Abstract
G-protein-coupled receptor kinase 2 (GRK2) levels are elevated in inflammation but its role is not clear yet. Here we show that GRK2 expression is dependent on NFκB transcriptional activity. In macrophages, LPS induces GRK2 accumulation in mitochondria increasing biogenesis. The overexpression of the carboxy-terminal domain of GRK2 (βARK-ct), known to displace GRK2 from plasma membranes, in macrophages induces earlier localization of GRK2 in mitochondria in response to LPS leading to increased mt-DNA transcription, reduced ROS production and cytokines expression. Our study shows the relevance of GRK2 subcellular localization in macrophage’s biology and its potential therapeutic properties in inflammation.
Keywords: GRK2, mitochondria, inflammation, subcellular localization, βARK-ct
Introduction
Although G protein coupled receptor kinases (GRKs) are classically considered as the regulators of GPCR signaling through desensitization (1, 2), it is evident that their role is more articulated within the cell. In particular, GRK2 can regulate signaling mediated by other membrane receptor families, such as tyrosine kinase receptors for IGF-1, Insulin, PDGF or EGF (3, 4). In addition, a growing number of non receptor substrates have been identified for GRK2 (5–7), showing its involvement in the regulation of diverse cellular functions through the phosphorylation of several substrates or in a phosphorylation-independent manner through protein-protein interaction (6, 8, 9).
These novel features of GRK2 allow the kinase to participate in basic cellular processes such as migration and cell cycle progression or cardiovascular cell functionality (10–13). Nevertheless, we can only partially appreciate the full role of the kinase which might change in different cell types. Indeed, GRK2 is highly expressed in the immune system being an important regulator of cell responses during inflammation (14). In particular, mice with GRK2 deletion in cells of myeloid lineage exhibited exaggerated inflammatory cytokine/chemokine production, and organ injury in response to lipopolysaccharide (LPS, a TLR4 ligand) due to macrophages hyperreaction to stimulants (15). The link between GRK2 levels and inflammation can be demonstrated both in human patients (16) and in a number of animal disease models (15, 17–21). All these evidences suggest a key role of GRK2 in inflammation even if it has not yet been clarified. It is known that inflammation produces mitochondrial damage in host cells, compromising cell survival and organ function (22). The damaging effects of inflammation on mitochondria are opposed to cellular programs that induce mitochondrial DNA (mtDNA) transcription and replication and mitochondrial protein synthesis (23, 24). We have recently demonstrated that GRK2 localizes into mitochondria and regulates biogenesis and ATP generation (25). In particular, in other cellular systems, the overexpression of GRK2 increases ATP cellular content by enhancing mitochondrial biogenesis and antagonizes ATP loss after hypoxia/reperfusion. Based on these data, the aim of our study is to verify whether the ability of GRK2 to regulate mitochondrial function plays a role in macrophage activation during inflammation.
Materials and Methods
Methods are as previously described (25–33). Extended details are available in Supplements.
Results
NFκB regulates GRK2 expression during inflammation
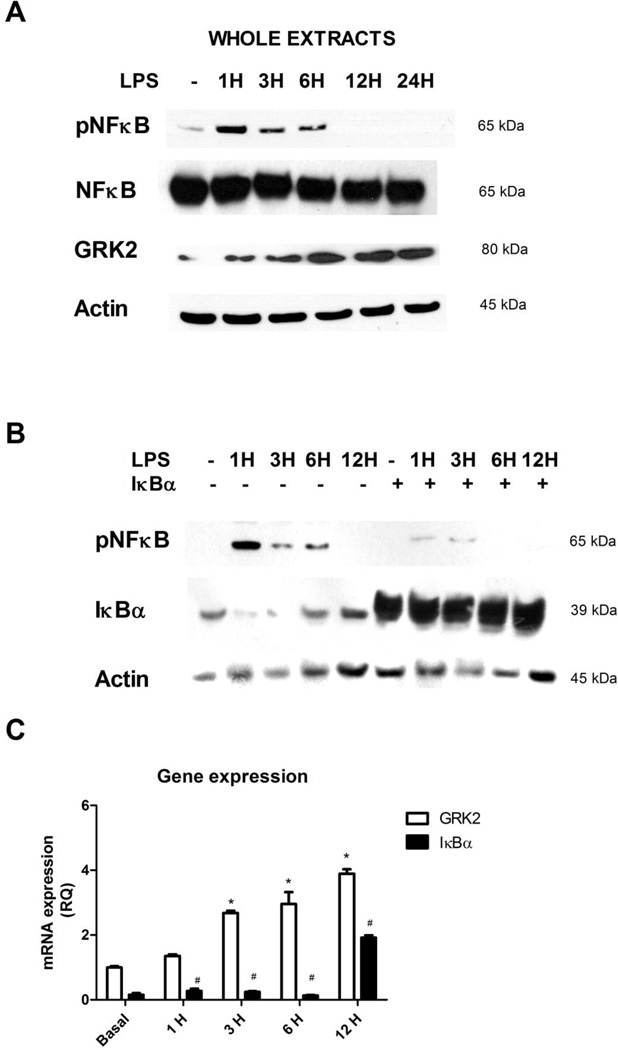
Given the key role of the transcription factor NFκB in the regulation of inflammatory processes, in cultured macrophages, Raw 264.7, we evaluated the activation of NFκB in response to LPS by western blot. The phosphorylation and activation of NFκB occurred early after LPS stimulation and decreased over time (Figure 1A). In these cells, LPS induced a time dependent increase of GRK2 protein levels (Figure 1A). This was in agreement with GRK2 gene expression which was evaluated by Real Time PCR. Indeed, the expression of GRK2 increased in a time dependent manner in response to LPS (Figure 1C). To assess if this phenomenon was dependent on NFκB transcriptional activity, we inhibited NFκB activity by overexpressing its known main regulator IκBα (Figure 1B). Such inhibition was confirmed by western blot analysis (Figure 1B) of the phosphorylated and activated form of NFκB. Real time PCR shows that the overexpression of IκBα reduced GRK2 gene expression in response to LPS (Figure 1C), suggesting that GRK2 expression is associated with NFκB activation in response to inflammation.
