Significance
Understanding volatile elements in the early solar system is a key step toward understanding the processes of planetary formation and the composition of Earth, but the origin of volatiles on Earth is not well understood. In this article, we present measurements of silicon isotope ratios in angrites, a class of meteorites dating from the first few million years after condensation of solids from the solar nebula. We show that the silicon isotope composition of angrites is consistent with a depletion of volatile elements on the angrite parent body through impact events during accretion. Such volatile-poor planetesimals may represent the material that formed the Earth.
Keywords: volatiles, accretion, isotopes, angrites, silicon
Abstract
Inner solar system bodies, including the Earth, Moon, and asteroids, are depleted in volatile elements relative to chondrites. Hypotheses for this volatile element depletion include incomplete condensation from the solar nebula and volatile loss during energetic impacts. These processes are expected to each produce characteristic stable isotope signatures. However, processes of planetary differentiation may also modify the isotopic composition of geochemical reservoirs. Angrites are rare meteorites that crystallized only a few million years after calcium–aluminum-rich inclusions and exhibit extreme depletions in volatile elements relative to chondrites, making them ideal samples with which to study volatile element depletion in the early solar system. Here we present high-precision Si isotope data that show angrites are enriched in the heavy isotopes of Si relative to chondritic meteorites by 50–100 ppm/amu. Silicon is sufficiently volatile such that it may be isotopically fractionated during incomplete condensation or evaporative mass loss, but theoretical calculations and experimental results also predict isotope fractionation under specific conditions of metal–silicate differentiation. We show that the Si isotope composition of angrites cannot be explained by any plausible core formation scenario, but rather reflects isotope fractionation during impact-induced evaporation. Our results indicate planetesimals initially formed from volatile-rich material and were subsequently depleted in volatile elements during accretion.
The Si isotope system is at the center of several important debates in cosmochemistry. Si isotope variations in meteorites and the terrestrial planets have been variously attributed to isotope fractionation during core formation (1–3) or incomplete condensation from the solar nebula (4, 5). However, little consensus has been reached regarding the origin of Si isotope variations in planetary materials.
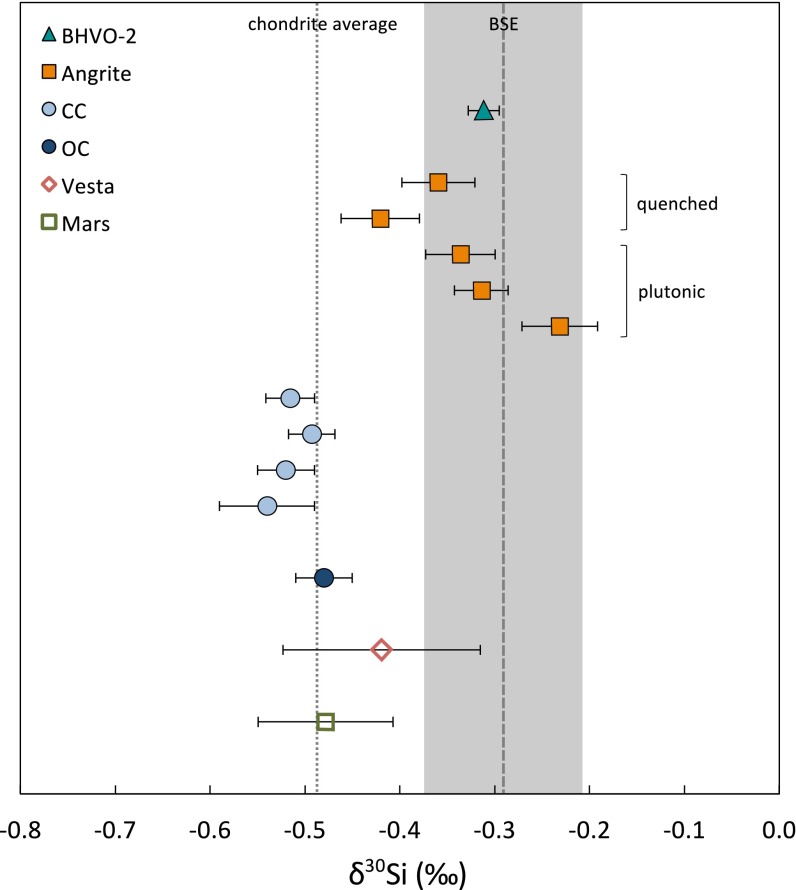
Here we present, to our knowledge, the first high-precision multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS) Si isotope data for a suite of angrites (Table 1 and Fig. 1). Angrites exhibit the largest Si isotope fractionations observed to date in achondritic meteorites compared with chondrites, with an average of δ30Si = −0.33 ± 0.12‰ (2 SD; see Table 1 legend for δ30Si definition). Additionally, select angrite samples have been measured directly against a terrestrial basalt (Table S1) to confirm these results. Our data show the Si isotope composition of angrites is similar to terrestrial basalts and is significantly offset from all major chondrite groups.
