Abstract
Background
The mechanism of stroke-induced immunodepression syndrome (SIDS) remains uncertain. Some studies suggest that hyperactivation of the sympathetic nervous system (SNS) may be the key factor underlying SIDS. Catecholamines impair early lymphocyte response, which can increase the risk of stroke-associated infection (SAI).
Material/Methods
Our study focused on dynamic changes of metanephrine (MN), normetanephrine (NMN), cytokines, and spleen volume in the rat middle cerebral artery occlusion (MCAO) model.
Results
After MCAO, there is hyperactivation of SNS and pro-/anti-inflammatory imbalance, indicating systemic immunodepression. In addition, rat spleen size was reduced. Correlation analysis indicated that MCAO-induced spleen size reduction correlated with the changes in MN, NMN, and cytokines. Blocking SNS with propranolol can partly reverse the immunodepression and the reduction in spleen volume.
Conclusions
Taken together, these findings suggest that acute ischemic stroke induces over-activation of the SNS, which lowers the threshold of infection and increases the risk of infection.
MeSH Keywords: Cerebral Infarction, Cytokines, Sympathetic Nervous System, Splenic Neoplasms
Background
Infectious complications, predominantly chest and urinary tract infections, have been reported in 23–65% of all patients within the first few days after stroke [1]. While acute stroke patients may survive the initial brain insult, many succumb to subsequent infections over time. There is increasing evidence that brain-induced immunodepression makes patients more vulnerable to infections [2,3]. Prass et al. demonstrated in a mouse model that focal cerebral ischemia induces long-lasting depression of cell-mediated immunity associated with spontaneous bacteremia and pneumonia [4]. A clinical study indicated that in patients with acute ischemic stroke the risk of infection increased, which is associated with an early hyperactivation of the sympathetic adrenomedullary pathway [5].
There is a rich bidirectional communication existing between the central nervous system and the immune system [6]. This functional interaction could pave the way to immunological manifestations as the result of central nervous system injury, and vice versa. Sympathetic nerves that innervate lymphoid tissues provide one of the major outflows from the brain to regulate tissue repair and host defense.
Catecholamines from the adrenal medulla are metabolized by catechol-O-methyl-transferase (COMT) to form metanephrine (MN) and normetanephrine (NMN). Because extracellular norepinephrine (NE) is metabolized rapidly [7], MN and NMN have several advantages over the determination of catecholamines in plasma or urine, with the advantages that they are easily obtained, have much longer half-lives than the plasma concentrations of their parent compounds, and are less susceptible to the effects of body posture, medications, or renal function, yet there are strong correlations between MN, NMN, and plasma catecholamines [5,8]. In this experiment, we used the serum levels of MN and NMN to determine SNS activity.
The underlying mechanism that results in widespread immunodepression and concurrent systemic infections after stroke induction in animals or humans is not well understood. Our study focused on the hyperactivation of the SNS and changes in the immune system in the context of experimental focal cerebral ischemia. First, we performed a detailed characterization of the changes in the SNS, peripheral blood cytokine levels, and the spleen induced by cerebral infarction. Second, the effects of propranolol on these measures of the consequences of MCAO were determined.
Material and Methods
Animals and MCAO surgery
A total of 72 age-matched, sexually mature, male Sprague-Dawley rats weighing 220–250 g were used for experiments. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Southeast University. The surgical procedure of middle cerebral artery occlusion (MCAO) did not exceed 10 min and was induced as previously described [4]. In brief, rats were anaesthetized through intraperitoneal injection of chloral hydrate (30–50 mg/kg), a monofilament was introduced into the common carotid artery, advanced to the origin of the middle cerebral artery (MCA), and left there for 60 min until reperfusion. During surgery and MCAO, rectal temperature was maintained at 37.0–37.5°C with a heating pad. Rats were kept in heated cages for 2 h and rectal temperature was frequently measured. Animals were then returned to their home cages and allowed free access to food and water. These precautions eliminate stress in cage mates. In sham-operated animals, after inserting the filament to the MCA origin, the filament was immediately withdrawn by 2.0 mm to avoid ischemia. Occlusion and reperfusion were verified by 7.0T MRI (Peri Flux 4001 Master; Perimed), TTC stain, and HE stain.