Figure 1. GRK2 levels and effects on inflammation.
A) The activation of NFκB and GRK2 levels were evaluated in cultured macrophages, Raw 264.7, in response to LPS (20 ng/ml) by western blot. The phosphorylation in Ser 536 and activation of NFκB occurred early after LPS stimulation and decreased over time. Total NFκB was used as loading control. LPS induced a time dependent increase of GRK2 protein levels. Images are representative of 3 independent experiments. B) To confirm the inhibition of NFκB activation by overexpression of IκBα, we evaluated IκBα levels and NFκB phosphorylation and activation by western blot. The overexpression of IκBα, induced by transient transfection of IκBα plasmid, is able to inhibit the activation of NFκB in response to LPS. Images are representative of 3 independent experiments. C) GRK2 gene expression was evaluated by Real Time PCR. GRK2 increased in a time dependent manner in response to LPS (20 ng/ml) and its expression was reduced by IκBα-dependent inhibition of NFκB activity; * p<0.05 vs basal, # p<0.05 vs LPS. Results are the mean of 5 independent experiments and are presented as mean±SEM.
Subcellular localization of GRK2 in response to LPS stimulation
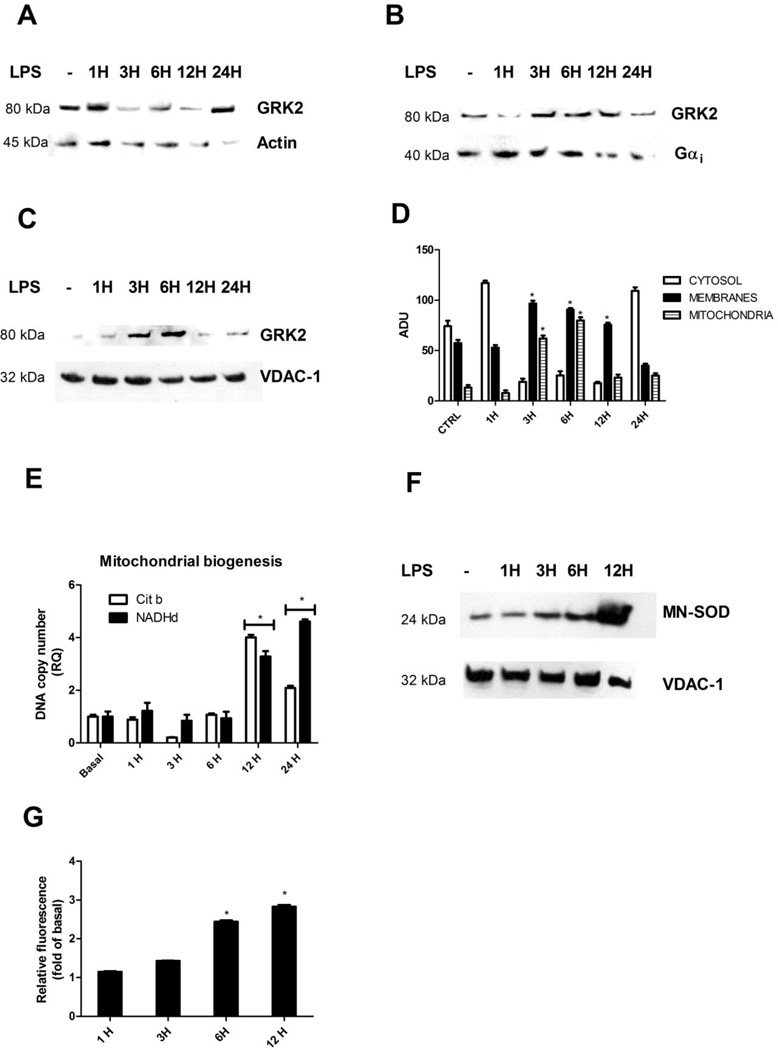
Since GRK2 levels were increased by LPS stimulation, we analyzed the subcellular distribution of this kinase. To this aim we purified membranes, cytosolic and mitochondrial extracts from macrophages after LPS stimulation. Western blot analysis of actin, histone 3 and calnexin levels was performed in purified extracts to exclude cross contamination of the different subcellular fractions (data not shown). Figure 2 shows that GRK2 is mainly localized in cytosol both basally and after 1 hour of stimulation with LPS (Fig. 2A). After 3 hour of stimulation GRK2 moves to both plasma membrane (Figure 2B) and mitochondria (Figure 2C), decreasing in the cytosol (Figure 2A). At 3 and 6 hours of stimulation with LPS, GRK2 significantly accumulated into mitochondria (Figure 2C). Quantifications are shown in Figure 2D. This suggests a time dependent regulation of the subcellular localization of GRK2 in mitochondria and a particular effect of the kinase during sustained inflammation. Such phenomenon is selective for GRK2 since we demonstrated that GRK5, which is known to be involved in inflammatory responses (34), does not localize in mitochondria (Supplementary Figure 1). In order to confirm the increase of GRK2 levels in mitochondria in response to LPS, we performed a kinase assay in mitochondrial extracts from macrophages basally and after 6 hours of stimulation with LPS, using rhodopsin as substrate. Rhodopsin phosphorylation increased in response to LPS respect to control (Supplementary Figure 2), thus confirming LPS dependent increase of GRK2 levels in mitochondria.
Figure 2. GRK2 levels and its subcellular localization during inflammation.
