Abstract
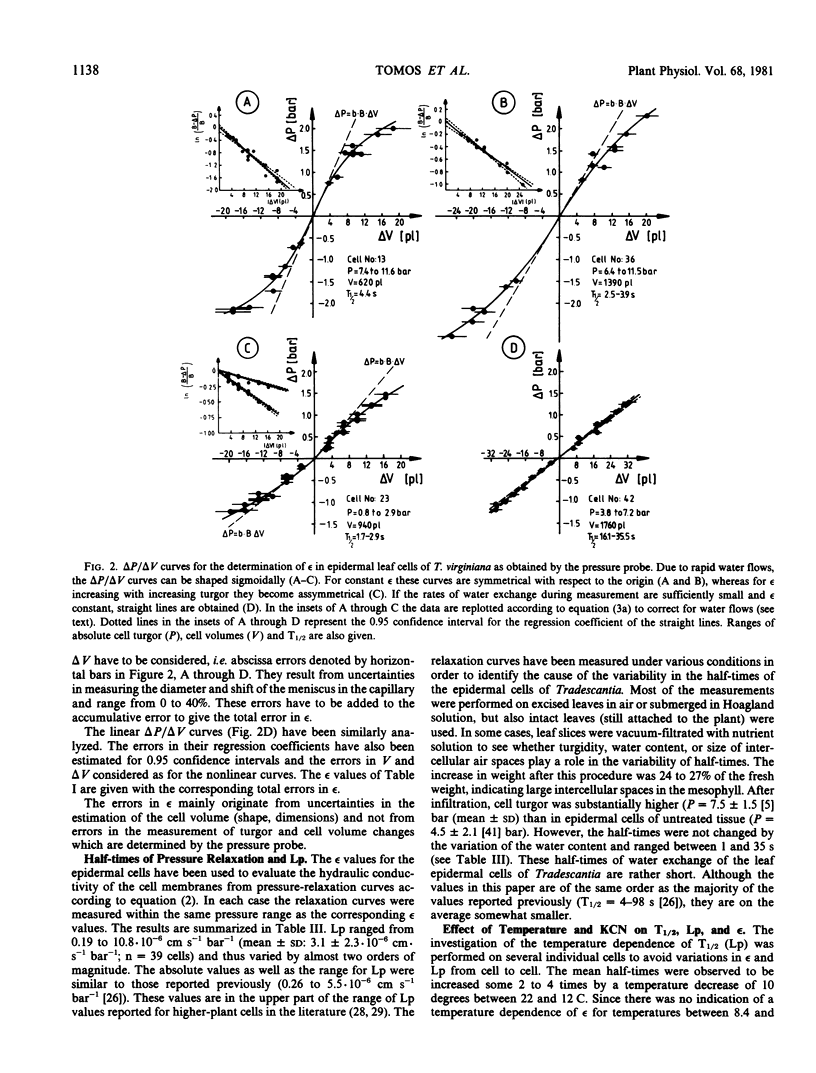
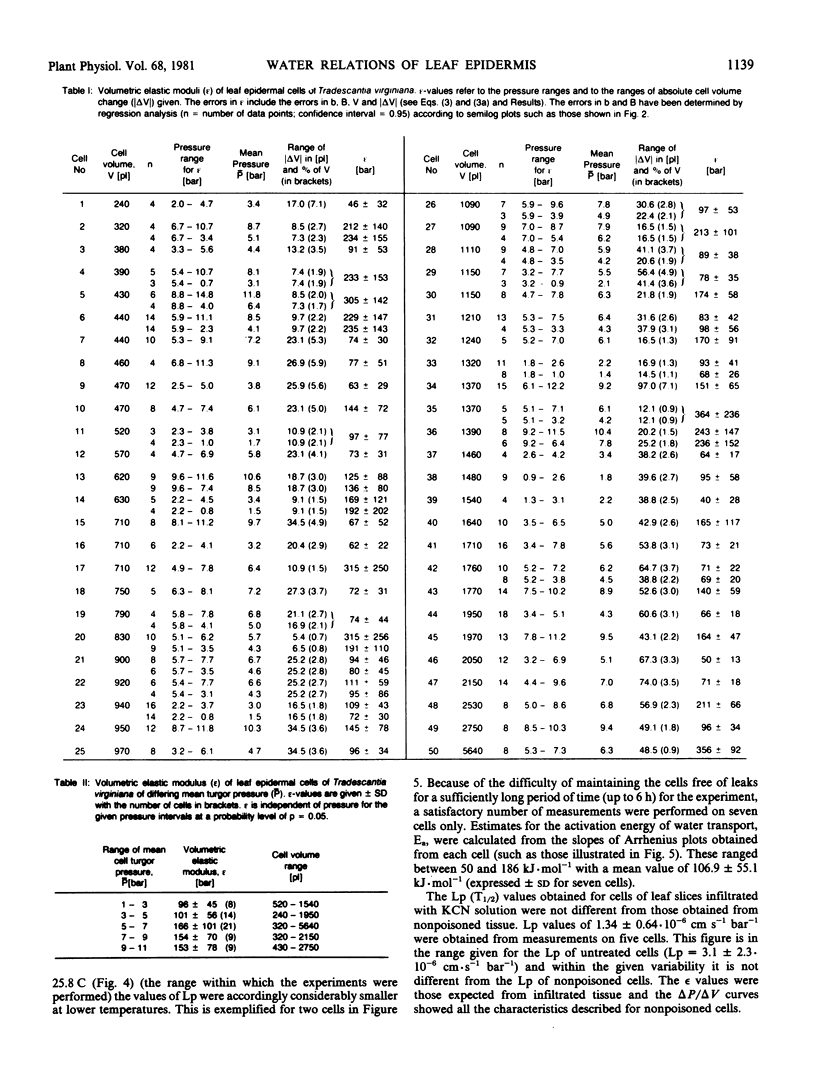
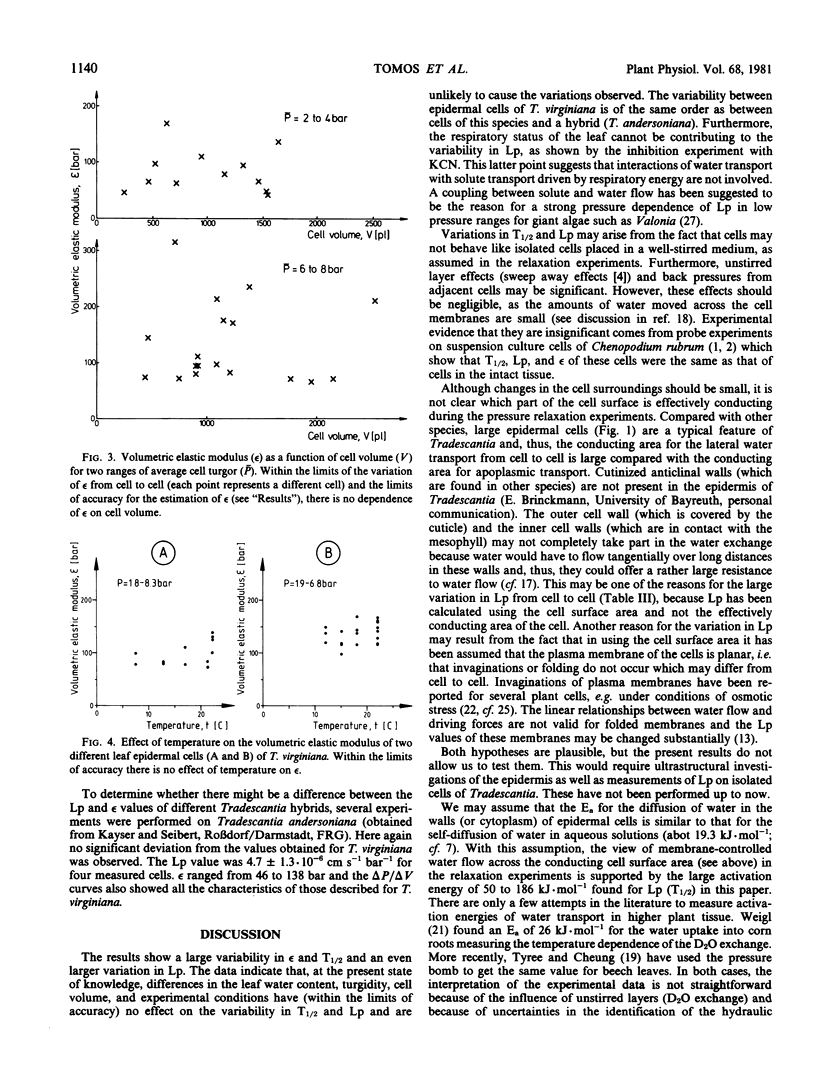
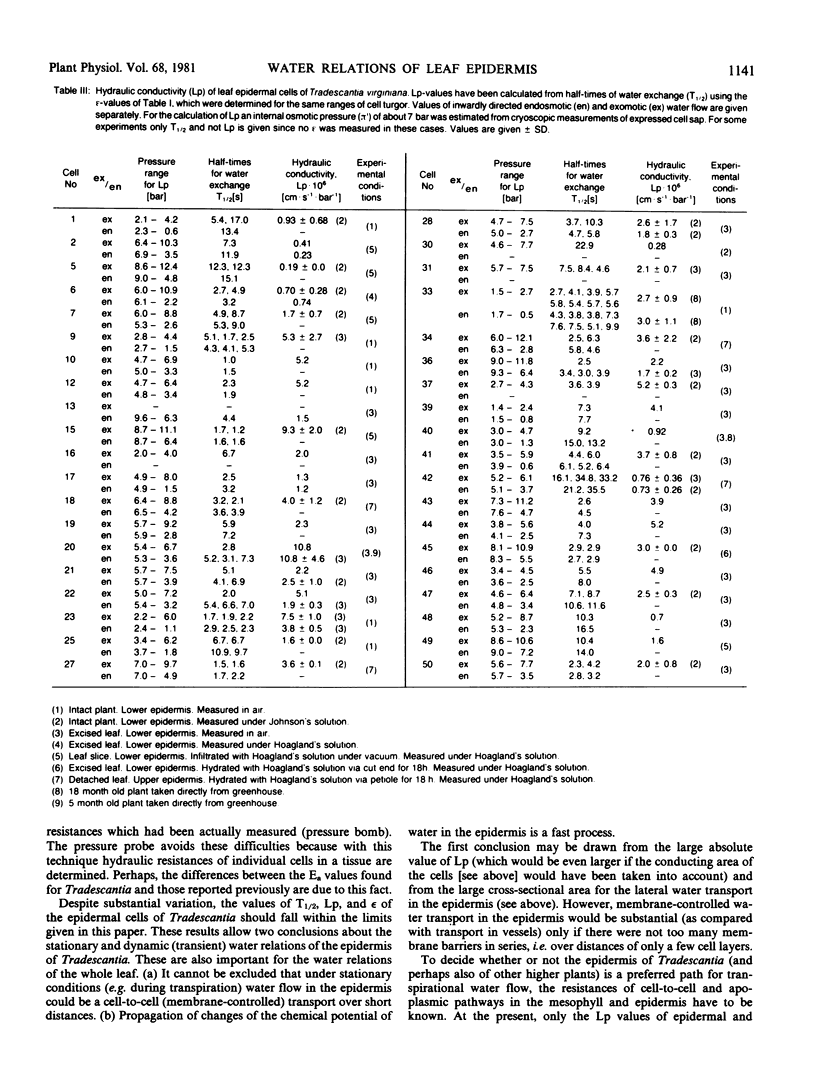
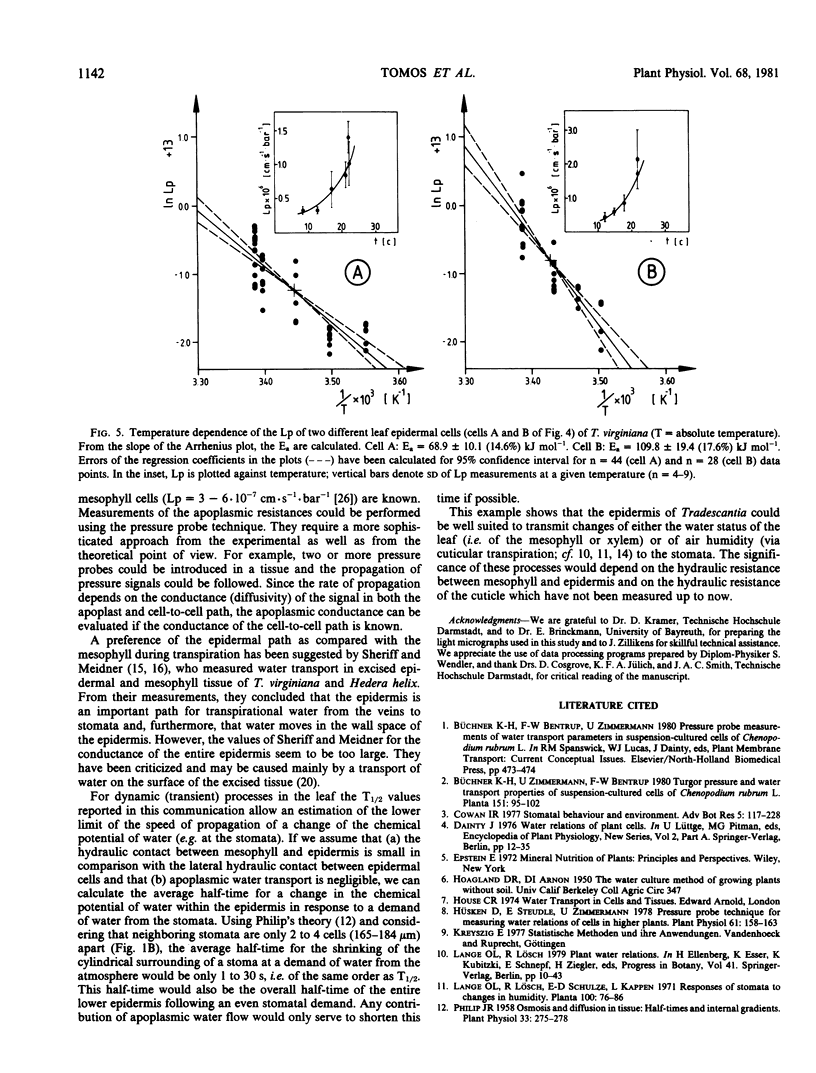
Water-relation parameters (cell turgor pressure [P], volumetric elastic modulus [ε] and hydraulic conductivity [Lp]) of individual leaf epidermal cells of Tradescantia virginiana have been determined with the pressure-probe technique. Turgor was 4.5 ± 2.1 [41] bar (mean ± sd; in brackets the number of cells) and ranged from 0.9 to 9.6 bar. By vacuum infiltration with nutrient solution, it was raised to 7.5 ± 1.5 [5] bar (range: 5.3-8.8 bar). There was a large variability in the absolute value of ε of individual cells. ε ranged from 40 to 360 bar; mean ± sd: 135 ± 83 bar; n = 50 cells. ε values of individual cells seemed to be rather independent of changes in