Significance
Stem cell therapy is an emerging approach to the treatment of heart failure. Endogenous or transplanted stem cells have limited repair capacity due to damage by exposure to stress. Agonists of growth hormone-releasing hormone receptor (GHRH-R) have been previously shown to increase the number of endogenous cardiac stem cells (CSCs) after myocardial infarction; enhance vasculogenesis of mesenchymal stem cells; and improve growth, engraftment, and survival of transplanted pancreatic islets in experimental models. This study shows that CSCs isolated from different species express GHRH-R and agonists of GHRH-R stimulate their proliferation and survival. Our findings support the potential use of GHRH agonists for endogenous stem cell stimulation and preconditioning prior to transplantation to improve CSC mobilization, survival, and probably enhancement of their angiomyogenic potential in the infarcted myocardium.
Keywords: cardiac stem cells, growth hormone-releasing hormone, agonists, cell proliferation, cell survival
Abstract
The beneficial effects of agonists of growth hormone-releasing hormone receptor (GHRH-R) in heart failure models are associated with an increase in the number of ckit+ cardiac stem cells (CSCs). The goal of the present study was to determine the presence of GHRH-R in CSCs, the effect of GHRH-R agonists on their proliferation and survival, and the mechanisms involved. We investigated the expression of GHRH-R in CSCs of different species and the effect of GHRH-R agonists on their cell proliferation and survival. GHRH-R is expressed in ckit+ CSCs isolated from mouse, rat, and pig. Treatment of porcine CSCs with the GHRH-R agonist JI-38 significantly increased the rate of cell division. Similar results were observed with other GHRH-R agonists, MR-356 and MR-409. JI-38 exerted a protective effect on survival of porcine CSCs under conditions of oxidative stress induced by exposure to hydrogen peroxide. Treatment with JI-38 before exposure to peroxide significantly reduced cell death. A similar effect was observed with MR-356. Addition of GHRH-R agonists to porcine CSCs induced activation of ERK and AKT pathways as determined by increased expression of phospho-ERK and phospho-AKT. Inhibitors of ERK and AKT pathways completely reversed the effect of GHRH-R agonists on CSC proliferation. Our findings extend the observations of the expression of GHRH-R by CSCs and demonstrate that GHRH-R agonists have a direct effect on proliferation and survival of CSCs. These results support the therapeutic use of GHRH-R agonists for stimulating endogenous mechanisms for myocardial repair or for preconditioning of stem cells before transplantation.
Heart failure is the worldwide leading cause of death from cardiovascular disease and the end result of a number of chronic illnesses (1). Pharmacologic therapy plays an important, but limited, role in controlling heart failure, and, for end stage patients, heart transplantation remains the ultimate option. Stem cell therapy is an emerging and promising approach for the treatment of heart failure. Clinical studies have demonstrated encouraging results and improvement of myocardial function after transplantation of different stem cell types (2–6).
Tissue-resident stem cells have been identified in many organs, including the heart (7–10). Cardiac stem cells (CSCs) have been isolated from animal and human myocardium and are able to reconstitute the injured heart under experimental conditions (2, 3, 11–15), suggesting the possibility of activation of endogenous repair mechanisms for myocardial regeneration. Several studies have demonstrated the use of growth factors and cytokines for endogenous stem cell stimulation through enhancement of mobilization, survival, proliferation, and differentiation (16–19). Similarly, preconditioning of stem cells by genetic modification or priming with cytokines, growth factors, small molecules, and other pharmacological approaches, before transplantation, has significant effects on survival and engraftment (16, 20, 21).
Activation of hormonal signaling pathways has been pursued as a potential avenue for the treatment of heart failure, with a primary focus on the role of these pathways in cardiac growth, survival, and hemodynamics (22–25). The growth hormone (GH)/insulin-like growth factor axis is an important pathway involved in the regulation of cardiac growth and function (26, 27). Recent studies have shown that not only GH, but GH-releasing peptides such as growth hormone-releasing hormone (GHRH) and ghrelin are expressed in myocardial tissue and protect the heart from ischemia (22–24, 28). In addition to their effect on differentiated cells, these hormonal mediators have also been shown to play a role in stem cell function and fate, suggesting their possible use for stimulation of endogenous CSCs or their preconditioning before transplantation (29–34).