A-D) Cultured macrophages were stimulated with LPS and GRK2 expression was analyzed in membrane, cytosolic and mitochondrial extracts by western blot. GRK2 is mainly localized in cytosol both basally and after 1 hour of stimulation with LPS (20 ng/ml) (A). After 3 hour of stimulation GRK2 moves to both plasma membrane (B) and mitochondria (C), decreasing in the citosol (A). At 3 and 6 hours of stimulation with LPS, GRK2 significantly accumulated into mitochondria (C). Actin, Gαi and VDAC-1 were used as loading controls for cytosolic, membrane and mitochondrial samples, respectively. Images are representative of 5 independent experiments. and the media of quantifications are shown in bar graph (D) *p<0.05 vs basal. E) Mitochondrial biogenesis in macrophages was evaluated by Real Time PCR. Cytochrome b and NADH dehydrogenase-DNA copy number were evaluated in response to LPS (20 ng/ml). Mitochondrial biogenesis was impaired by LPS stimulation and was restored at later time points. * p<0.05 vs basal. Results are the mean of 5 independent experiments and are presented as mean±SEM. F-G) In agreement with results of mitochondrial biogenesis, mitochondrial mass, evaluated by western blot using the antibody against Mn-SOD (F) and fluorescence analysis using the fluorescent probe Mitotracker (G), analyzed in microplate reader, were increased in response to LPS; * p<0.05 vs basal. Results are the mean of 3 independent experiments.
Mitochondrial GRK2 accumulation associates with mitochondrial biogenesis
Our data confirm the recent demonstration that GRK2 enters into mitochondria and regulates mitochondrial biogenesis (25). Indeed, the overexpression of GRK2 increases mitochondrial biogenesis while GRK2 gene deletion exerts the opposite effect (25). It is known that inflammatory stimuli creates a cycle of oxidative stress that causes mitochondrial damage and initiates retrograde signals for the transcriptional activation of mitochondrial biogenesis (24). Therefore, we evaluated mitochondrial biogenesis in macrophages in response to LPS stimulation focusing on cytochrome b and NADH dehydrogenase-DNA copy number. In macrophages, LPS stimulation causes an impairment of mitochondrial biogenesis which is restored at later time points (12–24 hours) (Figure 2E). Accordingly, manganese superoxide dismutase (Mn-SOD), an anti-oxidant enzyme, increased in a time dependent manner in response to LPS (Figure 2F). Similar results were obtained by analyzing mitochondrial mass using a specific fluorescent probe (Mitotracker). Mitochondrial mass progressively increased in response to stimulation with LPS (Figure 2G).
βARK-ct induces GRK2 accumulation into mitochondria
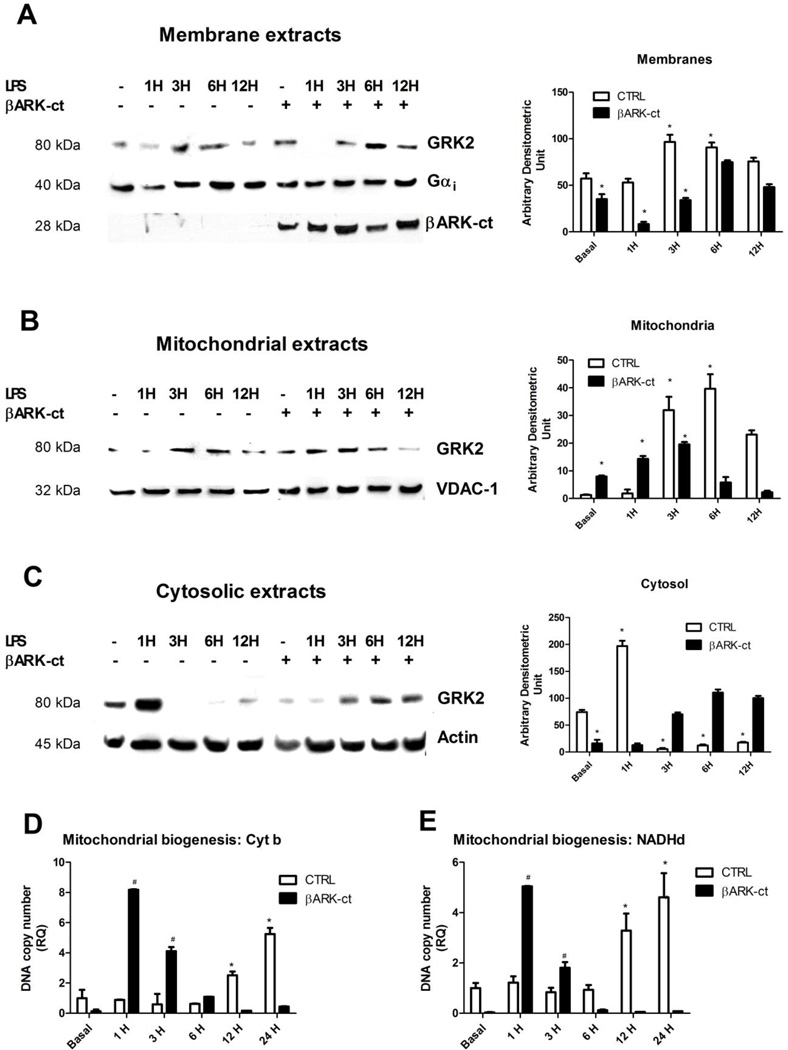
Based on previous results, we hypothesized that the late GRK2 accumulation into mitochondria might participate to the rescue of mitochondrial biogenesis after LPS stimulation. We therefore sought to enrich GRK2 in mitochondria. It has been previously demonstrated that GRK2 can be displaced from one cellular compartment to another using the overexpression of a truncated mutant of GRK2 reproducing its carboxy-terminal domain (βARK-ct) (35). Thus, we evaluated whether βARK-ct could move GRK2 out of the cell membrane towards mitochondria. To this aim, we overexpressed βARK-ct in macrophages by means of adenoviral mediated gene transfer (AdβARK-ct) and evaluated GRK2 levels on plasma membrane, cytosolic and mitochondrial extracts. Figure 3 shows that βARK-ct expression led to GRK2 moving from plasma membrane (Figure 3A) toward mitochondria (Figure 3B) and this phenomenon was increased in response to LPS (Figure 3A-B). Cytosolic GRK2 levels changed accordingly (Figure 3C). Such results were quantified and showed in bar graph (Figure 3). These data suggest that the displacement of GRK2 from plasma membrane leads to a different subcellular localization of this kinase.
