Significance
Gastrointestinal worms (helminths) infect more than 2 billion people, and vaccines are not yet available. Helminths elicit a type 2 immune response characterized by high serum IgE levels and increased numbers of IL-4– or IL-13–secreting effector cells including Th2 cells, eosinophils, basophils, and type 2 innate lymphoid cells. We determined the mechanism by which basophils contribute to protection against secondary infections with gastrointestinal helminths. Here we demonstrate that basophils are recruited into the small intestine of infected mice and orchestrate the local type 2 immune response in this tissue. Basophil-mediated protection required the presence of IgE and the expression of activating Fc receptors and IL-4/IL-13 in basophils. These findings could help the development of new vaccination strategies against helminths.
Keywords: basophils, helminths, IL-4, protective immunity, IgE
Abstract
Basophils orchestrate protection against reinfections with gastrointestinal helminths and ticks, but the underlying mechanisms remain elusive. We investigated the role of Fc receptors on basophils, the antibody isotypes IgG1 and IgE, and basophil-derived IL-4/IL-13 during challenge infections with Heligmosomoides polygyrus and Nippostrongylus brasiliensis. Using mixed bone marrow chimeras, we found that activating Fc receptors on basophils were required for protective immunity but not for regulation of basophil homeostasis. Furthermore, rapid worm expulsion was impaired in IgE-deficient but not in IgG1-deficient mice. Basophils promoted the recruitment of other effector cells into the small intestine and induced expression of the antihelminthic proteins resistin-like molecule β and mucin 5ac. Selective deletion of IL-4/IL-13 in basophils resulted in impaired worm expulsion. Collectively, our results indicate that IgE-mediated activation of basophils and the release of basophil-derived IL-4/IL-13 are critical steps in protective immunity against helminths. Therefore, development of effective vaccines against helminths should consider boosting the IL-4/IgE/basophil axis of the immune system.
Infections with gastrointestinal helminths present a major global health problem affecting ∼2 billion people worldwide (1). Although chemotherapy can be used to treat most of the infections, reinfections commonly occur within 12 mo after treatment, and increasing drug resistance is observed (2). To date no efficacious vaccines against intestinal helminths are available, and further studies are needed to improve our understanding of protective immune responses against these parasites.
Heligmosomoides polygyrus (Hp) and Nippostrongylus brasiliensis (Nb) are natural rodent parasites that are commonly used as models for human hookworm infections. Hp is a strictly enteric natural murine parasite; mice are infected orally with Hp L3-stage larvae that first enter the submucosa in the small intestine and emerge into the gut lumen about 1 wk after infection to mature into adult worms, mate, and produce eggs. During the first infection Hp establishes a chronic infection in C57BL/6 mice, but when worms are cleared by anthelminthic agents mice are protected from subsequent infections (3, 4). Nb has a very different life cycle. Mice are infected s.c. with Nb L3-stage larvae that migrate via the bloodstream to the lung and, after being coughed up and swallowed, ultimately end up in the small intestine where they mate and produce eggs about 5 d after infection. Worms are expelled by day 10 after infection, so protective immunity to secondary infection can be studied without the need to clear the primary infection with drugs.
Type 2 immune responses against helminths typically are characterized by an increase in Th2 cells, eosinophils, and basophils, accompanied by high levels of IgG1 and IgE. Work during the past few years has provided important insights into the regulation of protective immunity against helminths by cells of the innate immune system including mast cells, macrophages, basophils, and type 2 innate lymphoid cells (ILC2) (5–7).
Basophils express the high-affinity receptor for IgE (FcεRI) together with an activating (FcγRIIIA) and an inhibitory (FcγRIIB) receptor for IgG (8). Upon receptor cross-linking, activated basophils rapidly release effector molecules including histamine and proteases from cytoplasmic granules. They further produce various proinflammatory lipid mediators, chemokines, and the Th2-associated cytokines IL-4, IL-5, and IL-13 (9–11). Over the past years several genetic mouse models have been used to elucidate basophil functions in vivo. It has been shown that basophils cooperate with dendritic cells to initiate Th2 responses, promote alternative activation of macrophages, and contribute to acquired resistance against parasites (12–16). We and others have shown that basophils contribute to protective immune responses against secondary infection by Nb and ticks (13, 14, 17). Antibody-mediated depletion of basophils also was reported to result in partially impaired protection against Hp and Trichuris muris (18, 19). However, the mechanisms by which basophils mediate protection remained elusive.
The cytokines IL-4 and IL-13 are critical for protective immunity, because they induce signal transducer and activator of transcription 6 (STAT6)-regulated genes in different cell types which contribute to worm expulsion (20). It was further shown that protective immunity against secondary Hp infection depends mostly on memory CD4+ T cells and arginase-1 production by alternatively activated macrophages (AAM), which contribute to the formation of type 2 granulomas around the larvae in the submucosa (21–23). In addition, antibody-secreting B cells and parasite-specific antibodies play an important role in the expulsion of Hp (24–26). Furthermore, it has been demonstrated that transfer of immune serum could confer resistance to Hp, Nb, and Ascaris suum (27–30). However, how antibodies execute their protective effect remains unclear. One possibility is that they might act by activating Fc receptor-bearing cell types including macrophages, mast cells, and basophils. The role of basophils is particularly appealing in this context because basophils are a prominent source of IL-4 and IL-13, both of which induce AAM differentiation, goblet cell hyperplasia, collagen production, and secretion of effector molecules such as resistin-like molecule β (Relm-β) and mucin 5ac (Muc5ac) from intestinal epithelial cells.
In the current study we uncovered the main mechanism by which basophils confer protection against reinfection with Hp and Nb. We found that expression of activating Fc receptors and IL-4/IL-13 in basophils is required for protection. Furthermore, IgE-deficient, but not IgG1-deficient mice, showed impaired worm expulsion during secondary infection, indicating that IgE-induced release of IL-4/IL-13 from basophils is a critical step in the orchestration of downstream effector functions that ultimately lead to worm expulsion.
Results
Basophils Promote Protective Immunity Against Hp.
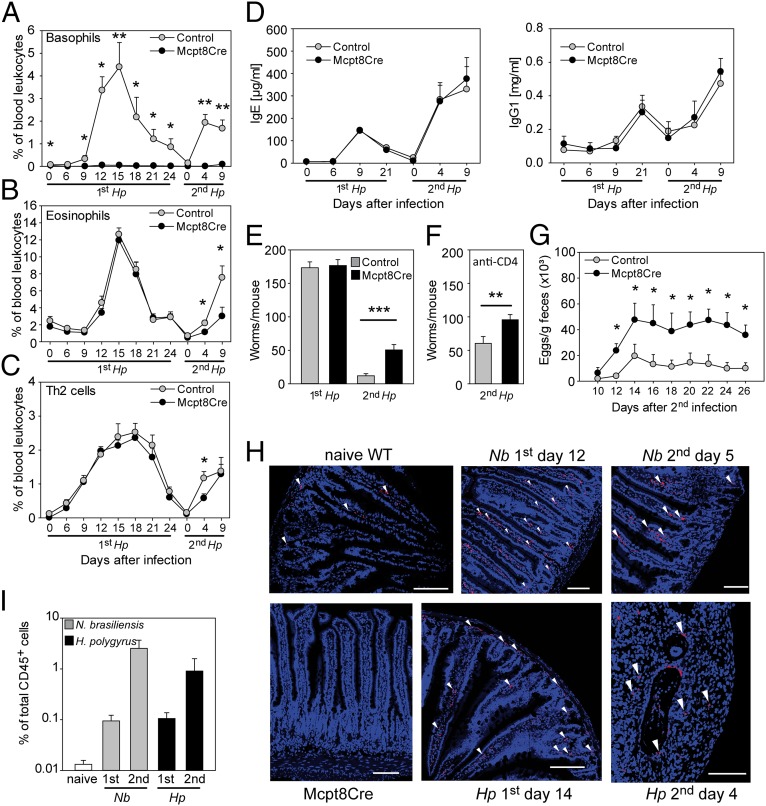
To address the role of basophils during infection with the strictly enteric helminth Hp, we used Mcpt8Cre BAC-transgenic mice in which basophils are constitutively and efficiently deleted because of Cre toxicity, but mast cells are not affected (13). Mcpt8Cre and control mice, both on the C57BL/6 background, were crossed to IL-4eGFP reporter mice (4get mice) (31) to facilitate detection of IL-4–expressing cell types by flow cytometry. After oral infection with 200 L3-stage Hp larvae, we monitored expansion of eosinophils, basophils, and Th2 cells in the blood. In control mice, basophils and eosinophils peaked at day 15 after the first infection, and Th2 cells reached a plateau at day 12 that persisted for 9 d (Fig. 1 A–C). Faster expansion of all three cell types was observed during the secondary response. As expected, basophils did not increase in infected basophil-deficient Mcpt8Cre mice, but the expansion of eosinophils and Th2 cells during primary infection was comparable to that in control mice (Fig. 1 A–C). However, Mcpt8Cre mice showed decreased frequencies of eosinophils (2.4-fold; P < 0.05) and Th2 cells (twofold; P < 0.05) shortly after reinfection, indicating that basophils induced the early memory type 2 immune response, as previously suggested by others (32).