Our group recently demonstrated that JI-38, a synthetic agonist of the growth hormone-releasing hormone receptor (GHRH-R), has cardioprotective effects and also stimulates cardiac reverse remodeling in a rodent model of myocardial infarction (22, 35, 36). These findings were associated with an increase in the number of CSCs in vivo. Exposure of purified rat ckit+ CSCs to JI-38 stimulated proliferation, suggesting a direct effect of this agonist on these cells (35). In the present work, we report that CSCs isolated from different species express GHRH-R. In addition, we demonstrate that recently made potent GHRH-R agonists, MR-356 and MR-409, stimulate proliferation of CSC and promote survival through mechanisms that involve activation of the ERK and AKT pathways.
Results
Cardiac Stem Cells Express GHRH Receptor.
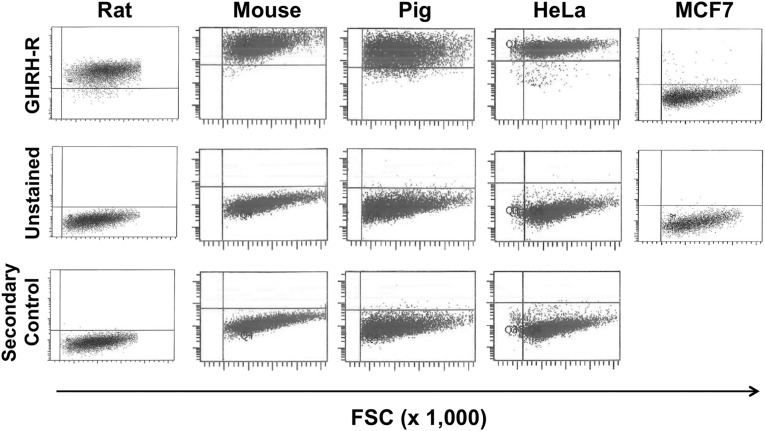
We investigated the expression of GHRH-R in ckit+ CSCs of different species by flow cytometric analysis. As shown in Fig. 1, GHRH-R is expressed in CSCs isolated from mouse, rat, and pig. The expression was compared with that in HeLa and MCF7 human cancer cell lines, which were used as positive and negative controls, respectively. Results showed that CSCs from all species tested were 96–98% positive for GHRH-R. HeLa cells were 100% positive for GHRH-R, whereas no expression was detected in the negative control, the MCF7 cells.
Fig. 1.
Expression of GHRH-R in cardiac stem cells. Fluorescence-activated cell sorting analysis of cardiac stem cells isolated from different species for analysis of GHRH-R expression. (Top) Cells stained for GHRH-R. (Middle) Unstained cells. (Bottom) Cells stained with secondary antibody as control. Cancer cell lines expressing (HeLa) or lacking (MCF7) GHRH-R were used as positive and negative controls, respectively. Results correspond to representative images from three different cell isolates for each individual species. FSC, forward scatter.
GHRH-R Agonists Stimulate Self-Renewal of CSCs.