Table 1.
Silicon isotope data for terrestrial and meteorite samples relative to NBS28 as the bracketing standard
| Sample | Group | δ29Si | 2 SD | 2 SE | δ30Si | 2 SD | 2 SE | n |
| BHVO-2 | Terrestrial basalt | −0.16 | 0.08 | 0.01 | −0.31 | 0.12 | 0.02 | 57 |
| Carbonaceous chondrites | ||||||||
| Allende | CV3 | −0.26 | 0.07 | 0.01 | −0.52 | 0.13 | 0.03 | 26 |
| Angrites | ||||||||
| D'Orbigny | Quenched | −0.16 | 0.06 | 0.02 | −0.36 | 0.13 | 0.04 | 12 |
| NWA1296 | Quenched | −0.19 | 0.08 | 0.03 | −0.42 | 0.13 | 0.04 | 10 |
| NWA2999 | Plutonic | −0.17 | 0.05 | 0.02 | −0.34 | 0.12 | 0.04 | 10 |
| NWA4590 | Plutonic | −0.13 | 0.04 | 0.01 | −0.31 | 0.09 | 0.03 | 10 |
| NWA4931 | Plutonic | −0.10 | 0.06 | 0.02 | −0.23 | 0.13 | 0.04 | 10 |
| Weighted mean | −0.14 | 0.06 | −0.33 | 0.12 | 5 | |||
δ30Si = [(30Si/28Si)sample/(30Si/28Si)standard − 1] × 1,000. All data are whole-rock analyses. 2 SD, 2 × SD; 2 SE, 2 × SD/√n, where n is number of measurements. NWA2999 and NWA4931 are paired specimens. Weights calculated as 1/sd2.
Fig. 1.
Silicon isotope compositions of meteorite and terrestrial samples. Data plotted are relative to the Si isotope standard NBS28. Filled symbols represent individual samples (±2 SE), and open symbols represent group averages (±2 SD). Carbonaceous chondrites (CC), ordinary chondrites (OC), and averages for Mars and Vesta include data from ref. 3. The dotted line represents the weighted average of carbonaceous chondrites, ordinary chondrites, and the silicate phase of enstatite chondrites from the literature (1–3, 5, 11–13). The shaded box represents the estimated Si isotope composition of BSE (±2 SD) from ref. 27. Angrites are significantly offset from chondrites and overlap with the estimate of BSE.