The animals were randomly assigned to the 3 experimental groups: sham-operated surgery, MCAO only (6 h, 12 h, 24 h, 48 h, 72 h, 1 week, and 2 weeks), and MCAO plus propranolol (72 h). Each group had 8 rats. The rats were housed 4–5 per cage and were kept under a 12-h dark/light cycle with free access to food and water for at least 1 week to acclimate them to our laboratory prior to experimentation.
Drug administration
Propranolol (Sigma-Aldrich, USA) was dissolved in 0.9% sodium chloride at 6 mg/ml and administered intraperitoneally (10 mg/kg body weight) immediately before and also 4 and 8 h after MCAO. The dose and injection interval were calculated according to previous descriptions of effective SNS blocking in rats [9]. Blood samples, brains and spleens were collected 72 h after MCAO. In sham-operated animals, only 0.9% sodium chloride was administered at the corresponding time point.
Magnetic resonance imaging
We employed a 7.0 Tesla animal micro-MRI system (Bruker, Germany) to visualize cerebral infarction. Rapid acquisition with relaxation enhancement (RARE) T1WI and T2WI sequences, spin echo diffusion-weighted imaging (SE DWI) sequence, and 3-dimensional fast low-angle shot imaging (3D FLASH) sequence were used (Figure 1).
Figure 1.
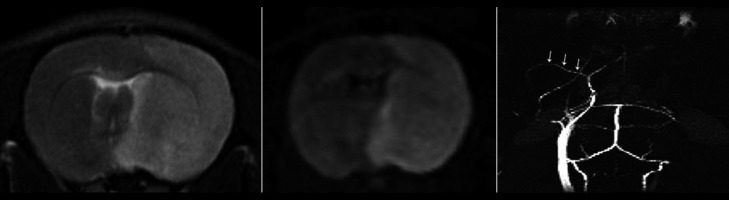
7.0T MRI and MRA in which (A) and (B), respectively, rat heads axial T2WI, DWI images revealed a right frontal lobe, parietal lobe and striatum, and high signal change for cerebral infarction. (C) shows the FLASH 3D image display on the left carotid artery and middle cerebral artery (arrow). The right carotid artery and middle cerebral artery are not shown.
Brain TTC stain and HE stain
After the collection of blood samples, all rats were killed by decapitation and the brains were quickly removed. Six 2.0-mm-thick coronal slices of brain were prepared with a brain matrix (ASI Instruments, Inc) and then were stained with 0.1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich, USA) in saline at 37°C for 15 min, as described previously [10]. On each slice, the unstained area represents ischemic brain (Figure 2). Subsequently, hematoxylin and eosin (HE) staining was also performed on these slices (Figure 3).
Figure 2.
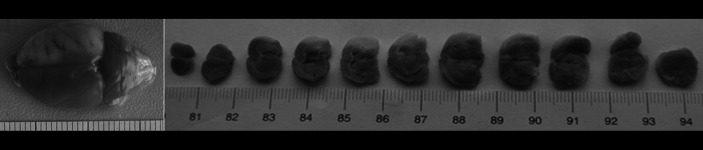
TTC stain: cerebral infarction area is white, and normal brain tissue is stained red.
Figure 3.
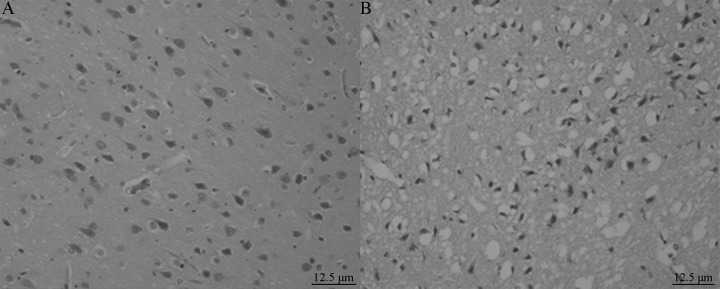
HE staining: MCAO rat brain tissue lateral neurons show serious shrinkage, nuclear pyknosis, and the nerve fiber network is obviously enlarged, with vacuolar changes.