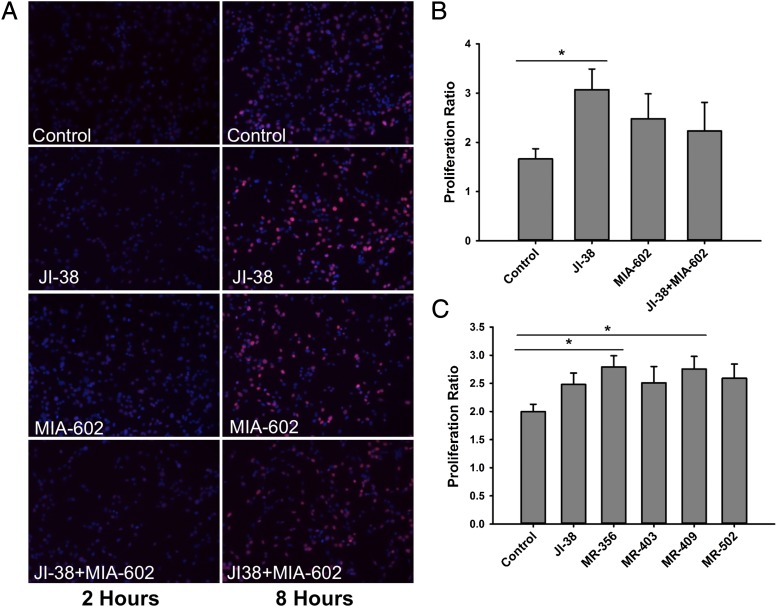
To determine if GHRH-R activation can improve CSC self-renewal, we initially tested the effect of the GHRH-R agonist, JI-38, on ckit+ porcine CSC proliferation. The rate of cell division was quantified by incorporation of the thymidine analogs 5-ethynyl-2′-deoxyuridine (EdU) or bromodeoxyuridine (BrdU). We demonstrate that incorporation of EdU in CSCs was significantly increased, approximately twofold, with JI-38 treatment (3.1 ± 0.4) vs. vehicle control (1.7 ± 0.2) (P < 0.05, n = 3) (Fig. 2). Pretreatment of CSCs with the GHRH-R antagonist MIA-602 produced no significant change but did show a trend toward reversal of the effects of the agonist JI-38 on proliferation rate (2.2 ± 0.6) (Fig. 2 A and B). These proliferation studies were further extended to other GHRH-R agonists: MR-356, MR-403, MR-409, and MR502. The agonists MR-356 and MR-409 significantly increased CSC proliferation relative to vehicle control by 36.7 ± 8.5% and 36.2 ± 12.2% (P < 0.05 for each, n = 14), although their effects did not differ. The agonists JI-38, MR-403, and MR-502 showed a trend of increase in CSC proliferation by 20.0 ± 5.7%, 12.9 ± 7.4%, and 23.4 ± 10.2%, respectively. However, this difference was not significant when all agonists were compared together and relative to the control (Fig. 2C).
Fig. 2.
Effect of GHRH-R agonists on proliferation of cardiac stem cells. (A) Representative immunofluorescence staining of porcine CSCs showing incorporation of EdU at 2 and 8 h after exposure to 100 nM of the GHRH-R agonist JI-38 and to 100 nM of the antagonist MIA-602. (B) Quantification of proliferation rate from fluorescence images in A (*P < 0.05, n = 3). (C) Effect of different GHRH-R agonists at 100 nM concentration on porcine CSC proliferation (*P < 0.05, n = 14). Values in B and C represent the proliferation ratio between 2 and 8 h. Error bars indicate SE.
GHRH-R Agonists Promote Survival of CSCs Following Exposure to Oxidative Stress.
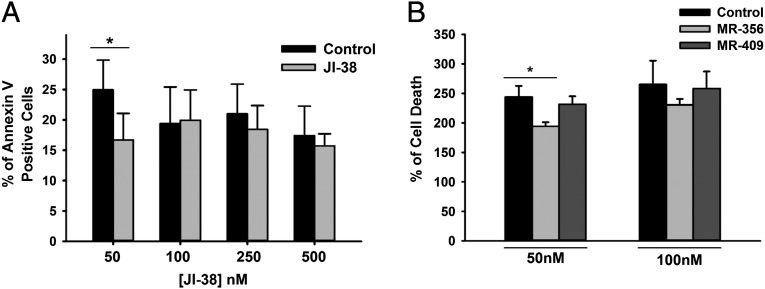
The protective effect of JI-38 on survival of porcine CSCs was determined during exposure to oxidative stress generated by 0.2 mM hydrogen peroxide. Pretreatment of CSCs with JI-38 before peroxide exposure reduced the number of cells positive for the apoptosis marker Annexin-V by 32.8 ± 4.4% relative to control (Fig. 3A) (P < 0.02, n = 4). This effect was observed only at the lower dose of the agonist (50 nM) compared with the higher dose (100 nM) required to stimulate cell proliferation (Fig. 3A). Cytotoxicity studies based on lactate dehydrogenase release from damaged cells confirmed that the agonist MR-356 also protected CSCs, reducing cell death by 14.14 ± 7.2% relative to vehicle control (Fig. 3B) (P < 0.02, n = 14). Similar to what was observed for JI-38, the lower agonist dose of 50 nM was more effective. No significant effect was observed for the agonist MR-409.
Fig. 3.