Fig. 1.
Basophils promote protective immunity against secondary infection with Hp. (A–C) Frequency of basophils, eosinophils, and Th2 cells in the blood at indicated time points after first and second infection with Hp. (D) Serum concentrations of IgE (Left) and IgG1 (Right) after Hp infection. (E) Number of adult worms in the small intestine at day 28 after the first infection and day 9 after the second infection. (F) Number of adult worms in the small intestine of CD4+ T-cell–depleted Mcpt8Cre-4get (black) and 4get control (gray) mice at day 9 after the second infection. (G) Number of Hp eggs in the feces at indicated time points after second infection (also see Fig. S1). (H) Immunofluorescence staining for mMCP-8 (red) to detect basophils (arrows) in granulomas in the small intestine at the indicated time points after the first and second Hp and Nb infections. Mcpt8Cre mice served as negative staining control. Nuclei were counterstained with DAPI (also see Fig. S1). (I) Frequency of basophils among CD45+ cells isolated from the lamina propria of naive WT mice (open bar) and after the first and second infections with Hp (black bars) and Nb (gray bars). Graphs show the mean + SEM from pooled experiments with 11 (A–C), six (D), nine (E), four to seven (F), and five or six (G) Mcpt8Cre-4get and 4get control mice per group. *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student t test.
Basophils have been reported to promote the survival of plasma cells in the spleen and to enhance humoral memory responses (33, 34). To investigate whether basophil deficiency results in lower antibody levels, we measured serum concentrations of IgE and IgG1 antibodies in Mcpt8Cre and control mice after first and second Hp infection (Fig. 1D). IgE and IgG1 antibody levels in Mcpt8Cre mice were comparable to those in control mice, and both strains showed a typical humoral memory response reflected by faster increase of both isotypes during secondary as compared with primary infection.
To determine the contribution of basophils during the protective immune response to Hp, we analyzed the worm burden after primary and secondary infection. Similar numbers of worms and eggs were found in Mcpt8Cre and control mice after primary infection (Fig. 1E and Fig. S1A), but Mcpt8Cre mice harbored significantly more worms than control mice after secondary infection (Fig. 1E), even when CD4+ T cells were depleted just before the second infection (Fig. 1F and Fig. S1B). Importantly, expulsion of worms was not simply delayed, because basophil-deficient mice still shed significantly more eggs in the feces several weeks after the secondary infection and therefore probably harbored more live adult worms than control mice (Fig. 1G).
The protective effect of basophils suggests that they may be in close contact with larval antigens. This contact could occur inside granulomas in the submucosa of the small intestine. Therefore, we stained frozen tissue sections of small intestines from naive and infected mice with the basophil-specific anti-mMCP8 (mouse mast cell protease 8) antibody (Fig. 1H and Fig. S1C). We detected few basophils via immunofluorescence staining in naive control mice but not in Mcpt8Cre mice. Lamina propria basophils increased during primary Hp and Nb infection in control mice but not Mcpt8Cre mice. Basophils were present in granulomas 4 d after the second infection with Hp. Importantly, they also were increased in the lamina propria during the second Nb infection; this increase had not been observed in earlier reports (17). Furthermore, basophil numbers increased in the lamina propria after primary and secondary infection with both Hp and Nb as measured by flow cytometry (Fig. 1I). In both infection models basophil recruitment was about 10-fold higher during secondary infections than in primary infections. The recruitment of basophils to the anatomical sites where the helminths are located suggests that they promote protective immunity by activating nearby effector cells including macrophages and goblet cells.
Basophils Promote Recruitment of Effector Cells and Expression of Antihelminthic Proteins in the Small Intestine.
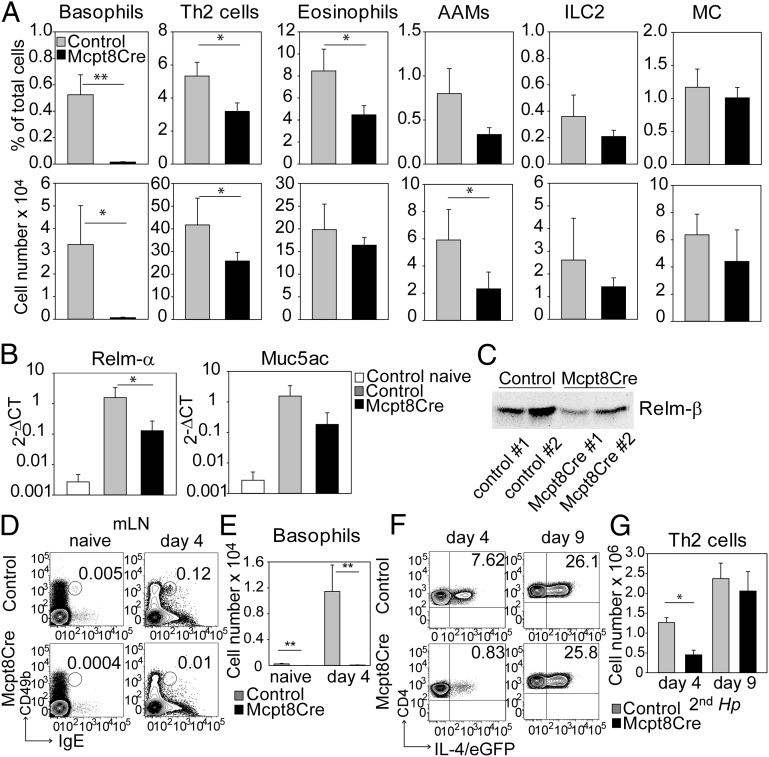
Because basophils were recruited efficiently to the lamina propria of the small intestine during the second infection with Hp, we investigated whether basophils promote recruitment of other effector cells. Fewer Th2 cells, eosinophils, and PD-L2+ AAMs were detected in Mcpt8Cre mice than in control mice, but ILC2s and mast cells were not reduced significantly in the lamina propria or mesenteric lymph nodes (Fig. 2A and Figs. S2 and S3). Expression of Relm-α, an established marker for AAMs, and Muc5ac, a glycoprotein secreted by goblet cells during helminth infections, also was decreased in Mpct8Cre mice as compared with controls (Fig. 2B). Relm-β also is secreted by goblet cells during infections with Hp and Nb in response to IL-4/IL-13, and this molecule has direct antihelminthic activity in vivo (35). Western blot analysis of feces of infected mice revealed decreased Relm-β secretion in Mcpt8Cre mice as compared with controls (Fig. 2C). Overall, these results indicate that the protective type 2 immune response early after reinfection with Hp is impaired in Mcpt8Cre mice.