Spleen preparation and HE stain
The spleen was immediately removed through a lateral abdominal incision after the collection of blood samples, rinsed in PBS (phosphate buffered solution) solution, and weighed. Then the spleen was fixed in 4% paraformaldehyde saline, embedded in paraffin wax, and a 10-micrometer-thick section was obtained by microtome dissection, stained with HE, and finally examined by light microcopy (Figures 4–6).
Figure 4.
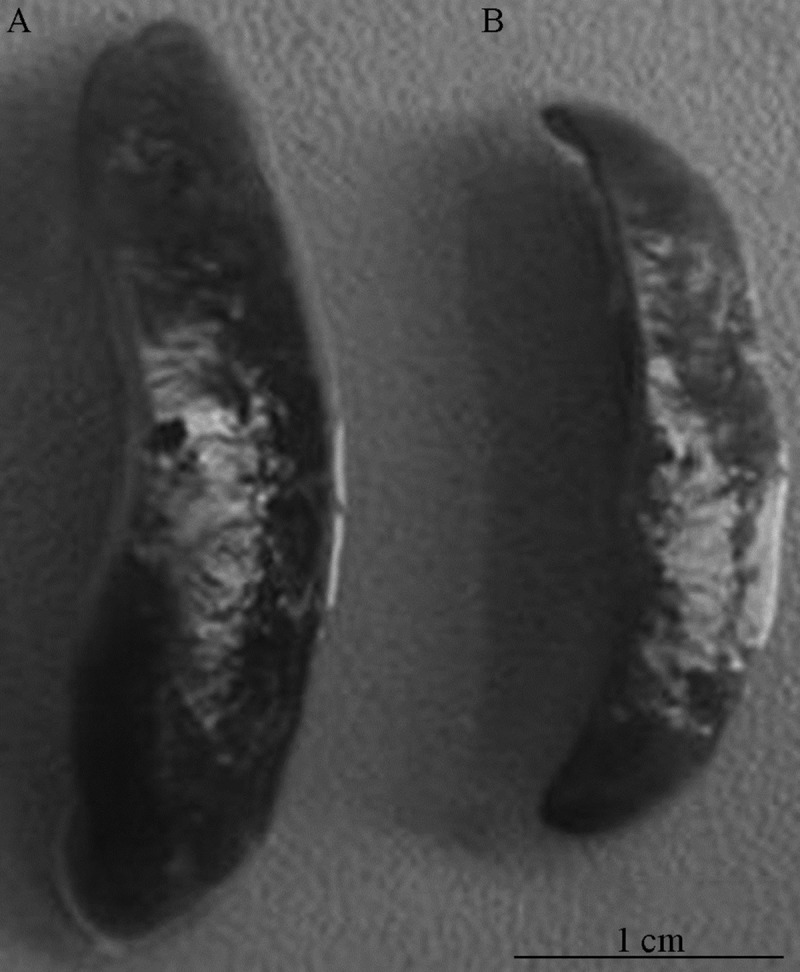
(A, B) The spleen volume significantly decreased 72 h after MCAO compared with the sham-operated group.
Figure 6.
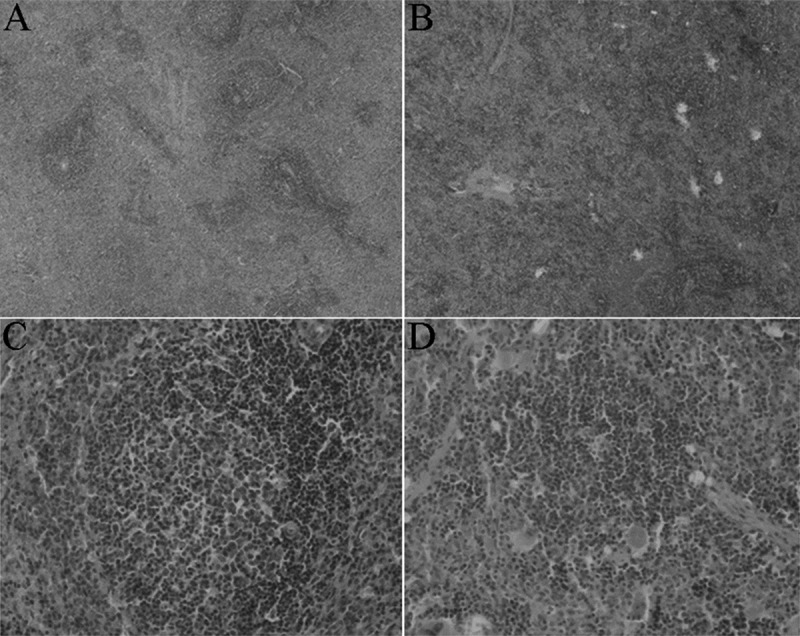
HE staining of spleen 72 h after MCAO, sham-operated operation treatment (A), and MCAO treatment (B) of SD rats. Original magnification 40× (a) and 200×. (C, D) HE staining showed that the spleen of MCAO rats was significantly reduced in spleen germinal centers, and did not form integrated germinal centers, only a loose aggregation of local lymphocytes, compared with sham-operated group.