Effect of GHRH-R agonists on cardiac stem cell survival. (A) Dose-dependent effect of the agonist JI-38. Values represent the percentage of Annexin V-positive cells 16 h after treatment with 0.2 mM of hydrogen peroxide (*P < 0.05, n = 4). (B) Effect of the agonists MR-356 and MR-409. Values represent the percentage of cell death (lactate dehydrogenase activity detected in culture supernatants), relative to control without peroxide, 48 h after 0.2 mM hydrogen peroxide treatment (*P < 0.05, n = 13–14). Error bars indicate SE.
Effect of GHRH-R Agonists on Proliferation of CSCs Is Mediated by the Activation of Erk and Akt Pathways.
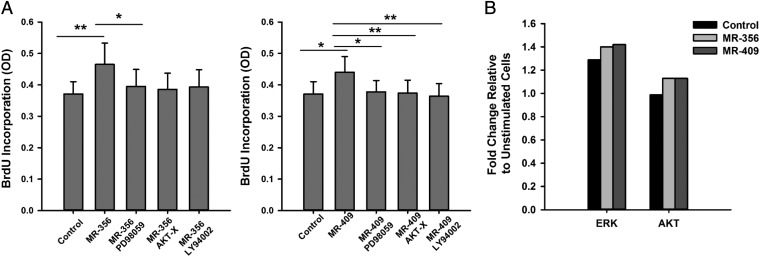
To determine the downstream mechanism of activation by the GHRH-R agonist on proliferation of CSCs, we investigated two potential mechanisms of action previously shown to be induced downstream of GHRH-R, the ERK and AKT pathways (24, 25, 37, 38). The involvement of these pathways in the induction of proliferation by GHRH-R agonists was tested by treatment of porcine CSCs with the inhibitors of ERK, PI3K, and AKT pathways (PD98059, LY294002, and AKT-X, respectively) before the addition of the agonists. The ERK inhibitor PD98059 significantly reversed the induction in proliferation of the agonists MR-356 and MR-409 by 85 ± 1.46% and 87.1 ± 3.48%, respectively (Fig. 4A) (P < 0.05 for each, n = 5). Inhibitors of the PI3K and AKT pathways significantly reversed the effect of the agonist MR-409 by 85.7 ± 3.83% and 83.1 ± 3.34%, respectively (Fig. 4A) (P < 0.005 for each, n = 5). However, although these inhibitors showed a trend toward reversal of the effect of agonist MR-356 by 86.0 ± 8.05% and 85.8 ± 5.39%, respectively, the results were not significantly different. Activation of ERK and AKT pathways was determined by ELISA. After overnight starvation, porcine CSCs were treated with either vehicle (control) or the agonists, and lysates were collected to determine the activation of ERK and AKT by time-course analysis of phosphorylation of ERK and AKT. Our results show that the agonists MR-356 and MR-409 activated ERK and AKT pathways, as determined by an increase in the expression of phospho-ERK and phospho-AKT by 9–11 and 15% relative to vehicle control, respectively (Fig. 4B) (n = 2). These findings indicate that GHRH-R agonists mediate their effects on CSCs through activation of the ERK and AKT pathways.
Fig. 4.
Activation of AKT and ERK signaling after stimulation of cardiac stem cells with GHRH-R agonists. (A) Effect of AKT (AKT-X), PI3K (LY294002), and ERK (PD98059) pathway inhibitors on CSC proliferation after GHRH-R agonist exposure. Values represent incorporated BrdU 8 h after stimulation with agonists. Pathway inhibitors were tested at 5 μM and GHRH-R agonists at 100 nM. Error bars indicate SE (*P < 0.05, **P < 0.005, n = 5). OD, optical density. (B) Detection of phosphorylated ERK and AKT pathways 10 min after stimulation with vehicle control (DMSO) and GHRH-R agonists by ELISA. Values represent fold change relative to unstimulated cells before agonist treatment (n = 2).