Figure 3. βARK-ct induces GRK2 accumulation into mitochondria.
A-C) βARK-ct was expressed in macrophages by means of adenoviral gene transfer (AdβARK-ct) and GRK2 levels were evaluated on plasma membrane and mitochondrial extracts by western blot. βARK-ct reduced GRK2 expression on plasma membrane (A) and increased GRK2 localization into mitochondria both in basal condition and after stimulation with LPS (20ng/ml) (B). Gαi and VDAC were used as loading controls for plasma membrane and mitochondrial extracts, respectively. βARK-ct was visualized using the specific antibody against the carboxy-terminal domain of GRK2. Cytosolic localization of GRK2 is shown in panel C. Actin was used as loading control. Images are representative of 3 independent experiments and the media of quantifications are shown in bar graph * p<0.05 vs basal. D-E) Mitochondrial biogenesis was evaluated by Real time PCR. Cytochrome b (C) and NADH dehydrogenase (D) DNA copy numbers were analyzed in response to LPS (20ng/ml) in presence or absence of βARK-ct. Mitochondrial DNA copy numbers increased after 12 and 24 hours of stimulation with LPS and βARK-ct anticipated such increase at 1 and 3 hours after stimulation; * p<0.05 vs basal, # p<0.05 vs LPS. Results are the mean of 5 independent experiments and are presented as mean±SEM.
Mitochondrial GRK2 regulates mitochondrial biogenesis
We then evaluated the effect of βARK-ct dependent mitochondrial localization of GRK2 in inflammation. In particular, we evaluated mitochondrial biogenesis by Real Time PCR in response to LPS in presence or absence of βARK-ct. Mitochondrial DNA copy number of cytochrome b (Figure 3C) and NADH dehydrogenase (Figure 3D) were both increased after 12 and 24 hours of stimulation with LPS and βARK-ct anticipated such increase at 1 and 3 hours after stimulation (Figure 3C-D).
Mitochondrial GRK2 inhibits ROS production and inflammatory cytokines expression
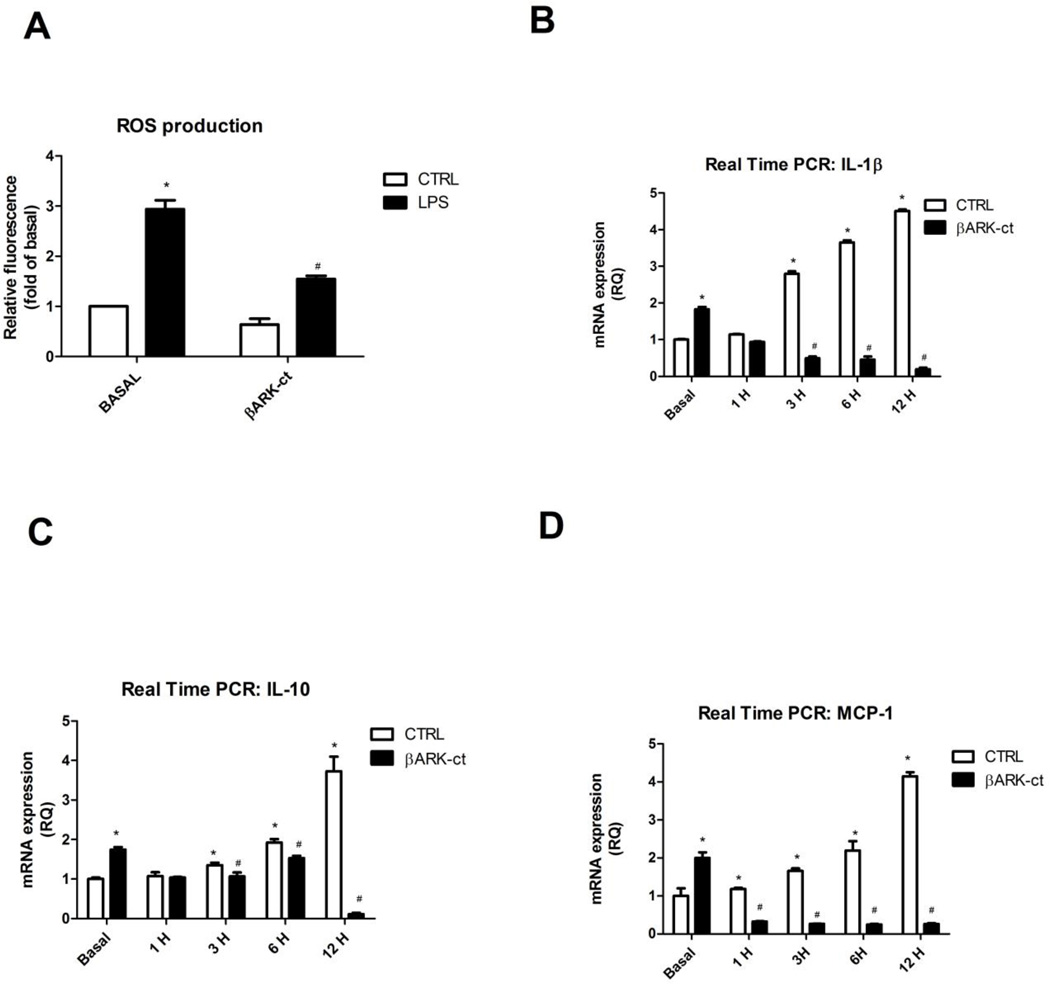
As an alternative to demonstrate the positive effect of GRK2, we analyzed reactive oxygen species (ROS) production in macrophages. To this aim, RAW264.7 were treated with DCFH for 15 min and then stimulated with LPS. ROS production in LPS-activated macrophages progressively increased over time (Figure 4A). The treatment with βARK-ct reduced ROS production basally and in response to LPS, with a decline starting from the 6th hour of stimulation (Figure 4A). The inhibition of ROS production induced by βARK-ct was associated to a reduction of cytokines production. Indeed, βARK-ct inhibited IL-1β(Figure 4B), IL-10 (Figure 4C) and MCP-1 (Figure 4D) gene expression in response to LPS, evaluated by Real Time PCR. All these data suggest that the mitochondrial localization of GRK2 is key for inhibition of inflammation.