The angrites display a range of δ30Si values from −0.42 ± 0.04‰ to −0.23 ± 0.04‰ (2 SE). These data may indicate a relationship between Si isotope composition and petrologic group; the plutonic angrites have slightly heavier compositions relative to quenched samples. The generally unbrecciated and unshocked textures of angrites (with the possible exception of NWA2999 and its pairs) suggest that they did not experience impact processing following ejection from their parent body (6). Variable modal abundances of anorthite have been observed in angrites, and plagioclase minerals are generally heavier in Si isotope composition compared with olivine and pyroxene (7). However, the two angrites analyzed here with the lowest modal abundance of anorthite are in fact two of the samples with the highest δ30Si values (the paired specimens NWA2999 and NWA4931). Plutonic angrites have coarse cumulate textures, and high proportions of cumulates are expected to drive Si isotope compositions toward lighter values, but again, the plutonic angrites in fact have the heaviest Si isotope compositions. Finally, the Si isotope composition of angrites could reflect an abundance of calcium–aluminum-rich inclusion (CAI) material enriched in heavy Si isotopes. Although angrites have been found to exhibit small Si isotope anomalies when normalized for equilibrium isotope fractionation (8), they do not show large anomalies in the neutron-rich isotopes of Ca and Ti as found in CAIs (9), so an enrichment of CAIs in angrite precursor material is unlikely. Therefore, these factors suggest that the Si isotope variations of angrites are not dominated by postejection impacts, modal mineralogy, or cumulate formation, and the δ30Si value of angrites reflects the composition of their parent magmas.
To calculate the Si isotopic offset between angrites and chondrites, we take the weighted average of carbonaceous chondrites, ordinary chondrites, and enstatite chondrites from MC-ICP-MS literature data (1–3, 5, 10–12) and obtain δ30Sichondrite = −0.48 ± 0.14‰ (2 SD). Only measurements of the nonmetallic phase for enstatite chondrites are included in this average, as the metal contains several wt% of isotopically light Si, most likely inherited during condensation of Fe–Si alloys under reducing conditions in the solar nebula (5). On average, angrites are offset by δ30Si = 0.15‰ from the Si isotope composition of chondritic meteorites.
One explanation for this difference is isotope fractionation due to partitioning of Si between silicate and metal during core formation on the angrite parent body (APB), which would preferentially enrich the core in the light isotopes of Si (1, 13, 14). Silicon becomes more siderophile at high temperature and pressure and low oxygen fugacity (fO2). Therefore, one core formation scenario that could explain the Si isotope composition of angrites is a planet-sized parent body that experienced metal–silicate equilibration at the base of a deep magma ocean, similar to conditions during terrestrial core formation. However, the early crystallization ages for angrites of ∼4.56 billion years ago argue for a small parent body that experienced early accretion, differentiation, and cooling only a few million years after CAI formation (15, 16).
If angrites are from a small parent body, most likely a large asteroid (or a family of related asteroids), then core formation would have occurred at relatively low temperature and pressure, and reducing conditions are required to partition Si into the metal phase. The amount of Si in the core needed to account for the isotopic offset between angrites and chondrites can be calculated using a mass balance and by assuming that the APB has a chondritic Si isotope bulk composition. Furthermore, because the elemental partitioning of Si between silicate and metal is only possible under specific conditions of pressure, temperature, and fO2, the amount of Si in the core calculated from the isotopic results can be used to model core formation conditions (3) and assess whether such a scenario is plausible based on parent body constraints (see Table S2 for calculation parameters and Fig. S1 for model results). Siderophile element abundances are consistent with core formation on the APB for a core mass fraction of 8% and fO2 of one log-unit below the iron-wüstite (IW) buffer, i.e., IW−1 (17). However, a small core necessitates a large proportion of Si (>17 wt%) to explain the isotopic composition of angrites, and extremely reducing conditions (<IW−5) are needed to partition such a large amount of Si into the metal. Alternatively, a large core mass fraction of 35% requires less Si (3–5 wt%) and therefore less reducing conditions to partition Si into the metal (∼IW−4), but in this case, oxidizing conditions (>IW+1) are needed to match siderophile element abundances.
Partitioning of significant amounts of Si into a planetary core may be an efficient pump for oxidizing the planet’s mantle through the reaction 2Femetal + SiO2silicate = 2FeOsilicate + Simetal. However, calculations of oxidation potential based on the core formation scenarios discussed above show that there is a disparity of several fO2 log units between the redox conditions required to explain the Si isotope results and the FeO content of angrite basalts in all cases. The disparity between the oxidized state of the angrites and the reduced conditions required to incorporate sufficient Si into the core is too great to be explained by this oxidation mechanism within realistic core formation parameters.