Discussion
Our studies demonstrate that CSCs from different species express GHRH-R and that synthetic agonists of this receptor promote proliferation and survival of CSCs. These findings suggest the potential use of GHRH-R agonists in the activation of endogenous mechanisms of cardiac repair. The expression of GHRH-R, classically described in the pituitary, has been more recently demonstrated in various other tissues, including the heart (39, 40). The somatotropic role of GHRH in the regulation of synthesis and release of growth hormone is well established (41, 42). However, little is known regarding the extrapituitary role of GHRH (43). Some studies have demonstrated the tissue stimulatory effect of GHRH and its potential involvement in cardiac disease (43). The activation of hormonal signaling pathways has been pursued as a potential approach to the treatment of heart failure, and growth hormone and its releasing peptides, such as ghrelin, exhibit cardioprotective effects (22–27, 35, 44–46). Our findings are in agreement with studies previously performed using cardiac myocytes. Treatment of cardiac myocytes with synthetic rat GHRH protected against cell death induced by serum deprivation and by isoproterenol and improved ventricular function in isolated rat heart subjected to ischemia-reperfusion injury (24). The protective effect of activation of GHRH-R in the heart was further supported by previous in vivo work of our group where the GHRH-R agonist JI-38 promoted cardiac repair and improvement of ventricular function in rats after myocardial infarction (22, 35, 36). In addition to improved cardiac performance in acute and chronic infarction models, administration of the GHRH agonist JI-38 in a chronic stage of injury reversed ventricular remodeling (35).
The beneficial effects of JI-38 in rat models of myocardial infarction were accompanied by an increase in the number of ckit+ CSCs, suggesting that GHRH-R agonists may produce a local stimulation of these cells, directly or indirectly (22, 35). We have previously shown that exposure of purified rat ckit+ CSCs in vitro to JI-38 increased their rate of cell division, suggesting a direct effect of the GHRH agonist on these cells (35). These results are supported by our current study showing that purified CSCs from different species express GHRH-R and that other GHRH-R agonists also promote CSC proliferation. In addition to effects on differentiated cells, stimulation of hormone-mediated pathways also plays a role in stem cell function and fate. However, most previous studies focused on GH and its secretagogue, ghrelin, and the role of GHRH signaling in stem cell function remains largely unexplored. Infusion of GH into mice activated a population of neural stem cells and induced neurogenesis (47). Ghrelin, a GH secretagogue, affects adult progenitors and embryonic stem cells. Johansson et al. demonstrated that ghrelin and the synthetic GH secretagogue hexarelin stimulate neural progenitor proliferation (32). However, only hexarelin, which acts on the ghrelin receptor, protected these progenitors from growth deprivation-induced cell death (32). Targeted deletion of ghrelin in mice reduced the number of neural progenitors in the hippocampus whereas treatment with ghrelin rescued neurogenesis through mechanisms that involve stimulation of proliferation, migration, and differentiation of neural progenitors (33, 34). In the cardiovascular system, ghrelin guides migration of adult endothelial progenitor cells through receptor-mediated activation of PI3K/AKT/NO signaling (29) and induces cardiac differentiation of embryonic stem cells by a mechanism that involves activation of the ERK pathway (30). Furthermore, recent work from our group showed that the GHRH-R agonist JI-38 enhances the vasculogenic potential of mesenchymal stem cells (31).
The cardioprotective effects of GHRH-R agonists appear to be receptor-mediated, as they can be reversed by specific antagonists (22, 24, 35). In purified CSCs, we also observed a partial reversal of the effect of the agonist, although these results were not significant. The GHRH-R is a G protein-coupled receptor known to activate at least three major signaling pathways: cAMP/PKA, MAPK/ERK, and PI3K/AKT (24, 38). Accordingly, we found that treatment of CSCs with agonists of GHRH-R induced ERK and AKT phosphorylation. Our results suggest that the proliferative effect of GHRH-R agonists in CSCs is triggered by activation of ERK and AKT pathways, as specific inhibitors of these pathways blocked this effect. The relevance of ERK and AKT signaling for CSC self-renewal and survival has been previously demonstrated. Activation of these pathways by stem cell preconditioning also improves survival after transplantation (48, 49). Overexpression of nuclear-targeted AKT in mice induces an increase in the number of myocyte-committed ckit+ progenitor cells, with activation of AKT being associated with upregulation of myocardial expression of several growth factor receptor systems known to promote growth and survival (50). Inactivation of AKT signaling in CSCs not only decreased the proliferation of these cells in vitro, but also induced cell death (51), suggesting an essential role of this pathway in CSC maintenance. The role of ERK in CSCs is controversial. Choi et al. demonstrated that the ERK pathway is activated when human CSCs undergo replicative senescence and that ERK inhibition rescues this effect (52). This result is at variance with several studies, including ours, showing that ERK activation favors proliferation and survival (53–55). These differences are likely due to additional effectors that play a role during replicative senescence. Altogether, these reports confirm an essential role for AKT and ERK in promoting stem cell proliferation and survival, which are critical for successful engraftment of transplanted cells.