Fig. 2.
Basophils promote the type 2 response during secondary infection with Hp. (A) Frequency (Upper) and total number (Lower) of basophils, Th2 cells, eosinophils, AAMs, ILC2, and mast cells (MC) among total cells recruited to the lamina propria 4 d after the second infection with Hp as determined by flow cytometry. Gating strategies for the different cell populations are shown in Fig. S2. (B) Quantitative RT-PCR analysis of expression of Relm-α (FIZZ1) and Muc5ac in the small intestines of naive control mice and 4 d after secondary infection with Hp. (C) Western blot analysis of Relm-β expression in the feces of infected control and Mcpt8Cre mice 4 d after the second infection with Hp. Forty micrograms of protein lysate per mouse was used for blotting. (D) Detection of basophils in mLNs in naive mice and at day 4 after the second infection. Numbers indicate the frequency of gated cells (also see Fig. S3). (E) Number of basophils in mLNs in naive mice and at day 4 after the secondary infection of Mcpt8Cre-4get mice (black bar) and 4get control mice (gray bar). (F) Dot plots show the frequency of Th2 cells in mLNs of infected 4get control mice (Upper) and Mcpt8Cre-4get mice (Lower) at 4 and 9 d after the second infection. Numbers indicate the frequency of cells in each quadrant. (G) Number of Th2 cells in mLNs after the second infection of Mcpt8Cre-4get (black bars) and 4get control mice (gray bars). Bar graphs show the mean + SEM from two or three pooled experiments with six to 10 mice per group (A, E, and G) or the mean + SD from one experiment with three mice per group (B). *P < 0.05; **P < 0.01 by unpaired Student t test.
Basophils Provide Early IL-4 for Th2 Polarization During Secondary Infection.
Because Hp is a strictly enteric parasite, the mesenteric lymph nodes (mLN) draining the intestinal tract are the major sites for initiation of the immune response. Analysis of reinfected mice revealed that basophils were recruited to the mLN as early as day 4 after secondary infection (Fig. 2 D and E). Interestingly, in the absence of basophils, Th2 polarization was significantly impaired in both frequency and total number early after infection, confirming our earlier observation of reduced frequencies of Th2 cells in the blood of Mcpt8Cre mice. However, by day 9 after infection, Th2 cells in mLN in Mcpt8Cre and control mice reached comparable frequencies and total numbers (Fig. 2 F and G). Hence, we hypothesized that basophils provide IL-4 shortly after reinfection to drive early Th2 polarization and thereby contribute to worm expulsion, as suggested earlier by others (9, 32).
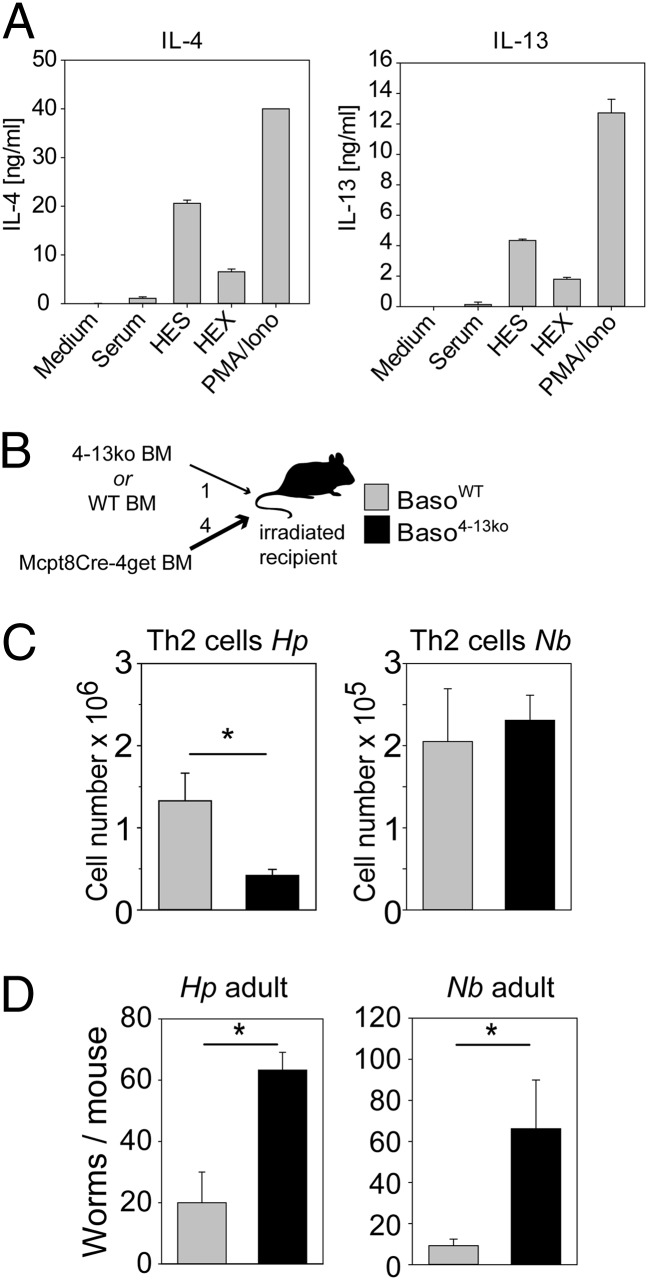
Indeed, ex vivo-sorted basophils loaded with Hp-immune serum released large amounts of IL-4 and IL-13 when incubated with Hp extracts or excretory/secretory products (Fig. 3A). To determine whether basophil-derived IL-4 is required for early Th2 polarization, we generated mixed bone marrow (BM) chimeras (MBMCs) with a 4:1 ratio of BM cells from basophil-deficient Mcpt8Cre mice and IL-4/IL-13–deficient (4-13ko) mice or WT control mice (Fig. 3B). In these MBMCs the basophil compartment was reconstituted only with basophils originating from BM of IL-4/IL-13 KO (Baso4-13ko) mice or WT (BasoWT) mice, whereas the great majority of all other hematopoietic cell types and all nonhematopoietic cells were WT. We then infected these chimeric mice twice with Hp or Nb and analyzed the mice 4 and 9 d after reinfection. In line with the results from basophil-deficient Mcpt8Cre mice, early Th2 differentiation after secondary Hp infection was observed only when basophils were capable of producing IL-4/IL-13 (Fig. 3C). However, basophil-derived IL-4/IL-13 was dispensable for Th2 polarization during secondary infection with Nb, indicating that basophils generally are not required to promote rapid memory Th2 responses against helminths (Fig. 3C and Fig. S4). Importantly, Baso4-13ko mice were not able to clear secondary infections with Hp and Nb efficiently (Fig. 3D). This inability demonstrates that basophil-derived IL-4/IL-13 is required for optimal worm expulsion during a secondary response in both infection models, although the migratory pathways of Hp and Nb are very different. With the next set of experiments we addressed the role of Fc receptors on basophils in basophil homeostasis and protective immunity against both helminths.
Fig. 3.