In all cases, our calculations suggest that unrealistic redox conditions during core formation are required to partition sufficient Si into the APB core to produce the observed heavy Si isotope enrichment in the angrites. Regardless of model parameters, extremely reducing conditions during core formation are required, in contrast to the high FeO content and fO2 calculated from siderophile element abundances in angrites. These results suggest that Si isotope fractionation during core formation cannot explain the heavy Si isotope enrichments observed in angrites compared with chondrites, and therefore a volatility-related process best explains the Si isotope composition of angrites.
Incomplete or fractional condensation of elements from the solar nebula has been proposed as an explanation for the volatile abundances of planetary bodies in the inner solar system, and Si is one of the most volatile major elements. However, the postulated condensation processes responsible for creating the Mg/Si variability between chondrite groups are expected to give rise to Si isotope fractionation (2), but no resolvable δ30Si variations are observed across the range of Mg/Si for different chondrite groups (Fig. 2A). Furthermore, the nonchondritic Mn/Na ratio in angrites argues against a nebular cause of volatile loss (18). These arguments indicate that a parent body, not nebular, process was responsible for creating the heavy Si isotope enrichment observed in angrites.
Fig. 2.
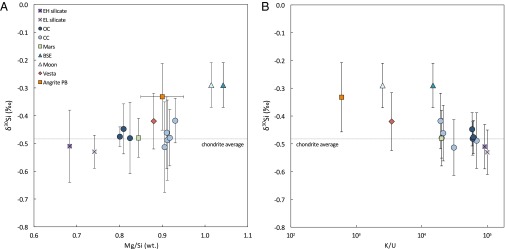
Silicon isotope variations in planetary objects and indicators of volatile depletion. (A) δ30Si versus elemental Mg/Si wt ratio. Carbonaceous chondrites, ordinary chondrites, and the silicate phases of enstatite chondrites have similar Si isotope compositions but span a wide range of Mg/Si values. In contrast, differentiated bodies exhibit increasing δ30Si with a higher Mg/Si ratio, suggesting that parent body processes are responsible for their Si isotope composition. (B) δ30Si versus K/U ratio. Bodies with low K/U ratios also exhibit low δ30Si, consistent with Si isotope fractionation during evaporative mass loss. Data for carbonaceous, ordinary, and enstatite chondrites as well as Mars, Earth, Moon, and Vesta are weighted group averages from the literature (1–3, 5, 10–12).
Evaporative loss following high-energy impacts during accretion may be the cause of volatile element depletions in terrestrial planets and asteroids (19, 20). In a plot of δ30Si versus a volatile–refractory element ratio, such as K/U, the most volatile depleted bodies are the most enriched in heavy Si isotopes (Fig. 2B). Evaporation experiments of silicate liquids under vacuum have shown significant heavy Si isotope enrichments in the residue (21), and evaporation under an impact-generated planetary atmosphere with higher partial pressure of Si could explain the smaller degree of isotope fractionation observed in angrites than expected based on experimental results under vacuum (22). Evaporative loss of Si may also have implications for Fe, Mg, and elements with higher volatilities, so it may be noteworthy that angrites are enriched in heavy Fe (23), and possibly Mg (24), isotopes relative to chondrites. Finally, based on Hf–W systematics, the APB likely experienced a protracted period of internal differentiation (25), and multiple early impact events coupled with incomplete mantle rehomogenization may explain the dichotomy of Si isotope compositions observed between the plutonic and quenched angrite groups.
Our results show planetesimals initially formed from material rich in moderately volatile elements, which were lost during accretion through early impact events. These volatile-depleted materials may represent the primary building blocks of Earth, consistent with heterogeneous terrestrial accretion models (26). Additionally, if Earth accreted from such material, our results imply the bulk Earth may be isotopically heavier in Si isotopes than previously assumed based on a chondritic model. This would lower the Si content required in Earth’s core based on the Si isotopic offset between Earth’s mantle and bulk Earth. This could reconcile the disparity between the Si content in Earth’s core estimated from Si isotope analyses (∼5–10 wt%) ( 27) and recent experimental geophysics results (<4 wt%) (28, 29).