The limitations of pharmacologic therapy in the control of heart failure have been the driving force in the search for alternative approaches to improve chronic heart disease and its associated morbidity and mortality. Stem cell therapy is an emerging and promising approach to the treatment of heart failure (56, 57). Clinical studies have demonstrated encouraging results and improvement of myocardial function after transplantation of different types of stem cells (2, 3, 58, 59). Nevertheless, the advancement of regenerative medicine has faced several obstacles, most of which relate to the fact that highly potent cell sources for repair, engraftment, and survival after transplantation are not definitively identified (60–62). Enhancement of the regenerative potential of stem cells by means of improved engraftment and survival has recently received attention from numerous research groups. The local stimulation and protection of CSCs from damage after myocardial injury may be a key determinant of clinical outcome in patients. Several studies have demonstrated the use of growth factors and cytokines for stimulation of endogenous stem cells to enhance mobilization, survival, proliferation, and differentiation (16, 33). Similarly, preconditioning of stem cells before transplantation, by genetic modification or by priming with cytokines, growth factors, small molecules, or other pharmacological approaches, has shown significant effects on survival and engraftment (17, 18, 50, 63, 64). Interestingly, one common characteristic among these studies is that improvement of engraftment and survival of stem cells was associated with activation of ERK and AKT signaling, two pathways that, in our study, were stimulated by GHRH-R agonists. In our present study, we show that induction of CSC proliferation and survival by GHRH-R agonists was associated with simultaneous stimulation of both ERK and AKT pathways, placing these compounds in a leading position as preconditioning agents for cell transplantation. The preconditioning effect of GHRH-R agonists has been previously demonstrated in transplantation of pancreatic islet where pretreatment of islets with the GHRH-R agonists JI-36 and MR-403 improved growth, engraftment, and survival (65, 66).
In conclusion, our results suggest the potential use of GHRH-R agonists as a novel therapeutic approach for in situ stimulation of endogenous CSCs in vivo and as preconditioning agents for stem cell transplantation. Previous studies of our group demonstrating the cardioprotective effects of the GHRH-R agonist class of compounds, combined with our current findings, further support the potential application of these analogs for the treatment of chronic heart disease and prevention of heart failure.
Methods
A detailed description of the methods can be found in SI Methods. All procedures for cell isolation from animal sources were carried out in accordance with protocols reviewed and approved by the University of Miami Animal Care and Use Committee in compliance with the Guide for the Care and Use of Laboratory Animals. ckit+ CSCs were isolated from mouse, rat, and pig myocardium biopsies and analyzed for the expression of GHRH-R by fluorescence-activated cell sorting. The effect of the GHRH-R agonists JI-38, MR-356, MR-403, MR-409, and MR-502 on cell proliferation and survival and the mechanisms involved were assessed in porcine CSCs. Data were analyzed for significance using one-way ANOVA and repeated measures ANOVA with Tukey’s Multiple Comparison as post hoc test. For a given parameter, P < 0.05 was considered significant. All tests were carried out using GraphPad software.
Supplementary Material
Acknowledgments
We thank Dr. Wayne Balkan for critical reading of this manuscript and the University of Miami, Sylvester Comprehensive Cancer Center, Flow Cytometry Core for technical assistance with FACS analysis. This work was funded by National Heart, Lung, and Blood Institute Grant R01-HL107110 (to J.M.H.) and the Starr Foundation (J.M.H.); by the Medical Research Service of Veterans Affairs Department and the Department of Medicine, Division of Hematology/Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine (A.V.S.); and by the L. Austin Weeks Endowment for Research (N.L.B.).
