. doi: 10.1079/ber2003238. [DOI] [PubMed] [Google Scholar]
- 31.Matsuura K, Kobayashi N, Yashiro T. Seasonal patterns of egg production in field colonies of the termite Reticulitermes speratus (Isoptera: Rhinotermitidae) Popul Ecol. 2007;49(2):179–183. [Google Scholar]
- 32.Suzanne M, Perrimon N, Noselli S. The Drosophila JNK pathway controls the morphogenesis of the egg dorsal appendages and micropyle. Dev Biol. 2001;237(2):282–294. doi: 10.1006/dbio.2001.0384. [DOI] [PubMed] [Google Scholar]
- 33.Vargo EL. Polymorphism at trinucleotide microsatellite loci in the subterranean termite Reticulitermes flavipes. Mol Ecol. 2000;9(6):817–820. doi: 10.1046/j.1365-294x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 34.Dronnet S, Bagneres AG, Juba TR, Vargo EL. Polymorphic microsatellite loci in the European subterranean termite, Reticulitermes santonensis Feytaud. Mol Ecol Notes. 2004;4(1):127–129. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.