Figure 4. Mitochondrial GRK2 inhibits ROS production and inflammatory cytokines expression.
A-D) RAW264.7 were treated with DCFH for 15 min and then stimulated with LPS (20ng/ml) for different time points. ROS production in LPS-activated macrophages was progressively increased with time (A). βARK-ct reduced ROS production both basally and after LPS stimulation (A). The inhibition of ROS production by βARK-ct was associated to a reduction of cytokines gene expression (B-D). Indeed, βARK-ct inhibited IL-1β (B), IL-10 (C) and MCP-1 (D) gene expression, evaluated by Real Time PCR, in response to LPS; * p<0.05 vs basal, # p<0.05 vs LPS. Results are the mean of 5 independent experiments and are presented as mean±SEM.
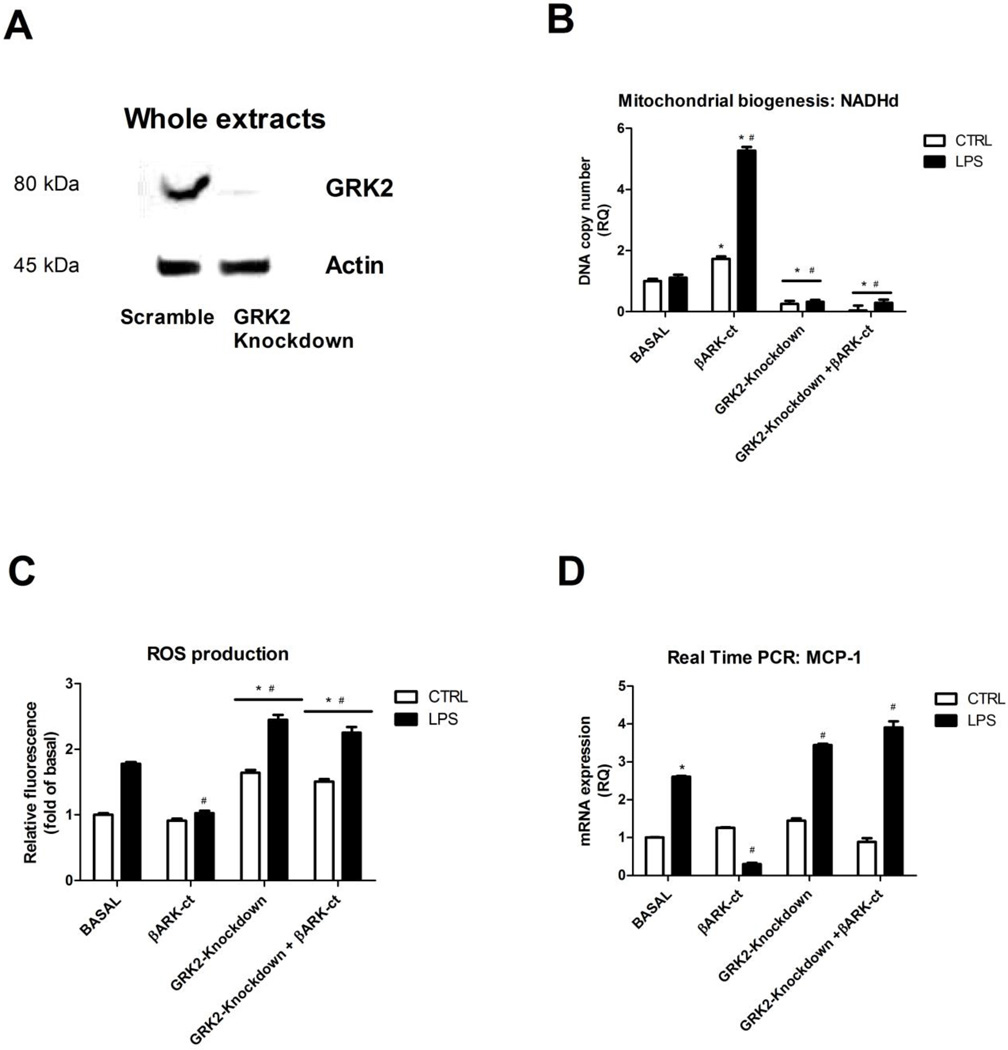
GRK2 silencing enhances inflammatory responses
So far our data do not allow to distinguish if the beneficial effects of βARK-ct were due to GRK2 accumulation into mitochondria rather than to GRK2 independent effect of βARK-ct. Therefore, we induced GRK2 gene silencing by means of specific siRNA. Gene silencing was evaluated by western blot analysis. Transfection of the specific siRNA induces a significant reduction of GRK2 expression respect to the transfection of Scramble (Figure 5A). Figure 5 shows that the silencing of GRK2 was inhibitory per se on mitochondrial biogenesis (Fig. 5B), increased ROS production (Fig. 5C) and increased cytokines gene expression (Fig. 5D) in response to LPS stimulation. These findings confirm the key role of GRK2 in inflammatory settings. Interestingly, none of these phenotypes could be restored by the concomitant overexpression of βARK-ct (Fig. 5B-D), thus suggesting that the effects of βARK-ct in response to inflammatory stimuli are not independent from GRK2 but rather depends on the ability of βARK-ct to regulate GRK2 subcellular localization. These data confirmed the protective role of GRK2 in inflammation due to its ability to restore mitochondrial biogenesis.