Finally, the Si isotope similarity between the Earth’s mantle and the Moon, and their collective difference from all other previously analyzed meteorite groups, has been used to argue for Si isotope homogenization in the proto-lunar disk following the Giant Impact or for the derivation of lunar material predominantly from the silicate Earth (29, 30). However, our results show it may indeed be plausible to expect an impactor with a Si isotope composition similar to that of the lunar mantle, as observed in the case of the angrites. This would preclude the need for postimpact Si isotope homogenization in the Earth–Moon system, as both the Earth’s mantle and the impactor may initially have similar Si isotope compositions. Therefore, the Si isotope composition of the Earth and the Moon may be consistent with Giant Impact models in which lunar material is derived primarily from the impactor. However, a homogenizing process may still be required to explain the Earth–Moon similarity for other isotope systems (e.g., O, Ti, W) (30), and it should be noted that angrites exhibit resolvable differences in O, Cr, and Ti isotope compositions compared with the Earth and Moon (15, 32, 33).
Methods
Sample fragments were crushed to a fine powder using an agate mortar and pestle. Aliquots of 10 mg were dissolved for Si isotope analysis. Samples were dissolved and purified for Si using an alkaline fusion and ion exchange chromatography method following that described in detail previously (1, 3). Sample aliquots were combined with 200 mg analytical grade NaOH flux in 99.99% (wt/wt) pure (trace metals basis) Ag crucibles and heated at 720 °C for 12 min in a muffle furnace. The fused sample–flux mixtures were acidified using HNO3 and passed through columns packed with 1.8 mL of BioRad AG50-X12 (200–400 mesh) ion exchange resin to quantitatively remove cationic matrix elements. All samples and standards were processed through the dissolution and chemical purification procedure. Silicon isotope compositions were measured against the quartz sand standard NBS28 (NIST RM8546) using standard sample bracketing to correct for instrumental mass bias on a Thermo Scientific Neptune Plus MC-ICP-MS at Washington University in St. Louis. All sample dissolutions were independently processed through column chemistry on a minimum of two separate occasions and analyzed for Si isotope composition during multiple analytical sessions. Data accuracy and reproducibility during analytical sessions were monitored using the US Geological Survey reference material Basalt, Hawaiian Volcanic Observatory, (BHVO-2) and the carbonaceous chondrite Allende.
To verify these results and directly compare the composition of angrites and a terrestrial basalt, the basalt standard BHVO-2 was used as the bracketing standard in a separate measurement session on the Thermo Scientific Neptune Plus MC-ICP-MS at the Institut de Physique du Globe de Paris (IPGP); these data are denoted δ30SiBHVO-2 and are presented in Table S1. Dissolutions of D’Orbigny, NWA4590, and BHVO-2 were chemically purified at IPGP using the same procedure described above. BHVO-2 was chosen as a bracketing standard because its Si isotope composition is well characterized in the literature and it has previously been found to have the same Si isotope composition as the estimated value for Bulk Silicate Earth (BSE) (27). The results are consistent with the data obtained through bracketing with the traditional Si isotope standard NBS28 and confirm the observed heavy Si isotope enrichments in angrites relative to chondrites.
Supplementary Material
Acknowledgments
We thank Bruce Fegley, Julien Siebert, Marc Chaussidon, Anat Shahar, and Alex Halliday for discussions as well as Klaus Mezger and two anonymous referees for their constructive comments on the manuscript. This is contribution IPGP 3583. E.A.P. thanks the Chateaubriand STEM Fellowship program for funding. F.M. thanks the Agence Nationale de la Recherche for a chaire d’Excellence Sorbonne Paris Cité (IDEX13C445) and for the UnivEarthS Labex program (ANR-10-LABX-0023 and ANR-11-IDEX-0005-02), the Institut National des Sciences de l'Univers for a Programme National de Planétologie grant, and the Institut Universitaire de France. P.S.S. acknowledges postdoctoral support through the Marie Curie FP7 International Outgoing Fellowship “Isovolc.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418889111/-/DCSupplemental.