Basophil-derived IL-4 and IL-13 promote early Th2 polarization and worm expulsion during secondary infection with Hp. (A) IL-4 and IL-13 production of ex vivo-sorted splenic basophils (purity >98%) of Hp-infected WT mice stimulated with HEX or HES. (B) Schematic of the generation of mixed MBMCs. Mcpt8Cre-4get BM was mixed at a 4:1 ratio with BM from either WT mice (CD45.1) or 4-13ko mice and injected i.v. into lethally irradiated recipient mice. (C) Number of Th2 cells (CD4+, intracellular IL-4+) in mLN (Hp) or lung (Nb) of MBMCs with normal (BasoWT; gray bars) or IL-4/IL-13–deficient (Baso4-13ko; black bars) basophils 4 d after a second infection with Hp and 5 d after a second infection with Nb (also see Fig. S4). (D) Number of worms in the small intestines of chimeric mice 9 (Hp) or 5 (Nb) days after the second infection of Baso413ko(black bars) and BasoWT (gray bars) mice. Bar graphs show the mean + SEM from two experiments with six or seven mice per group. *P < 0.05; **P < 0.01 by unpaired Student t test.
Fc Receptors Are Not Required for Expansion and Decline of Basophils During Helminth Infection.
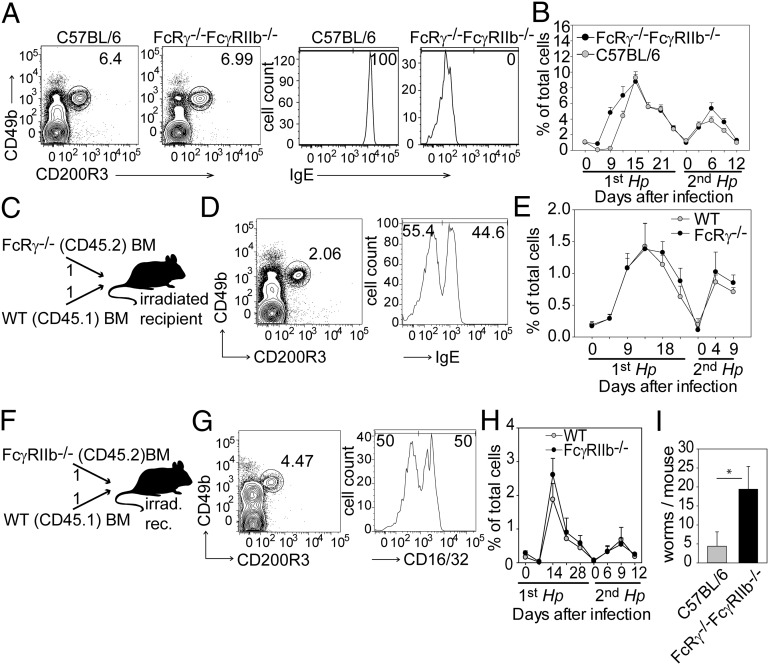
Murine basophils express the inhibitory IgG receptor FcγRIIB and activating receptors for IgG (FcγRIIIA) and IgE (FcεRI) in which the FcRγ chain is required for induction of down-stream signaling events (8). A previous study reported impaired Hp-induced basophilia in antibody-deficient and FcRγ−/− mice, suggesting that activating Fc receptors on basophils or other cells promote basophil expansion (18). Furthermore, cross-linking of activating Fc receptors on basophils induces the release of IL-4 and IL-13 which could be a mechanistic link by which antibodies contribute to protective immunity against Hp. We therefore decided to analyze directly the role of Fc receptors in basophil homeostasis and protective immunity. First, we monitored basophilia during primary and secondary infection of mice with functional deficiency in all activating and inhibitory Fc receptors (FcRγ−/−FcγRIIb−/−). In these mice basophils can be identified reliably by their surface expression of CD49b and CD200R3 (Fig. 4A). During the first infection basophils expanded faster in FcRγ−/−FcγRIIb−/− mice than in control mice and peaked around day 15 after infection (Fig. 4B). Basophil levels declined to basal levels 3 wk after infection in both KO and control mice, and we observed no differences during expansion after secondary infection. In contrast to a previous report (18), we also found unimpaired basophilia in FcRγ−/− mice (Fig. S5). To corroborate these findings, we generated MBMCs with equal ratios of FcRγ−/− and WT (CD45.1) BM cells (Fig. 4C), allowing us to study the role of activating Fc receptors on basophils in the expansion and decline of basophils in a competitive in vivo setting. In these MBMCs, basophils (CD49b+CD200R3+) originating from WT (CD45.1+) or FcRγ−/− (CD45.2+) could be distinguished further by surface staining of IgE (Fig. 4D) or CD45.1 (Fig. 4E). We observed that CD45.1+ (FcRγ+/+) and CD45.1− (FcRγ−/−) basophils expanded and declined in equal measure during the course of primary and secondary Hp infection (Fig. 4E). Additionally, we generated MBMCs with equal ratios of BM from FcγRIIb−/− and CD45.1 mice (Fig. 4F). In this competitive setting basophils from both donors also developed normally and expanded and declined with the same kinetics after Hp infection (Fig. 4 G and H). This result clearly demonstrates that Hp-induced basophilia is not regulated by activating or inhibitory Fc receptors. However, FcRγ−/−FcγRIIb−/− mice had increased worm burdens on day 9 after secondary infection, demonstrating that Fc receptors play an important role in protective immunity against Hp (Fig. 4I). However, it remained to be determined whether Fc receptors are required on basophils or other cell types to confer protection.
Fig. 4.
Fc receptors are required for protection against Hp but not for basophil expansion. (A) Dot plots show basophils in the blood 15 d after first infection. Histograms were gated on basophils and show staining of surface-bound IgE. Numbers indicate frequency of gated cells. (B) Detection of basophils in the blood at the indicated time points after infection with Hp in FcRγ−/−FcγRIIb−/− mice (black symbols) and C57BL/6 mice (gray symbols). (C) Schematic of the generation of MBMCs. CD45.1-WT and CD45.2-FcRγ−/− BM cells were mixed 1:1 and injected i.v. into lethally irradiated recipient mice. (D) The dot plot shows basophils in the blood of MBMCs described in C 9 d after the second infection. The number indicates the frequency of gated cells. The histogram was gated on basophils and shows staining of surface-bound IgE. (E) Frequency of WT CD45.1+ basophils (gray symbols) and FcRγ−/− CD45.2+ basophils (black symbols) in the blood of MBMCs described in C at the indicated time points after infection. (F) Schematic of generation of MBMCs. CD45.1-WT and CD45.2-FcγRIIb−/− BM cells were mixed 1:1 and injected i.v. into lethally irradiated recipient mice. (G) The dot plot shows basophils in the blood of MBMCs described in F 9 d after the second infection. The number indicates the frequency of gated cells. The histogram was gated on basophils and shows the expression of FcγRIIb on 50% of all basophils. (H) Frequency of WT-CD45.1+ basophils (gray symbols) and FcγRIIb−/−-CD45.2 basophils (black symbols) in the blood of MBMCs described in F at the indicated time points after infection. (I) Number of adult worms in the small intestines 12 d after secondary infection. Graphs show the mean + SD from one experiment with nine (B), five (E and I), or three (H) mice per group. *P < 0.05. Also see Fig. S6.
Expression of Activating Fc Receptors on Basophils Promotes Immunity Against Hp.