Footnotes
Conflict of interest statement: N.L.B. and J.M.H. have ownership in Biscayne Pharmaceuticals Inc., which has licensed the technology. R.-Z.C. and A.V.S. are co-inventors on a patent on GHRH agonists assigned to the University of Miami, but the present paper reports only basic experimental studies. Other authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420375111/-/DCSupplemental.
References
- 1.Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Williams AR, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127(2):213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugh AR, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11) Suppl 1:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suncion VY, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114(8):1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare JM, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldman AW, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: Till death do us part. Biogerontology. 2013;14(6):573–590. doi: 10.1007/s10522-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7(6):656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Barile L, Messina E, Giacomello A, Marbán E. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50(1):31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Hatzistergos KE, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latham N, et al. Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation. 2013;128(11) Suppl 1:S105–S112. doi: 10.1161/CIRCULATIONAHA.112.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oskouei BN, et al. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl Med. 2012;1(2):116–124. doi: 10.5966/sctm.2011-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welt FG, et al. Effect of cardiac stem cells on left-ventricular remodeling in a canine model of chronic myocardial infarction. Circ Heart Fail. 2013;6(1):99–106. doi: 10.1161/CIRCHEARTFAILURE.112.972273. [DOI] [PubMed] [Google Scholar]
- 14.Bolli R, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128(2):122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malliaras K, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63(2):110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pons J, et al. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376(2):419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Hahn JY, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51(9):933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Pasha Z, et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77(1):134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106(11):1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang FL, et al. Cardiomyocyte-specific overexpression of human stem cell factor improves cardiac function and survival after myocardial infarction in mice. Circulation. 2009;120(12):1065–1074. doi: 10.1161/CIRCULATIONAHA.108.839068. [DOI] [PubMed] [Google Scholar]
- 21.Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 22.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107(6):2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granata R, Isgaard J, Alloatti G, Ghigo E. Cardiovascular actions of the ghrelin gene-derived peptides and growth hormone-releasing hormone. Exp Biol Med (Maywood) 2011;236(5):505–514. doi: 10.1258/ebm.2011.010365. [DOI] [PubMed] [Google Scholar]
- 24.Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83(2):303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 25.Penna C, et al. GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways. Endocrinology. 2013;154(4):1624–1635. doi: 10.1210/en.2012-2064. [DOI] [PubMed] [Google Scholar]
- 26.Fazio S, Palmieri EA, Biondi B, Cittadini A, Saccà L. The role of the GH-IGF-I axis in the regulation of myocardial growth: From experimental models to human evidence. Eur J Endocrinol. 2000;142(3):211–216. doi: 10.1530/eje.0.1420211. [DOI] [PubMed] [Google Scholar]
- 27.Cittadini A, et al. Importance of an intact growth hormone/insulin-like growth factor 1 axis for normal post-infarction healing: Studies in dwarf rats. Endocrinology. 2001;142(1):332–338. doi: 10.1210/endo.142.1.7913. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G, et al. Ghrelin and cardiovascular diseases. Curr Cardiol Rev. 2010;6(1):62–70. doi: 10.2174/157340310790231662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Chen Q, Wang L, Li G. Ghrelin induces cell migration through GHSR1a-mediated PI3K/Akt/eNOS/NO signaling pathway in endothelial progenitor cells. Metabolism. 2013;62(5):743–752. doi: 10.1016/j.metabol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Gao M, et al. Ghrelin induces cardiac lineage differentiation of human embryonic stem cells through ERK1/2 pathway. Int J Cardiol. 2013;167(6):2724–2733. doi: 10.1016/j.ijcard.2012.06.106. [DOI] [PubMed] [Google Scholar]
- 31.Gomes SA, et al. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci USA. 2013;110(8):2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson I, et al. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149(5):2191–2199. doi: 10.1210/en.2007-0733. [DOI] [PubMed] [Google Scholar]
- 33.Li E, Kim Y, Kim S, Park S. Ghrelin-induced hippocampal neurogenesis and enhancement of cognitive function are mediated independently of GH/IGF-1 axis: Lessons from the spontaneous dwarf rats. Endocr J. 2013;60(9):1065–1075. doi: 10.1507/endocrj.ej13-0045. [DOI] [PubMed] [Google Scholar]