Figure 5. GRK2 silencing enhances inflammatory responses.
A-D) GRK2 gene silencing was induced by transfection of the specific siRNA. Western blot analysis was performed to evaluate the rate of reduction of GRK2. The loss of GRK2 reduced mitochondrial biogenesis (B), increased ROS production (C) and increased cytokines gene expression (D) in response to LPS stimulation (20ng/ml) and βARK-ct treatment is not able to restore these phenotypes when GRK2 is silenced. Results are the mean of 5 independent experiments and are presented as mean±SEM.; * p<0.05 vs basal, # p<0.05 vs LPS.
Discussion
G-protein coupled receptor kinase 2 (GRK2) is a member of a kinase family originally known for its role in the phosphorylation and desensitization of G-protein coupled receptors (1, 2). In the last decade new functions for this kinase have been evidenced in several conditions (cardiovascular, inflammatory diseases or cancer) characterized by impaired levels of GRK2 (10, 12, 13, 36). These findings suggest that GRK2 could be a potential interesting diagnostic marker and therapeutic target for several conditions. This pleiotropism of GRK2 is possibly due to some emerging properties of the kinase as well as to yet unexplored features. We focused on the recently discovered ability of the kinase to localize in mitochondria and, in particular, the effect of mitochondrial localization of GRK2 in inflammation. It is already known that GRK2 levels increase in inflammation. Our study though provides a series of significant advancements in knowledge. First of all we demonstrate that GRK2 regulation in macrophages is dependent on NFκB transcriptional activity. This result is in agreement with previous reports which show a similar NFκB dependent expression also for other GPCR kinases (37). The different role of GRK2 in the regulation of intracellular signaling depends on specific stimuli, cell type or physiological context (12), but also the subcellular localization is a fine predictor of its function. Indeed, GRK2 regulates GPCR activation when it is localized on plasma membrane (38, 39); it regulates intracellular signaling in a phosphorylation-dependent or - independent manner when it is localized in the cytosol (40); GRK2 regulates mitochondrial biogenesis when it accumulates into mitochondria (25). Thus, GRK2 function is strictly correlated to its subcellular localization, as it was previously demonstrated also for other kinases (26, 41, 42). The second major finding is that GRK2 accumulates into mitochondria in response to inflammatory stimuli in a time dependent fashion activating mitochondrial biogenesis and this phenomenon is selective for GRK2 since GRK5, also known to be involved in inflammation, does not localize in mitochondria both basally and after LPS stimulation. Giving the emerging key role of GRK2 in mitochondria which is able to enhance ATP accumulation, mitochondrial mass and consequent resistance to ischemic insult, the possibility to regulate its subcellular localization represents an efficient therapeutic strategy for all those conditions in which cell is under stress. Here we use βARK-ct as regulator of GRK2 displacement within the cell during inflammation. Indeed, an important evidence of this study is that the overexpression of βARK-ct hastens the localization of GRK2 into mitochondria in response to LPS-induced inflammation. Stimulation of the innate immune system by activation of toll-like receptors (TLR) generates pro-inflammatory mediators, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), necessary for optimal host defense, but which also contribute to mitochondrial damage through oxidative stress and other mechanisms (24). To protect its energy supply, host cells sense mitochondrial damage and initiate mitochondrial biogenesis under the control of an inducible transcriptional program that also activates antioxidant and anti-inflammatory gene expression (24). Accordingly to our previous observations (25), here we demonstrated that GRK2 moves to mitochondria to exert an anti-inflammatory effect in macrophages by restoring mitochondrial function. βARK-ct ameliorates this effect because it anticipates GRK2 localization into mitochondria leading to an increase of mt-DNA transcription, a reduction of ROS production and inflammatory cytokines expression in response to LPS stimulation. A third important finding of our work is that the positive effect of βARK-ct treatment cannot be limited to the reduction of GRK2 dependent desensitization. The key role of βARK-ct-induced inhibition of GRK2 in cardiovascular diseases is known and βARK-ct is often proposed as promising gene therapy strategy to increase contractility and function of the failing heart (43). Here we show that the role of βARK-ct is not limited to the displacement of GRK2 from plasma membrane and consequently up-regulation of GPCR signaling. Indeed, besides the loss of GPCRs desensitization, βARK-ct accumulates GRK2 in different cellular compartments. This finding is in agreement with recent literature suggesting that βARK-ct ameliorates cardiac metabolism and cardiac contractility in experimental myocardial infarction (44). However, our data appear to be in contrast with those reported very recently by Chen et al. (45). Indeed, these Authors show that βARK-ct prevents stress-induced translocation of GRK2 to mitochondria in neonatal rat ventricular myocytes and decreases myocytes apoptosis. Differences in the timing and the experimental model might explain these opposing results; GRK2 could have different effects depending on specific cell type and stimulus, as it happens for other proteins: NFκB for instance has a pro-apoptotic function in cardiac cells and is anti-apoptotic in other cell types (26, 46). Furthermore, our data are in agreement with Patial and co-authors which demonstrated that GRK2 deletion in myeloid cells induces inflammation in vivo (15). Altogether, these findings confirm the key role of GRK2 in the regulation of inflammation. A possible limitation of this study could be the experimental model, the RAW264.7 cells. These cells are macrophage like cell line which maintains many of the properties of macrophages (No production, phagocytosis, extreme sensitivity to TLR agonists and motility). However, differences in response of this cell line compared to naive macrophages, might occur and should be taken into account.