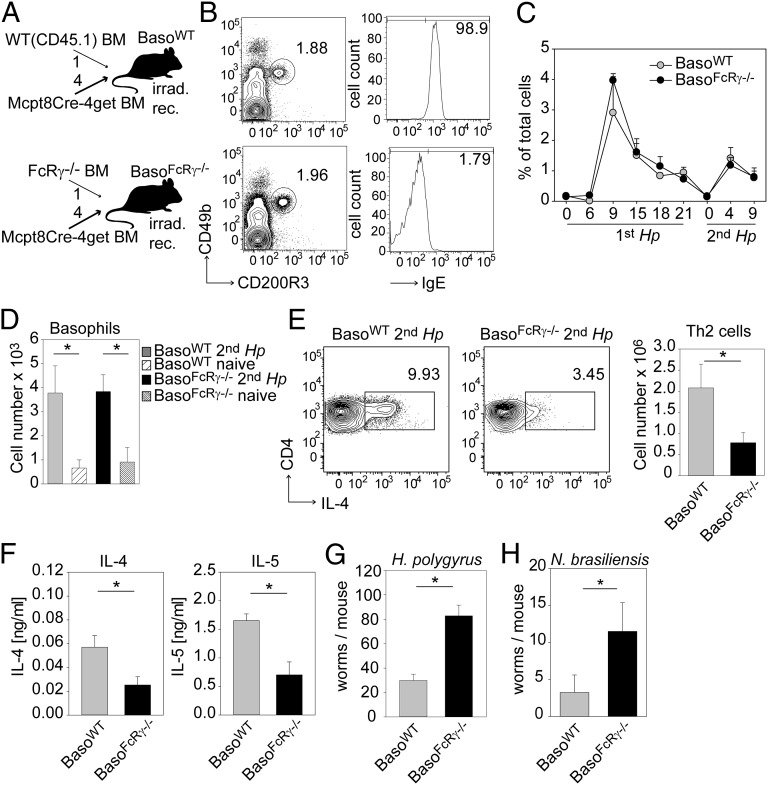
To address whether Fc receptor-mediated activation of basophils is required for protective immunity, we mixed Mcpt8Cre BM at a ratio of 4:1 with BM from either FcRγ−/− or CD45.1 mice to create MBMCs in which basophils lack activating receptors for IgG1 and IgE (Fig. 5A). In both BasoFcRγ−/− and BasoWT mice basophils were detectable at comparable frequencies and were derived exclusively from FcRγ−/− or CD45.1 donor mice, as determined by surface staining of IgE (Fig. 5B). As expected from the previous analysis of 1:1 MBMCs, basophil expansion after Hp infection was comparable in BasoFcRγ−/− and BasoWT mice (Fig. 5C).
Fig. 5.
Fc-receptor expression on basophils promotes Th2 polarization and worm expulsion during secondary infection with Hp. (A) Schematic of MBMC generation. Mcpt8Cre-4get BM was mixed at a ratio of 4:1 with BM from either WT (CD45.1) mice (Upper) or FcRγ−/− mice (Lower) and was injected i.v. into lethally irradiated recipient mice to create mice with normal (BasoWT) or FcRγ-deficient (BasoFcRγ−/−) basophils. (B) Dot plots show basophils in the blood of chimeric mice described in A 4 d after second infection. Numbers indicate the frequency of gated cells. Histograms were gated on basophils and show staining of surface-bound IgE. (C) Frequency of basophils in the blood of BasoWT mice (gray symbols) and BasoFcRγ−/− mice (black symbols) at indicated time points after infection (also see Fig. S5). (D) Number of basophils in the mLN before (striped bars) and 4 d after (solid bars) the second infection of MBMCs. (E) Dot plots show intracellular staining of IL-4 on gated CD4+ T cells in mLNs of MBMCs with normal (BasoWT) and FcRγ-deficient (BasoFcRγ−/−) basophils 4 d after the second infection. (F) Cytokine levels from restimulated mLN cells from MBMCs were measured by ELISA for IL-4 and IL-5. (G) Number of adult worms in the small intestines of MBMCs 12 d after the second infection with Hp. (H) Number of adult worms in the small intestine of MBMCs 5 d after the second infection with Nb. Graphs show the mean + SEM from two pooled experiments with three to eight (C), four to eight (D), or eight (E–G) mice per group. *P < 0.05.
Basophils were recruited to mLNs with the same efficiency 4 d after reinfection in BasoFcRγ−/− and BasoWT mice (Fig. 5D). However, we observed a marked decrease in the frequency and numbers of Th2 cells when basophils could not be activated via Fc receptors (Fig. 5E). Additionally, we observed that T cells in the mLNs of infected BasoFcRγ−/− mice produced significantly less IL-4 and IL-5 after restimulation (Fig. 5F). Thus, antibody-mediated basophil activation promoted the Th2 response at this early time point. Finally, mice were able to clear worms efficiently only when basophils expressed activating Fc receptors (Fig. 5G). Impaired worm expulsion also was observed in BasoFcRγ−/− mice after secondary infection with Nb (Fig. 5H). In contrast, MBMCs in which basophils did not express the inhibitory Fc receptor (BasoFcγRIIb−/−) had no defect in worm clearance (Fig. S6). Therefore, we conclude that direct activation of basophils by helminths-specific antibodies is a major mechanism for protective immunity during challenge infection with Hp or Nb.
IgG1 Antibodies Are Dispensable for Protection Against Hp.
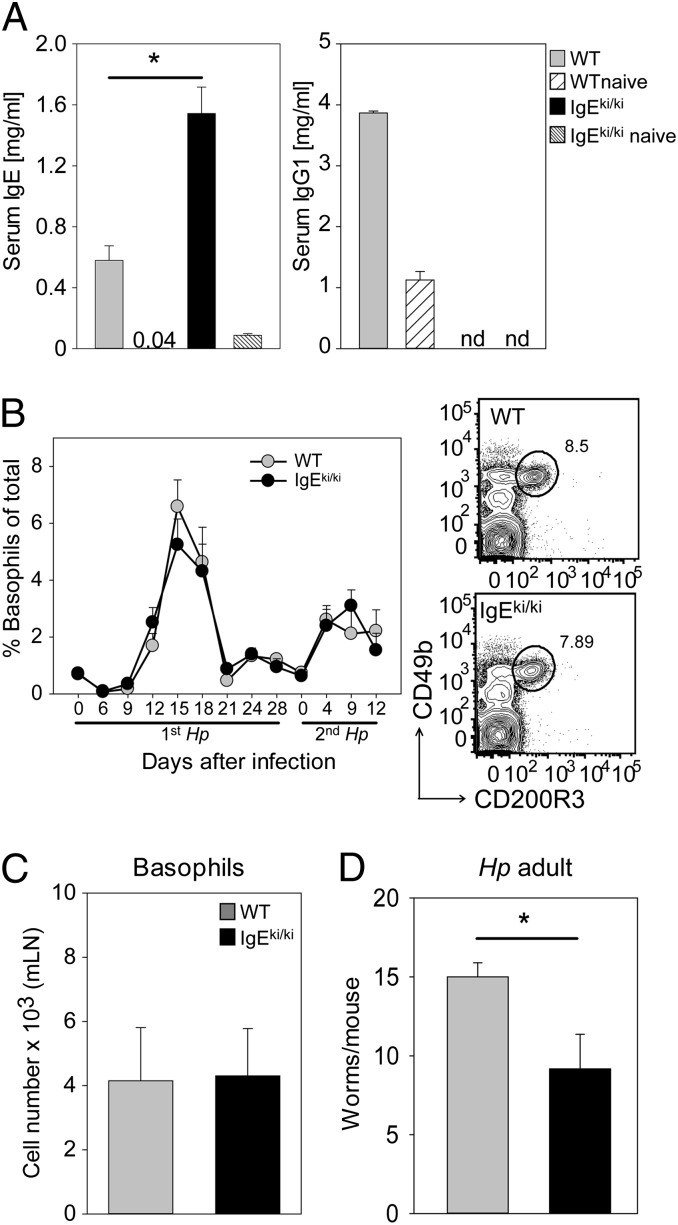
Given the important role of activating Fc receptors in basophil activation during secondary infection, we next investigated whether IgE or IgG1 antibodies are the major isotypes for basophil activation. Therefore, we used IgEki/ki mice in which the IgG1 heavy chain was replaced by the IgE heavy chain (36). As expected, these mice express elevated levels of serum IgE after secondary Hp infection as compared with control mice but are deficient for IgG1 (Fig. 6A). Of importance, Hp-induced basophilia (Fig. 6B) and basophil migration to mLNs was unaltered in the absence of IgG1 (Fig. 6C). Although IgG1 is missing in these mice, they expelled worms with significantly higher efficiency than WT mice (Fig. 6D). This result suggests that the elevated IgE levels in IgEki/ki mice could account for the improved protection.