- 34.Li E, et al. Ghrelin stimulates proliferation, migration and differentiation of neural progenitors from the subventricular zone in the adult mice. Exp Neurol. 2014;252:75–84. doi: 10.1016/j.expneurol.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Kanashiro-Takeuchi RM, et al. Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI) Proc Natl Acad Sci USA. 2012;109(2):559–563. doi: 10.1073/pnas.1119203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai R, et al. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides. 2014;52:104–112. doi: 10.1016/j.peptides.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo KE. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol Endocrinol. 1992;6(10):1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- 38.Pombo CM, Zalvide J, Gaylinn BD, Diéguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000;141(6):2113–2119. doi: 10.1210/endo.141.6.7513. [DOI] [PubMed] [Google Scholar]
- 39.Lin-Su K, Wajnrajch MP. Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor. Rev Endocr Metab Disord. 2002;3(4):313–323. doi: 10.1023/a:1020949507265. [DOI] [PubMed] [Google Scholar]
- 40.Christodoulou C, et al. Expression of growth hormone-releasing hormone (GHRH) and splice variant of GHRH receptors in normal mouse tissues. Regul Pept. 2006;136(1-3):105–108. doi: 10.1016/j.regpep.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Guillemin R, et al. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- 42.Frohman LA, Kineman RD. Growth hormone-releasing hormone and pituitary development, hyperplasia and tumorigenesis. Trends Endocrinol Metab. 2002;13(7):299–303. doi: 10.1016/s1043-2760(02)00613-6. [DOI] [PubMed] [Google Scholar]
- 43.Barabutis N, Schally AV. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 44.Tivesten A, et al. The growth hormone secretagogue hexarelin improves cardiac function in rats after experimental myocardial infarction. Endocrinology. 2000;141(1):60–66. doi: 10.1210/endo.141.1.7249. [DOI] [PubMed] [Google Scholar]
- 45.Broglio F, et al. Ghrelin: Much more than a natural growth hormone secretagogue. Isr Med Assoc J. 2002;4(8):607–613. [PubMed] [Google Scholar]
- 46.Marleau S, Mulumba M, Lamontagne D, Ong H. Cardiac and peripheral actions of growth hormone and its releasing peptides: Relevance for the treatment of cardiomyopathies. Cardiovasc Res. 2006;69(1):26–35. doi: 10.1016/j.cardiores.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Waters MJ, Blackmore DG. Growth hormone (GH), brain development and neural stem cells. Pediatr Endocrinol Rev. 2011;9(2):549–553. [PubMed] [Google Scholar]
- 48.Zhang Z, et al. Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res Cardiol. 2013;108(2):333. doi: 10.1007/s00395-013-0333-5. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, Fang B, Zhao X, Zhang G, Ma H. Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PLoS ONE. 2014;9(3):e90667. doi: 10.1371/journal.pone.0090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gude N, et al. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99(4):381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 51.Tateishi K, et al. Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun. 2007;352(3):635–641. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 52.Choi SH, et al. Regulation of ROS-independent ERK signaling rescues replicative cellular senescence in ex vivo expanded human c-kit-positive cardiac progenitor cells. Int J Cardiol. 2013;169(1):73–82. doi: 10.1016/j.ijcard.2013.08.076. [DOI] [PubMed] [Google Scholar]
- 53.Roskoski R., Jr ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacolo Res. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Steelman LS, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany, NY Online) 2011;3(3):192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotoh N. Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells. Curr Stem Cell Res Ther. 2009;4(1):9–15. doi: 10.2174/157488809787169048. [DOI] [PubMed] [Google Scholar]
- 56.Malliaras K, Marbán E. Cardiac cell therapy: Where we’ve been, where we are, and where we should be headed. Br Med Bull. 2011;98:161–185. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams AR, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108(7):792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hare JM, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Traverse JH, et al. Cardiovascular Cell Therapy Research Network (CCTRN) Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The TIME randomized trial. JAMA. 2012;308(22):2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traverse JH, et al. Cardiovascular Cell Therapy ResearchNetwork Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdel-Latif A, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 63.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011;51(4):619–625. doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer KM, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120(21):2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107(28):12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schubert U, et al. Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone. Proc Natl Acad Sci USA. 2013;110(6):2288–2293. doi: 10.1073/pnas.1221505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.