Enzyme-linked immunosorbent assay
Blood samples were collected by cardiac puncture at 6 h, 12 h, 24 h, 48 h, 72 h, 1 week, and 2 week after MCAO. Whole blood was centrifuged (7000 × g for 15 min), supernatants collected in 100 μl/capsules, and were snap frozen and stored at −80°C until ELISA analysis.
There is a circadian rhythm in plasma levels of catecholamines [11]. Therefore, all plasma samples were collected at 08:00–09:00 a.m. after MCAO at every time point to determine unconjugated levels of MN and NMN by commercially available, quantitative, competitive, enzyme-linked immunosorbent assay (ELISA) kits (R&D, USA). Blood samples were also collected to determine the level of IL-1β, INF-γ, and IL-10 in serum by available quantitative sandwich ELISA kits (R&D, USA). All use of ELISA kits was in strict accordance with the manufacturer’s protocols.
Statistical analysis
Data are presented as mean ±SD and were analyzed using Kruskal-Wallis analysis of variance (ANOVA) followed by pairwise comparison with Dunn’s method or Mann-Whitney U test and linear correlation analysis using SPSS 13.0 for Windows (SPSS Inc, Chicago, IL, USA). Differences in group survival were determined by Fisher’s exact test. Values of P<0.05 were considered significant.
Results
Cytokine serum levels
Compared with the sham-operated group, the serum levels of IL-1β in MCAO rats increased slightly at 6 h, at 12 h levels were significantly higher (P<0.01), then gradually reduced to a minimum at 72 h, although each time point was higher than the level of the sham-operated group thereafter (P<0.01). The serum levels of IFN-γ in MCAO rats began to rise at 6 h (P<0.01) to a peak at 12 h, dropping at 48 h to a level below that of the sham-operated group (P<0.01), with a minimum at 72 h with a slow recovery later, but at 2 weeks was still lower than in the sham-operated group (P<0.01). In contrast, the serum levels of IL-10 in MCAO rats decreased at 6 h (P<0.01), rising at 24 h to higher than the sham-operated group (P<0.01), with a peak at 72 h, then decreased slowly to the level of the sham-operated group at 2 weeks (Table 1).
Table 1.
The serum levels of IL-1β, INF-γ and IL-10 in MCAO and sham-operated group in different time points.
| IL-1β | INF-γ | IL-10 | |
|---|---|---|---|
| Sham | 64.6±9.65 | 20.95±2.66* | 37.57±1.24 |
| MCAO 6 h | 66.77±3.56 | 28.61±1.32* | 32.3±2.55* |
| MCAO 12 h | 161.26±7.31* | 30.78±2.3* | 39.39±1.59 |
| MCAO 24 h | 99.36±9.32* | 24.64±1.79* | 40.69±2.09* |
| MCAO 48 h | 84.61±5.58* | 15.83±1.4* | 45.09±1.72* |
| MCAO 72 h | 74.7±4.26* | 11.11±1.46* | 47.32±2.07* |
| MCAO 1 w | 106.65±5.28* | 15.13±2.63* | 45.39±2.14* |
| MCAO 2 w | 99.76±3.62* | 17.31±1.86* | 38.33±2.41 |
P<0.01, compared with the sham-operated group.