cell turgor. A critical assessment of the errors incurred in determining ε by the technique is included. The half-times of water exchange of individual cells ranged from 1 to 35 seconds, which gave values of 0.2 to 11 × 10−6 centimeters per second per bar for Lp (mean ± sd: 3.1 ± 2.3 × 10−6 centimeters per second per bar; n = 39 cells). The large range in Lp and ε is believed to be due to the difficulties in determining the effective surface area of water exchange of the cells. Lp is not influenced by active salt pumping driven by respiration energy inasmuch as it was not altered by 0.1 millimolar KCN. The temperature dependence of Lp (T½) was measured for the first time in individual higher-plant cells. Lp increased by a factor of 2 to 4, when the temperature was increased by 10 C. The activation energy of water exchange was found to be between 50 and 186 kilojoules per mole. Within the large range of variation it was found that T½, Lp, and ε did not change under various experimental conditions (intact and excised tissue, water content and turgidity, age, etc.). Similar results were obtained for the epidermal cells of Tradescantia andersoniana. The measurements suggest that the entire epidermis would respond very rapidly (i.e. with a half-time of 1 to 30 s) to a demand for water from the stomata.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hüsken D., Steudle E., Zimmermann U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978 Feb;61(2):158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip J. R. Osmosis and Diffusion in Tissue: Half-times and Internal Gradients. Plant Physiol. 1958 Jul;33(4):275–278. doi: 10.1104/pp.33.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson I. W., Licko V., Bartoli E. The nature of passive flows through tightly folded membranes. The influence of microstructure. J Membr Biol. 1973;11(4):293–308. doi: 10.1007/BF01869827. [DOI] [PubMed] [Google Scholar]
- Steudle E. Water-relation Parameters of Individual Mesophyll Cells of the Crassulacean Acid Metabolism Plant Kalanchoë daigremontiana. Plant Physiol. 1980 Dec;66(6):1155–1163. doi: 10.1104/pp.66.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigl J. Wasserstruktur und Permeation: Die Aktivierungsenergie und der molekulare Mechanismus der Wasserpermeation. Z Naturforsch B. 1967 Aug;22(8):885–890. [PubMed] [Google Scholar]
- Zimmermann U., Steudle E. The pressure-dependence of the hydraulic conductivity, the membrane resistance and membrane potential during turgor pressure regulation in Valonia utricularis. J Membr Biol. 1974;16(4):331–352. doi: 10.1007/BF01872422. [DOI] [PubMed] [Google Scholar]