Fig. 6.
IgG1 antibodies are dispensable for basophil expansion and protective immunity against Hp. (A) Serum concentrations of total IgE and IgG1 were measured by isotype-specific ELISA in WT mice (gray bars) and IgEki/ki mice (black bars) 12 d after the second infection with Hp. Naive mice (striped bars) served as controls. (B, Left) Frequency of basophils in the blood at the indicated time points after infection. (Right) Representative dot plots show basophils in WT (Upper) and IgEki/ki (Lower) mice. (C) Number of basophils in mLNs 4 d after the second infection. (D) Number of worms in the small intestine 12 d after the second infection. Graphs show the mean + SEM from pooled experiments with four to eight (A), five to eight (B), or five or six (C and D) mice per group. *P < 0.05; nd, not detected.
IgE Antibodies Promote Worm Expulsion.
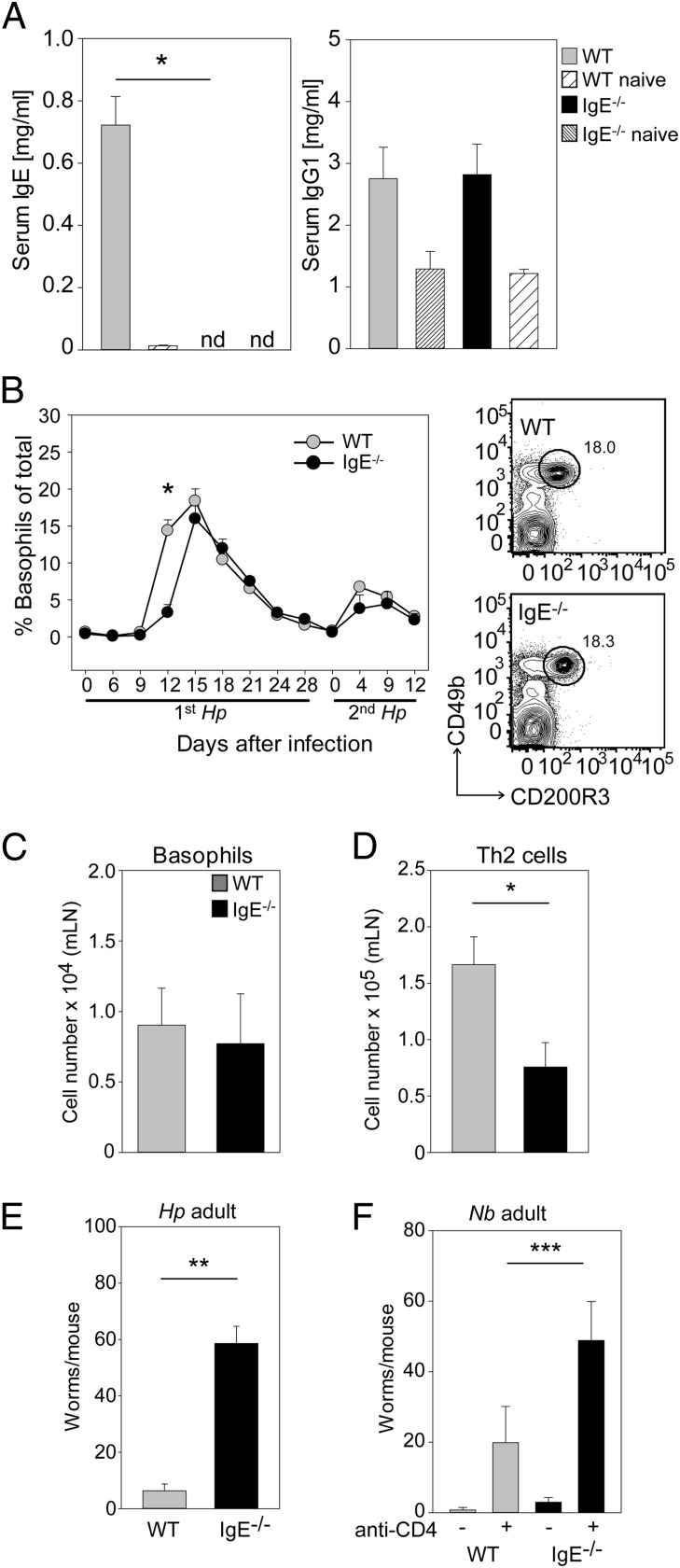
Because we observed improved worm clearance in IgEki/ki mice in which IgG1 is missing and IgE levels are elevated, we reasoned that protective immunity against Hp could be affected in IgE−/− mice. As expected, no IgE was detectable in the sera of infected IgE−/− mice, but IgG1 concentrations in IgE−/− and control mice were comparable (Fig. 7A). During the first infection we observed a minor delay in basophil expansion in IgE−/− mice, but by day 15 after infection basophils represent almost 20% of blood leukocytes in both mouse strains (Fig. 7B). During the second infection basophils expanded in both mouse strains with the same kinetics, peaking on day 4 after reinfection, and normal recruitment to the mLN was observed (Fig. 7C). Strikingly, IgE−/− mice exhibited a twofold reduction in Th2 polarization and 10-fold higher worm burdens during the challenge infection with Hp (Fig. 7 D and E). We also observed a protective function for IgE in the Nb model but only when CD4+ T cells had been depleted just before secondary infection (Fig. 7F). Therefore we conclude that IgE is necessary for worm expulsion by activating basophils via FcεRI to release IL-4 and IL-13 and thus initiate the downstream cascade of STAT6-regulated genes in other cell types that ultimately cause worm expulsion.
Fig. 7.
IgE-deficient mice show impaired protection against the second infection with Hp. (A) Serum concentrations of total IgE and IgG1 were measured by isotype-specific ELISA in WT mice (gray bars) and IgE−/− mice (black bars) 12 d after the second infection with Hp. Naive mice (striped bars) served as controls. (B, Left) Frequency of basophils in the blood at the indicated time points after infection. (Right) Representative dot plots show basophils in WT (Upper) and IgE−/− (Lower) mice. (C) Number of basophils in mLNs 4 d after the second infection. (D) Number of Th2 cells in mLNs 4 d after the second infection. (E) Number of worms in the small intestine 12 d after the second infection with Hp. (F) Number of adult worms in the small intestine with and without CD4 depletion 5 d after the second infection with Nb. Graphs show the mean + SEM from pooled experiments with four to eight (A), five to 11 (B), five or six (C–E), or seven or eight (F) mice per group. *P < 0.05; **P < 0.01.
Discussion
In the past few years nonredundant protective functions of basophils have been observed during secondary infections with helminths and ticks (13, 14, 17). However, the underlying mechanisms remained poorly understood. We addressed this issue and demonstrate here that the protective function of basophils operates mainly via activation of FcεRI and secretion of IL-4 and IL-13 from basophils.