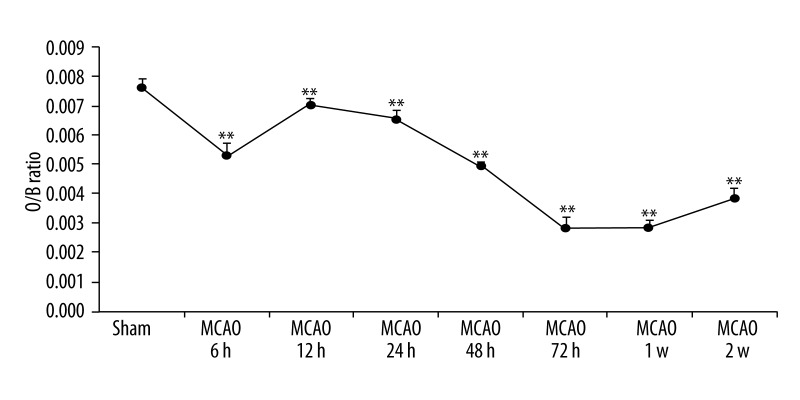
Spleen volume and HE staining
The spleen organ/body weight ratio of MCAO rats was significantly reduced after 6 h (P<0.01), with a small increase at 12 h (P<0.01), then fell again to a minimum at 72 h (P < 0. 01), but at 2 weeks after MCAO the spleen volume was still lower than in the control group (P<0.01) (Figures 4, 5). HE staining showed that a representative section of MCAO spleen had marked depletion of lymphoid tissue with lack of germinal centers, and a loose aggregation of local lymphocytes instead of integrated germinal centers compared with the sham-operated group. In contrast, representative sections of sham-operated MCAO spleens exhibited abundant lymphoid tissue and the central follicle contained a germinal center (Figure 6).
Figure 5.
Changes in MCAO rat organ/weight ratio of spleen.
MN and NMN serum levels
Serum levels of MN in cerebral infarction rats increased slightly at 6 h after MCAO, and at 12 h was significantly higher (P<0.05), with a peak at 72 h followed by a slow decline, but at 2 weeks after MCAO was still higher than in the sham-operated group (P<0.01). NMN levels in the serum began to increase 6 h after MCAO (P<0.01), to a peak at 72 h, then slowly decreased, but at 2 weeks was still higher than after MCAO in the sham-operated group (P<0.01) (Table 2).
Table 2.
The serum levels of metanephrine and normetanephrine in MCAO group and sham-operated group in different time points.
| MN | NMN | |
|---|---|---|
| Sham | 45.47±5.99 | 75.4±5.28 |
| MCAO 6 h | 48.58±7.32 | 84.39±6.25** |
| MCAO 12 h | 54.05±4.06* | 95.87±6.15** |
| MCAO 24 h | 82.13±5.14** | 96.15±6.44** |
| MCAO 48 h | 94.01±7.52** | 97.32±7.76** |
| MCAO 72 h | 101.95±10.64** | 100.64±5.31** |
| MCAO 1 w | 71.6±6.56** | 92.87±6.24** |
| MCAO 2 w | 55.47±7.57** | 84.34±6.54** |
P<0.05;
P<0.01, compared with the sham-operated group.
Correlation analysis
Correlation analysis indicated that IL-1β levels were negatively correlated with MN levels (r=−0.240, P=0.014), but not with NMN levels (r=−0.055, P=0.582). INF-γ levels were negatively correlated with MN and NMN levels (MN: r=−0.599, P=0.000; NMN: r=−0.245, P=0.012). IL-10 levels were positively correlated with MN and NMN levels (MN: r=0.756, P=0.000; NMN: r=0.570, P=0.000). IL-1β and INF-γ levels were positively correlated with the spleen O/B ratio (IL-1β: r=0.304, P=0.002; INF-γ: r=0.644, P=0.000). IL-10, MN, and NMN levels were negatively correlated with the spleen O/B ratio (IL-10: r=−0.492, P=0.000; MN: r=−0.576, P=0.000; NMN: r=−0.499, P=0.000).
Cytokine, MN and NMN serum levels after intraperitoneal injection of propranolol
Compared with MCAO at 72 h, pro-inflammatory cytokines IL-1β and INF-γ serum levels in the MCAO + propranolol group were significantly higher (P<0.01). Compared with MCAO 72 h, IL-10 serum levels in the MCAO + propranolol group were significantly lower (P<0.01). Compared with MCAO at 72 h, serum levels of MN and NMN in the MCAO + propranolol group were significantly lower (P<0.01) (Table 3).