References
- 1.Georg RB, Halliday AN, Schauble EA, Reynolds BC. Silicon in the Earth’s core. Nature. 2007;447(7148):1102–1106. doi: 10.1038/nature05927. [DOI] [PubMed] [Google Scholar]
- 2.Fitoussi C, Bourdon B, Kleine T, Oberli F, Reynolds BC. Si isotope systematics of meteorites and terrestrial peridotites: Implications for Mg/Si fractionation in the solar nebula and for Si in the Earth’s core. Earth Planet Sci Lett. 2009;287(1):77–85. [Google Scholar]
- 3.Pringle EA, Savage PS, Badro J, Barrat J-A, Moynier F. Redox state during core formation on asteroid 4-Vesta. Earth Planet Sci Lett. 2013;373:75–82. [Google Scholar]
- 4.Fitoussi C, Bourdon B. Silicon isotope evidence against an enstatite chondrite Earth. Science. 2012;335(6075):1477–1480. doi: 10.1126/science.1219509. [DOI] [PubMed] [Google Scholar]
- 5.Savage PS, Moynier F. Silicon isotopic variation in enstatite meteorites: Clues to their origin and Earth-forming material. Earth Planet Sci Lett. 2013;361:487–496. [Google Scholar]
- 6.Scott ERD, Bottke WF. Impact histories of angrites, eucrites, and their parent bodies. Meteorit Planet Sci. 2011;46(1):1878–1887. [Google Scholar]
- 7.Savage PS, Georg RB, Williams HM, Burton KW, Halliday AN. Silicon isotope fractionation during magmatic differentiation. Geochim Cosmochim Acta. 2011;75(2):6124–6139. [Google Scholar]
- 8.Pringle EA, Savage PS, Jackson MG, Barrat J-A, Moynier F. Si isotope homogeneity of the solar nebula. Astrophys J. 2013;779(2):123–127. [Google Scholar]
- 9.Lugmair GW, Galer SJG. Age and isotopic relationships among the angrites Lewis Cliff 86010 and Angra dos Reis. Geochim Cosmochim Acta. 1992;56(4):1673–1694. [Google Scholar]
- 10.Chakrabarti R, Jacobsen SB. Silicon isotopes in the inner Solar System: Implications for core formation, solar nebular processes and partial melting. Geochim Cosmochim Acta. 2010;74(2):6921–6933. [Google Scholar]
- 11.Armytage RMG, Georg RB, Savage PS, Williams HM, Halliday AN. Silicon isotopes in meteorites and planetary core formation. Geochim Cosmochim Acta. 2011;75(1):3662–3676. [Google Scholar]
- 12.Zambardi T, et al. Silicon isotope variations in the inner solar system: Implications for planetary formation, differentiation and composition. Geochim Cosmochim Acta. 2013;121:67–83. [Google Scholar]
- 13.Shahar A, et al. High-temperature Si isotope fractionation between iron metal and silicate. Geochim Cosmochim Acta. 2011;75(2):7688–7697. [Google Scholar]
- 14.Hin RC, Fitoussi C, Schmidt MW, Bourdon B. Experimental determination of the Si isotope fractionation factor between liquid metal and liquid silicate. Earth Planet Sci Lett. 2014;387:55–66. [Google Scholar]
- 15.Keil K. Angrites, a small but diverse suite of ancient, silica-undersaturated volcanic-plutonic mafic meteorites, and the history of their parent asteroid. Chem Erde. 2012;72(3):191–218. [Google Scholar]
- 16.Schiller M, Baker JA, Bizzarro M. 26Al-26Mg dating of asteroidal magmatism in the young Solar System. Geochim Cosmochim Acta. 2010;74(16):4844–4864. [Google Scholar]
- 17.Righter K. 2008. Siderophile element depletion in the angrite parent body (APB) mantle: Due to core formation? 39th Lunar and Planetary Science Conference (Lunar and Planetary Institute, Houston), abstract no. 1936.