In conclusion, our study clarifies the involvement of GRK2 in inflammatory settings and underlines the importance of the regulation of protein subcellular localization to modulate intracellular signaling both in physiological and pathological contexts. In particular, the regulation of GRK2 mitochondrial translocation could be a potential therapeutic target for inflammatory diseases to restore impaired mitochondrial function and oxidative stress.
Supplementary Material
Acknowledgments
Funding for this study was provided by the Italian Ministry of University and Scientific Research (PRIN2009-prot. 2009EL5WBP_001) and the Italian Society of Hypertension.
Abbreviations
- GRK2
G protein coupled receptor kinase 2
- LPS
lypolysaccharide
- ROS
reactive oxygen species
- NFκB
Nuclear Factor kappa B
References
- 1.Penela P, Ribas C, Mayor F., Jr Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. (Translated from eng) Cell Signal. 2003;15(11):973–981. doi: 10.1016/s0898-6568(03)00099-8. (in eng). [DOI] [PubMed] [Google Scholar]
- 2.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. (Translated from eng) Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. (in eng). [DOI] [PubMed] [Google Scholar]
- 3.Hupfeld CJ, Olefsky JM. Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. (Translated from eng) Annu Rev Physiol. 2007;69:561–577. doi: 10.1146/annurev.physiol.69.022405.154626. (in eng). [DOI] [PubMed] [Google Scholar]
- 4.Cipolletta E, et al. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. (Translated from eng) Cardiovasc Res. 2009;84(3):407–415. doi: 10.1093/cvr/cvp252. (in eng). [DOI] [PubMed] [Google Scholar]
- 5.Peregrin S, et al. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. (Translated from eng) Curr Biol. 2006;16(20):2042–2047. doi: 10.1016/j.cub.2006.08.083. (in eng). [DOI] [PubMed] [Google Scholar]
- 6.Ribas C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. (Translated from eng) Biochim Biophys Acta. 2007;1768(4):913–922. doi: 10.1016/j.bbamem.2006.09.019. (in eng). [DOI] [PubMed] [Google Scholar]
- 7.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. (G-protein-coupled-receptor kinases mediate TNFalpha-induced NFkappaB signalling via direct interaction with and phosphorylation of IkappaBalpha. (Translated from eng) Biochem J. 425(1):169–178. doi: 10.1042/BJ20090908. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallese M, Mariggio S, D'Urbano E, Iacovelli L, De Blasi A. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated galphaq. (Translated from eng) Mol Pharmacol. 2000;57(4):826–831. (in eng). [PubMed] [Google Scholar]
- 9.Ferguson SS. Phosphorylation-independent attenuation of GPCR signalling. (Translated from eng) Trends Pharmacol Sci. 2007;28(4):173–179. doi: 10.1016/j.tips.2007.02.008. (in eng). [DOI] [PubMed] [Google Scholar]
- 10.Ciccarelli M, Cipolletta E, Iaccarino G. (GRK2 at the control shaft of cellular metabolism. (Translated from eng) Curr Pharm Des. 18(2):121–127. doi: 10.2174/138161212799040493. (in eng). [DOI] [PubMed] [Google Scholar]
- 11.Ciccarelli M, et al. (G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. (Translated from eng) Circulation. 123(18):1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr (The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. (Translated from eng) Br J Pharmacol. 160(4):821–832. doi: 10.1111/j.1476-5381.2010.00727.x. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penela P, Ribas C, Aymerich I, Mayor F., Jr New roles of G protein-coupled receptor kinase 2 (GRK2) in cell migration. (Translated from eng) Cell Adh Migr. 2009;3(1):19–23. doi: 10.4161/cam.3.1.7149. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vroon A, Heijnen CJ, Kavelaars A. GRKs and arrestins: regulators of migration and inflammation. (Translated from eng) J Leukoc Biol. 2006;80(6):1214–1221. doi: 10.1189/jlb.0606373. (in eng). [DOI] [PubMed] [Google Scholar]
- 15.Patial S, et al. (Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. (Translated from eng) J Cell Physiol. 226(3):627–637. doi: 10.1002/jcp.22384. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arraes SM, et al. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. (Translated from eng) Blood. 2006;108(9):2906–2913. doi: 10.1182/blood-2006-05-024638. (in eng). [DOI] [PubMed] [Google Scholar]
- 17.Giorelli M, Livrea P, Trojano M. Post-receptorial mechanisms underlie functional disregulation of beta2-adrenergic receptors in lymphocytes from Multiple Sclerosis patients. (Translated from eng) J Neuroimmunol. 2004;155(1–2):143–149. doi: 10.1016/j.jneuroim.2004.05.013. (in eng). [DOI] [PubMed] [Google Scholar]
- 18.Lombardi MS, et al. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. (Translated from eng) J Immunol. 2001;166(3):1635–1640. doi: 10.4049/jimmunol.166.3.1635. (in eng). [DOI] [PubMed] [Google Scholar]
- 19.Lombardi MS, et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. (Translated from eng) FASEB J. 1999;13(6):715–725. doi: 10.1096/fasebj.13.6.715. (in eng). [DOI] [PubMed] [Google Scholar]
- 20.Vroon A, et al. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. (Translated from eng) J Immunol. 2005;174(7):4400–4406. doi: 10.4049/jimmunol.174.7.4400. (in eng). [DOI] [PubMed] [Google Scholar]
- 21.Vroon A, Lombardi MS, Kavelaars A, Heijnen CJ. Changes in the G-protein-coupled receptor desensitization machinery during relapsing-progressive experimental allergic encephalomyelitis. (Translated from eng) J Neuroimmunol. 2003;137(1–2):79–86. doi: 10.1016/s0165-5728(03)00050-x. (in eng). [DOI] [PubMed] [Google Scholar]