Table 3.
The serum levels of IL-1β, INF-γ, IL-10, MN and NMN in MCAO 72 h group and MCAO + propranolol 72 h group.
| IL-1β | INF-γ | IL-10 | MN | NMN | |
|---|---|---|---|---|---|
| MCAO 72 h | 74.7±4.26 | 11.11±1.46 | 47.32±2.07 | 101.95±10.64 | 100.64±5.31 |
| MO + pr 72 h | 87.36±3.79* | 21.32±1.88* | 38.47±1.4* | 45.43±6.71* | 75.65±4.66* |
P<0.01, compared with the sham-operated group.
Changes of spleen volume after intraperitoneal injection of propranolol
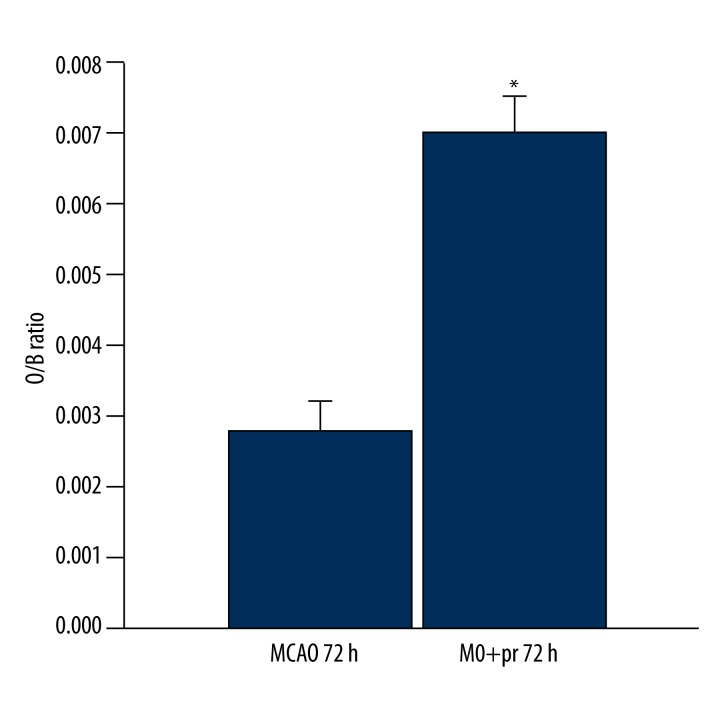
Compared with MCAO at 72 h, spleen volume in the MCAO + propranolol group was significantly increased (P<0.01) (Figure 7).
Figure 7.
The organ/body weight ratio (O/B ratio) of spleen in the MCAO 72 h group and MCAO + propranolol 72 h group. * P<0.01.
Discussion
As early as 1974, it was known that critical stroke could induce an immunodepression state [12]. Stroke-induced changes in the immune system have mainly been characterized as alterations in the immune cells and cytokines [4,13,14]. However, the mechanisms leading to an increased susceptibility to immunodepression after stroke are still poorly understood [15]. Data show that catecholamines can inhibit pro-inflammatory cytokines and potentiate ant-inflammatory cytokines production to suppress cellular immunity [16,17]. This response could shut down defense mechanisms and render the organism susceptible to infection [18]. The differences between MCAO rats and sham-operated rats in our experiment were striking and consistent in level of serum cytokines, spleen size, and SNS activity. As expected, we observed that MN and NMN serum levels increased after cerebral infarction, indicating the hyperactivation of the sympathetic nervous system (SNS), and that the level of pro-inflammatory cytokines IL-1β and INF-γ increased and the anti-inflammatory cytokine IL-10 decreased, indicating immunodepression. This phenomenon occurs early after MCAO and lasts several days, and is obvious at 72 h after MCAO.