We found that basophil-deficient Mcpt8Cre mice have an impaired protective immune response to secondary infections with the strictly enteric helminth Hp. Most helminth infections induce strong IgE and IgG1 responses, and B cells play an important role in protective immunity against various helminths (37). In humans, high serum IgE levels were reported to correlate positively with protection against Ascaris lumbricoides, Schistosoma mansoni, and Trichuris trichiura (38–40). However, only a limited role for IgE was described in most murine helminth infections (37). For the Hp model it was reported that protection is dependent largely on IgG1 but not IgE (24). This finding was based on the observations that (i) protective immunity could be transferred with purified IgG fractions from immune mice and (ii) no adult worms were found 14–20 d after reinfection of IgE−/− and BALB/c control mice. However, we show here that protection was not impaired in IgG1-deficient IgEki/ki mice, indicating that IgG1 is not required. Because larvae emerge into the lumen of the small intestine by day 7 after infection and mature rapidly to egg-producing adults, we reasoned that it is important to determine worm counts at an earlier time point. When we analyzed mice at day 12 after reinfection, we found 10-fold higher worm counts (58.6 ± 6.1) in IgE−/− mice than in BALB/c controls (6.3 ± 2.4). Furthermore, expulsion was more efficient in IgEki/ki mice with higher serum IgE levels than in control mice. This finding indicates that IgE mediates rapid worm expulsion and thereby limits the spread of the disease.
FcRγ−/− mice were reported to lack basophilia in response to Hp infection, indicating that immune complexes act directly on basophils or their precursors to enhance proliferation and survival (18). However, our results challenge this concept, because we observed normal basophilia in FcRγ−/− mice, IgEki/ki mice, and IgE−/− mice after Hp or Nb infection. Furthermore, basophils from WT mice had no competitive advantage over FcRγ−/− basophils in MBMCs. We identified basophils by flow cytometry using established surface markers, whereas the other study counted basophils in blood smears of FcRγ−/− mice; that method has the inherent problem that murine basophils, in contrast to their human counterparts, contain very few granules and are difficult to identify by histological staining (41).
The cytokines IL-4 and IL13 play important roles during the initiation and execution of type 2 responses against Hp and Nb (20). As we show here, sensitized basophils secreted IL-4 and IL-13 in response to Hp extracts and excretory/secretory products. Interestingly, CD4+ T cells are dispensable for protection against secondary Nb infection as long as IgE-sensitized basophils are present, but they contribute to worm expulsion after secondary Hp infection (23). By depleting CD4+ T cells before secondary Hp infection of WT and Mcpt8Cre mice, we found that basophils and CD4+ T cells play nonredundant roles and contribute to protective immunity by about the same extent. Both cell types are recruited into larval granulomas in the intestinal submucosa and release IL-4/IL-13 which promote the differentiation of AAMs and deposition of collagen, among other effects. AAMs have been shown to play an important role in protection against secondary Hp infection (23). Basophils were shown to promote AAM differentiation in the skin in a model of allergic skin inflammation and during secondary infection with Nb (16, 17). As we show here, basophil-derived IL-4/IL-13 enhanced the Th2 response in mLNs early after secondary Hp infection and accounted for basophil-mediated protective immunity in the Hp and Nb model.
By using depleting antibodies and constitutively basophil-deficient Mcpt8Cre mice, we have shown previously that basophils contribute to protective immunity against Nb independently of mast cells and memory Th2 cells (13, 42). Further, it was shown that basophils increase faster after secondary infection than after primary Nb infection, independently of IL-4 (43). Basophils also appeared to be more activated after migration into tissues of Nb-infected mice (12, 43). After secondary Nb infection about 20–30% of injected larvae are trapped in the skin of immune mice, and this effect recently has been shown to depend largely on IgE-activated basophils (17) [although previous studies (44, 45) also reported important roles for eosinophils in larval trapping in the skin]. However, the majority of larvae leave the skin of immune mice and are killed or trapped on their way to the lung by mechanisms that remain unclear (46). Although only a few worms reach the intestine and mature to the adult form, we consistently observed significantly more worms in the small intestine of basophil-deficient mice by day 5 after secondary Nb infection, and we show here that this effect requires the expression of IL-4/IL-13 and FcRγ on basophils. Even a few adult worms are sufficient to keep the life cycle intact and spread the infection to naive hosts.
We demonstrate that basophils are detectable by immunofluorescence staining and flow cytometry in the lamina propria even in naive mice, and the frequencies increased 10-fold after primary infection and another 10-fold after secondary infection with Nb or Hp. Importantly, the number of eggs in the feces after secondary Hp infection remained significantly higher for 2–3 wk in basophil-deficient Mcpt8Cre mice than in controls, indicating that basophils indeed play a biologically relevant role in limiting environmental spread of the infection.
Considering these results together, we elucidated the mechanisms by which basophils become activated and contribute to protective immunity against gastrointestinal helminths. Using MBMCs generated with donor cells from constitutively basophil-deficient mice and different KO mice, we demonstrate that activating Fc receptors on basophils are not required for basophil expansion but are important for IL-4/IL-13 secretion and the induction of down-stream effector mechanisms leading to worm expulsion. Human basophils have been shown to remain decorated with helminths-specific IgE even years after deworming and relocation to nonendemic regions (47). This persistence argues for the existence of long-lived IgE-producing plasma cells, and efficient vaccination strategies therefore should try to promote the generation and enhance the survival of these cells to keep basophils sensitized for long periods of time.
Materials and Methods
Mice.
Mcpt8Cre BAC-transgenic mice (13) on the BALB/c background were used for MBMCs with IL-4/IL-13−/− mice (48), and mice on the C57BL/6 background were used in all other experiments. These mice show constitutive and specific deletion of basophils resulting from Cre toxicity. IgEki/ki_C57BL/6 mice (36) were generated by replacing the first four exons of the IgG1 heavy chain with the corresponding IgE sequence. IgE−/− BALB/c mice (49), 4get_C57BL/6 mice (31), and FcRγ−/−, FcγRIIb−/−, and FcRγ−/−FcγRIIb−/− mice (all C57BL/6) (50, 51) have been described previously. CD45.1-B6 (B6.SJL-Ptprca Pepcb/BoyJ) and CD45.1-BALB/c [CBy.SJL(B6)-Ptprca/J] mice were obtained from The Jackson Laboratory. C57BL/6 and BALB/c mice were obtained from Charles River Laboratories. All animals were bred and maintained according to institutional guidelines under specific pathogen-free conditions. All animal experiments were approved by the Federal Government of Lower Franconia and performed in accordance with German animal protection law and European Union guidelines 86/809.
Parasite Infection, Enumeration of Eggs and Worms, and CD4 Depletion.
Mice were infected orally with 200 L3 Heligmosomoides polygyrus bakeri. After 3 wk mice were treated orally with 1 mg pyrantel pamoate (Sigma-Aldrich), followed by a second infection 2 wk later. The s.c. infection with 500 Nippostrongylus brasiliensis L3 larvae was performed as previously described (13). Parasite eggs were enumerated using a modified MacMaster counting chamber. Worm burdens were determined by counting live worms in the small intestine using a dissecting microscope. Hp extracts (HEX) and excretory/secretory products (HES) were generated as previously described (3). For T-cell–depletion experiments 400 µg anti-CD4 (clone GK1.5; BioXCell) antibody was injected i.v. 1 d before the second infection.
Flow Cytometry.
Single-cell suspensions were generated by mechanical disruption of spleens and mesenteric lymph nodes. Small intestines were incubated with 30 mM EDTA in PBS, washed extensively in PBS, and digested using Collagenase D (Roche) and DNaseI (Sigma) to obtain cell suspensions from the lamina propria. Antibodies used for flow cytometry are listed in Table S1. Basophils were identified as CD4−CD49b+CD200R3+ or CD4−CD49b+IgE+, mast cells as CD4−IL-4/eGFP+CD117+IgE+, eosinophils as CD4−Siglec-F+SSChi cells, AAMs as F4/80+MHCII+PD-L2+, ILC2 as lin−CD45+GATA3+KLRG1+ICOS+, and Th2 cells as CD4+IL-4/eGFP+ cells or CD4+ intracellular IL-4+ cells.
MBMCs.