- 22.Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. (Translated from eng) Crit Care Med. 2007;35(9 Suppl):S441–S448. doi: 10.1097/01.CCM.0000278049.48333.78. (in eng). [DOI] [PubMed] [Google Scholar]
- 23.Haden DW, et al. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. (Translated from eng) Am J Respir Crit Care Med. 2007;176(8):768–777. doi: 10.1164/rccm.200701-161OC. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piantadosi CA, Suliman HB. (Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. (Translated from eng) Biochim Biophys Acta. 1820(4):532–541. doi: 10.1016/j.bbagen.2012.01.003. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fusco A, et al. (Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. (Translated from eng) Cell Signal. 24(2):468–475. doi: 10.1016/j.cellsig.2011.09.026. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorriento D, et al. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. (Translated from eng) Proc Natl Acad Sci U S A. 2008;105(46):17818–17823. doi: 10.1073/pnas.0804446105. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. (Translated from eng) Proc Natl Acad Sci U S A. 2005;102(5):1442–1447. doi: 10.1073/pnas.0409532102. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iaccarino G, et al. AKT participates in endothelial dysfunction in hypertension. (Translated from eng) Circulation. 2004;109(21):2587–2593. doi: 10.1161/01.CIR.0000129768.35536.FA. (in eng). [DOI] [PubMed] [Google Scholar]
- 29.Ciccarelli M, et al. (Impaired neoangiogenesis in beta(2)-adrenoceptor gene-deficient mice: restoration by intravascular human beta(2)-adrenoceptor gene transfer and role of NFkappaB and CREB transcription factors. (Translated from eng) Br J Pharmacol. 162(3):712–721. doi: 10.1111/j.1476-5381.2010.01078.x. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cittadini A, et al. Insulin-like growth factor-1 protects from vascular stenosis and accelerates re-endothelialization in a rat model of carotid artery injury. (Translated from eng) J Thromb Haemost. 2009;7(11):1920–1928. doi: 10.1111/j.1538-7836.2009.03607.x. (in eng). [DOI] [PubMed] [Google Scholar]
- 31.Iaccarino G, et al. Beta2-adrenergic receptor polymorphisms and treatment-induced regression of left ventricular hypertrophy in hypertension. (Translated from eng) Clin Pharmacol Ther. 2006;80(6):633–645. doi: 10.1016/j.clpt.2006.09.006. (in eng). [DOI] [PubMed] [Google Scholar]
- 32.Iaccarino G, et al. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. (Translated from eng) Circ Res. 2005;97(11):1182–1189. doi: 10.1161/01.RES.0000191541.06788.bb. (in eng). [DOI] [PubMed] [Google Scholar]
- 33.Sorriento D, et al. A new synthetic protein, TAT-RH, inhibits tumor growth through the regulation of NFkappaB activity. (Translated from eng) Mol Cancer. 2009;8:97. doi: 10.1186/1476-4598-8-97. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patial S, et al. (G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. (Translated from eng) J Cell Physiol. 226(5):1323–1333. doi: 10.1002/jcp.22460. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daaka Y, et al. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. (Translated from eng) Proc Natl Acad Sci U S A. 1997;94(6):2180–2185. doi: 10.1073/pnas.94.6.2180. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penela P, et al. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. (Translated from eng) Cardiovasc Res. 2006;69(1):46–56. doi: 10.1016/j.cardiores.2005.09.011. (in eng). [DOI] [PubMed] [Google Scholar]
- 37.Islam KN, Koch WJ. Involvement of nuclear factor kappaB (NF-kappaB) signaling pathway in regulation of cardiac G protein-coupled receptor kinase 5 (GRK5) expression. (Translated from eng) J Biol Chem. 2012;287(16):12771–12778. doi: 10.1074/jbc.M111.324566. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. (Translated from eng) Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. (in eng). [DOI] [PubMed] [Google Scholar]
- 39.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. (Translated from eng) Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. (in eng). [DOI] [PubMed] [Google Scholar]
- 40.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. (G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. (Translated from eng) Pharmacol Ther. 133(1):40–69. doi: 10.1016/j.pharmthera.2011.08.001. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martini JS, et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. (Translated from eng) Proc Natl Acad Sci U S A. 2008;105(34):12457–12462. doi: 10.1073/pnas.0803153105. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomel R, et al. The localization of protein kinase Cdelta in different subcellular sites affects its proapoptotic and antiapoptotic functions and the activation of distinct downstream signaling pathways. (Translated from eng) Mol Cancer Res. 2007;5(6):627–639. doi: 10.1158/1541-7786.MCR-06-0255. (in eng). [DOI] [PubMed] [Google Scholar]
- 43.Lymperopoulos A. (GRK2 and beta-arrestins in cardiovascular disease: Something old, something new. (Translated from eng) Am J Cardiovasc Dis. 1(2):126–137. (in eng). [PMC free article] [PubMed] [Google Scholar]
- 44.Raake PW, et al. (AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. (Translated from Eng) Eur Heart J. doi: 10.1093/eurheartj/ehr447. (in Eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M, et al. (Prodeath signaling of g protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. (Translated from eng) Circ Res. 112(8):1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karin M, Lin A. NF-kappaB at the crossroads of life and death. (Translated from eng) Nat Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. (in eng). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.