The spleen receives a rich supply of sympathetic innervation. Norepinephrine and β2-adrenergic receptor (β2AR) stimulation by infection and injury has been strongly implicated in the regulation of the immune response [19], and it is conceivable that brain injury might also transmit sympathetic signals to the spleen. For example, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of Parkinson’s disease and in the MCAO rat model of stroke, it has been reported that there is gross reduction in spleen size and function [20]. Reduced cellularity in the spleen was also observed in mice and rats after stroke in 2 previous reports [4,21]. In the present study, we provide direct experimental proof of spleen atrophy after MCAO. Correlation analysis indicated that the spleen volume change shows a significant relationship with cytokine, MN, and NMN serum levels. Furthermore, we demonstrated that intraperitoneal injection of the β-adrenoreceptor antagonist, propranolol, immediately before and twice after MCAO, drastically reversed the spleen volume reduction and systemic immunodepression. We infer that this immunodepression state could be due to SNS hyperactivation, which then results in increased peripheral lymphocyte apoptosis, particularly in the spleen, and eventually leads to immunodepression.
Interestingly, this reversibility of spleen atrophy is not complete, suggesting that the mechanism of spleen volume reduction deserves further study. However, the present study, at least in part, indicates that SNS over-activation during ischemic stroke may play a major role in spleen volume changes.
The possible limitations of our study are that there are no results of spleen immunohistochemistry, which could demonstrate changes in the SNS and the β2-adrenergic receptor (β2AR) in situ. Another limitation is that we did not use more comprehensive parameters of immune status and used no infection parameters. We look forward to overcoming these limitations in future studies.
Conclusions
In conclusion, this study provides more information about SIDS. There are striking and consistent changes in cytokines, spleen, and SNS activity after stroke. Hyperactivation of the SNS is followed by persistent peripheral immunodepression. Increasing knowledge of these mechanisms may open new therapeutic avenues in the treatment of patients with stroke.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
Source of support: The National Natural Science Foundation of China (NO. 81271336) and Nanjing Municipal Science and Technology Bureau of international cooperation project (NO. 201201105)
References
- 1.Langhorne P, Stott DJ, Robertson L, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–29. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 2.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 3.Kamel H, Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol. 2012;69:576–81. doi: 10.1001/archneurol.2011.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prass K, Meisel C, Höflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–36. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamorro A, Amaro S, Vargas M, et al. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 7.Mitchell K, Oke AF, Adams RN. In vivo dynamics of norepinephrine release-reuptake in multiple terminal field regions of rat brain. J Neurochem. 1994;63:917–26. doi: 10.1046/j.1471-4159.1994.63030917.x. [DOI] [PubMed] [Google Scholar]
- 8.Raber W, Raffesberg W, Bischof M, et al. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med. 2000;160:2957–63. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- 9.Woiciechowsky C, Schöning B, Daberkow N, et al. Brain IL-1beta increases neutrophil and decreases lymphocyte counts through stimulation of neuroimmune pathways. Neurobiol Dis. 1999;6:200–8. doi: 10.1006/nbdi.1999.0242. [DOI] [PubMed] [Google Scholar]
- 10.Joshi CN, Jain SK, Murthy PS. An optimized triphenyltetrazolium chloride method for identification of cerebral infarcts. Brain Res Brain Res Protoc. 2004;13:11–17. doi: 10.1016/j.brainresprot.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Haim A, Zisapel N. The impact of beta-adrenergic blockade on daily rhythms of melatonin and body temperature of golden spiny mice Acomys russatus. Life Sci. 1997;61:703–9. doi: 10.1016/s0024-3205(97)00535-3. [DOI] [PubMed] [Google Scholar]
- 12.Howard RJ, Simmons RL. Acquired immunologic deficiencies after trauma and surgical procedures. Surg Gynecol Obstet. 1974;139:771–82. [PubMed] [Google Scholar]
- 13.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia W, Han J, Huang G, Ying W. Inflammation in ischaemic brain injury: current advances and future perspectives. Clin Exp Pharmacol Physiol. 2010;37:253–58. doi: 10.1111/j.1440-1681.2009.05279.x. [DOI] [PubMed] [Google Scholar]
- 15.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–53. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 16.Bellinger DL, Millar BA, Perez S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter U, Kolbaske S, Patejdl R, et al. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol. 2013;20:153–59. doi: 10.1111/j.1468-1331.2012.03818.x. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro A, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–10. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 19.Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- 20.Benner EJ, Mosley RL, Destache CJ, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:9435–40. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gendron A, Teitelbaum J, Cossette C, et al. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 2002;955:85–97. doi: 10.1016/s0006-8993(02)03368-1. [DOI] [PubMed] [Google Scholar]