- 18.O’Neill HSC, Palme H. Collisional erosion and the non-chondritic composition of the terrestrial planets. Philos Trans A Math Phys Eng Sci. 2008;366(1883):4205–4238. doi: 10.1098/rsta.2008.0111. [DOI] [PubMed] [Google Scholar]
- 19.Halliday AN, Porcelli D. In search of lost planets—The paleocosmochemistry of the inner solar system. Earth Planet Sci Lett. 2001;192(4):545–559. [Google Scholar]
- 20.Paniello RC, Day JMD, Moynier F. Zinc isotopic evidence for the origin of the Moon. Nature. 2012;490(7420):376–379. doi: 10.1038/nature11507. [DOI] [PubMed] [Google Scholar]
- 21.Knight KB, et al. Silicon isotopic fractionation of CAI-like vacuum evaporation residues. Geochim Cosmochim Acta. 2009;73(2):6390–6401. [Google Scholar]
- 22.Young ED, Nagahara H, Mysen BO, Audet DM. Non-Rayleigh oxygen isotope fractionation by mineral evaporation: Theory and experiments in the system SiO2. Geochim Cosmochim Acta. 1998;62(1):3109–3116. [Google Scholar]
- 23.Wang K, et al. Iron isotope fractionation in planetary crusts. Geochim Cosmochim Acta. 2012;89:31–45. [Google Scholar]
- 24.Larsen KK, et al. Evidence for magnesium isotope heterogeneity in the solar protoplanetary disk. Astrophys J. 2011;735(2):L37. [Google Scholar]
- 25.Kleine T, Hans U, Irving AJ, Bourdon B. Chronology of the angrite parent body and implications for core formation in protoplanets. Geochim Cosmochim Acta. 2012;84:186–203. [Google Scholar]
- 26.Schönbächler M, Carlson RW, Horan MF, Mock TD, Hauri EH. Heterogeneous accretion and the moderately volatile element budget of Earth. Science. 2010;328(5980):884–887. doi: 10.1126/science.1186239. [DOI] [PubMed] [Google Scholar]
- 27.Savage PS, Armytage RMG, Georg RB, Halliday AN. High temperature silicon isotope geochemistry. Lithos. 2014;190-191:500–519. [Google Scholar]
- 28.Siebert J, Badro J, Antonangeli D, Ryerson FJ. Terrestrial accretion under oxidizing conditions. Science. 2013;339(6124):1194–1197. doi: 10.1126/science.1227923. [DOI] [PubMed] [Google Scholar]
- 29.Badro J, Côté AS, Brodholt JP. A seismologically consistent compositional model of Earth’s core. Proc Natl Acad Sci USA. 2014;111(21):7542–7545. doi: 10.1073/pnas.1316708111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armytage RMG, Georg RB, Williams HM, Halliday AN. Silicon isotopes in lunar rocks: Implications for the Moon’s formation and the early history of the Earth. Geochim Cosmochim Acta. 2012;77:504–514. [Google Scholar]
- 31.Pahlevan K. Isotopes as tracers of the sources of the lunar material and processes of lunar origin. Philos Trans A Math Phys Eng Sci. 2014;372(2024):20130257. doi: 10.1098/rsta.2013.0257. [DOI] [PubMed] [Google Scholar]
- 32.Greenwood RC, Franchi IA, Jambon A, Buchanan PC. Widespread magma oceans on asteroidal bodies in the early Solar System. Nature. 2005;435(7044):916–918. doi: 10.1038/nature03612. [DOI] [PubMed] [Google Scholar]
- 33.Trinquier A, Birck J-L, Allègre CJ. Widespread 54Cr heterogeneity in the inner Solar System. Astrophys J. 2007;655(2):1179–1185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.