BM cells from tibia and femur were prepared, washed, and mixed at indicated ratios. Recipient C57BL/6 or BALB/c mice were lethally irradiated with 1,100 rad followed by reconstitution with 2 × 106 mixed BM cells. Mice were treated with antibiotic-containing water (2 g/L neomycin sulfate, 100 mg/L polymyxin B sulfate; Sigma-Aldrich) for 8 wk after reconstitution.
Basophil Stimulation, Restimulation of mLN Cells, and ELISA.
Sort-purified (>98%) basophils (105) from spleens of Hp-infected C57BL/6 mice were loaded with immune serum from reinfected mice and incubated for 24 h with HES, HEX, or phorbol12-myristate13-acetate/ionomycin.
The mLN cells (5 × 106 per well) were restimulated for 48 h in 96-well plates coated with purified 1 µg/mL anti-CD3 and 5 µg/mL anti-CD28 antibodies (eBioscience). IL-4, IL-5, and IL-13 were measured by standard ELISA. Serum IgE concentrations were determined as described (42). Serum IgG1 was determined using the SBA clonotyping system (Southern Biotech).
Quantitative RT-PCR.
Quantitative RT-PCR was performed on the 7900HT Fast Real-Time PCR system (Applied Biosystems-Life Technologies). Primers used are listed in Table S2.
Protein Isolation and Western Blot Analysis.
Proteins were isolated from fecal pellets of infected mice using Nonidet P-40 lysis buffer (50 mM Tris⋅HCl, 100 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 20 µg/mL leupeptin, 16 µg/mL aprotinin, 0.2 mM PMSF). Anti-Relm-β antibody (PeproTech) and HRP-conjugated anti–rabbit-IgG antibody (Jackson ImmunoResearch Laboratories) were used for detection after standard Western blotting.
Immunofluorescence Staining of Basophils.
Cryosections of small intestine tissue that had been fixed in 4% (wt/vol) paraformaldehyde were incubated for 1 h in 1% H2O2. Sections were stained with purified rat anti-mouse mMCP8 (clone TUG8; BioLegend). For visualization, sections were incubated with HRP-conjugated goat anti-rat antibody (Jackson ImmunoResearch Laboratories) followed by Tyramide-Cy3 (PerkinElmer). Nuclei were counterstained with DAPI and mounted with Fluoromount G (Southern Biotech). Pictures were acquired on an LSM 700 microscope (40× original magnification) and analyzed using ZEN software (Carl Zeiss).
Statistical Analysis.
Mann–Whitney u tests and Student t tests were performed with SigmaPlot 12.3 software (Systat Software). P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank A. Matthies, D. Döhler, L. Handl, and K. Castiglione for technical assistance; K. Oeser and B. Krljanac for critical comments on the manuscript; D. Schönhöfer from the Core Unit Cell Sorting and Immunomonitoring Erlangen for cell sorting; L. Gundel and M. Kirsch for animal husbandry; and C. Bogdan for continuous support. This work was supported by Deutsche Forschungsgemeinschaft Grant Vo944/7-1 (to D.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412663111/-/DCSupplemental.
References
- 1.Bethony J, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Appleton CC, Mosala TI, Levin J, Olsen A. Geohelminth infection and re-infection after chemotherapy among slum-dwelling children in Durban, South Africa. Ann Trop Med Parasitol. 2009;103(3):249–261. doi: 10.1179/136485909X398212. [DOI] [PubMed] [Google Scholar]
- 3.Camberis M, Le Gros G, Urban J., Jr 2003. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19:Unit 19.12.2–19.12.27.
- 4.Robinson M, Wahid F, Behnke JM, Gilbert FS. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): Dose-dependent expulsion of adult worms. Parasitology. 1989;98(Pt 1):115–124. doi: 10.1017/s0031182000059758. [DOI] [PubMed] [Google Scholar]
- 5.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepworth MR, et al. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci USA. 2012;109(17):6644–6649. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filbey KJ, et al. Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol Cell Biol. 2014;92(5):436–448. doi: 10.1038/icb.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassard L, Jönsson F, Arnaud S, Daëron M. Fcγ receptors inhibit mouse and human basophil activation. J Immunol. 2012;189(6):2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- 9.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200(4):507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seder RA, et al. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci USA. 1991;88(7):2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174(2):1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12(6):527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnmacht C, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33(3):364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Wada T, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120(8):2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11(7):608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa M, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38(3):570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Obata-Ninomiya K, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013;210(12):2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbst T, et al. Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci USA. 2012;109(37):14954–14959. doi: 10.1073/pnas.1117584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10(7):697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkelman FD, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 21.Urban JF, Jr, Katona IM, Finkelman FD. Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol. 1991;73(4):500–511. doi: 10.1016/0014-4894(91)90074-7. [DOI] [PubMed] [Google Scholar]
- 22.Urban JF, Jr, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci USA. 1991;88(13):5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12(8):955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy KD, et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4(4):362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 25.King IL, Mohrs K, Mohrs M. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J Immunol. 2010;185(10):6138–6145. doi: 10.4049/jimmunol.1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojciechowski W, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30(3):421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury PB, Stromberg BE, Soulsby EJ. Immune mechanisms to Ascaris suum in inbred guinea-pigs. I. Passive transfer of immunity by cells or serum. Immunology. 1977;32(4):405–411. [PMC free article] [PubMed] [Google Scholar]
- 28.Behnke JM, Parish HA. Expulsion of Nematospiroides dubius from the intestine of mice treated with immune serum. Parasite Immunol. 1979;1(1):13–26. doi: 10.1111/j.1365-3024.1979.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 29.Brindley PJ, Dobson C. Specificity of passive serum protection against Nippostrongylus brasiliensis and Nematospiroides dubius in mice. Aust J Exp Biol Med Sci. 1983;61(Pt 1):37–45. doi: 10.1038/icb.1983.4. [DOI] [PubMed] [Google Scholar]
- 30.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113(12):2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 31.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15(2):303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 32.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200(7):857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denzel A, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9(7):733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez Gomez M, et al. Basophils support the survival of plasma cells in mice. J Immunol. 2010;185(12):7180–7185. doi: 10.4049/jimmunol.1002319. [DOI] [PubMed] [Google Scholar]
- 35.Herbert DR, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lübben W, et al. IgE knock-in mice suggest a role for high levels of IgE in basophil-mediated active systemic anaphylaxis. Eur J Immunol. 2013;43(5):1231–1242. doi: 10.1002/eji.201242675. [DOI] [PubMed] [Google Scholar]
- 37.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32(2):80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McSharry C, Xia Y, Holland CV, Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. 1999;67(2):484–489. doi: 10.1128/iai.67.2.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faulkner H, et al. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: The importance of IgE. J Infect Dis. 2002;185(5):665–672. doi: 10.1086/339005. [DOI] [PubMed] [Google Scholar]
- 40.Dunne DW, et al. Immunity after treatment of human schistosomiasis: Association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22(6):1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 41.Lee JJ, McGarry MP. When is a mouse basophil not a basophil? Blood. 2007;109(3):859–861. doi: 10.1182/blood-2006-06-027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184(1):344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 43.van Panhuys N, et al. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011;186(5):2719–2728. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knott ML, et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37(12):1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Daly CM, Mayrhofer G, Dent LA. Trapping and immobilization of Nippostrongylus brasiliensis larvae at the site of inoculation in primary infections of interleukin-5 transgenic mice. Infect Immun. 1999;67(10):5315–5323. doi: 10.1128/iai.67.10.5315-5323.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvie M, et al. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun. 2010;78(9):3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitre E, Nutman TB. IgE memory: Persistence of antigen-specific IgE responses years after treatment of human filarial infections. J Allergy Clin Immunol. 2006;117(4):939–945. doi: 10.1016/j.jaci.2005.12.1341. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189(10):1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oettgen HC, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370(6488):367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 50.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76(3):519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 51.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13(2):277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.